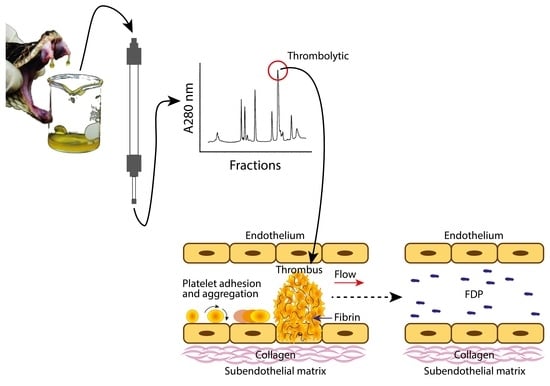
Direct Fibrinolytic Snake Venom Metalloproteinases Affecting Hemostasis: Structural, Biochemical Features and Therapeutic Potential
Abstract
:1. Introduction
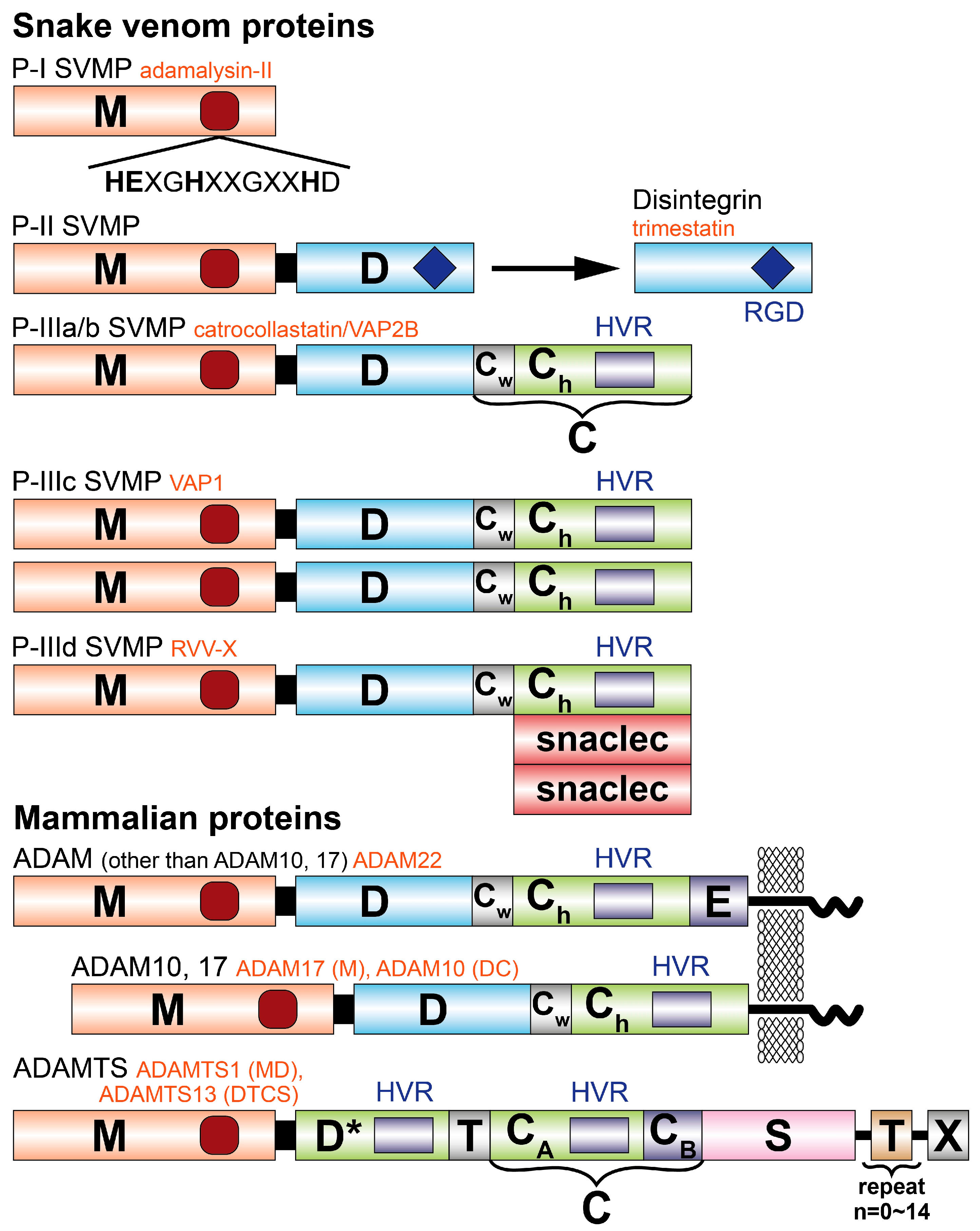
2. Structure and Classification of Snake Venom Metalloproteinases
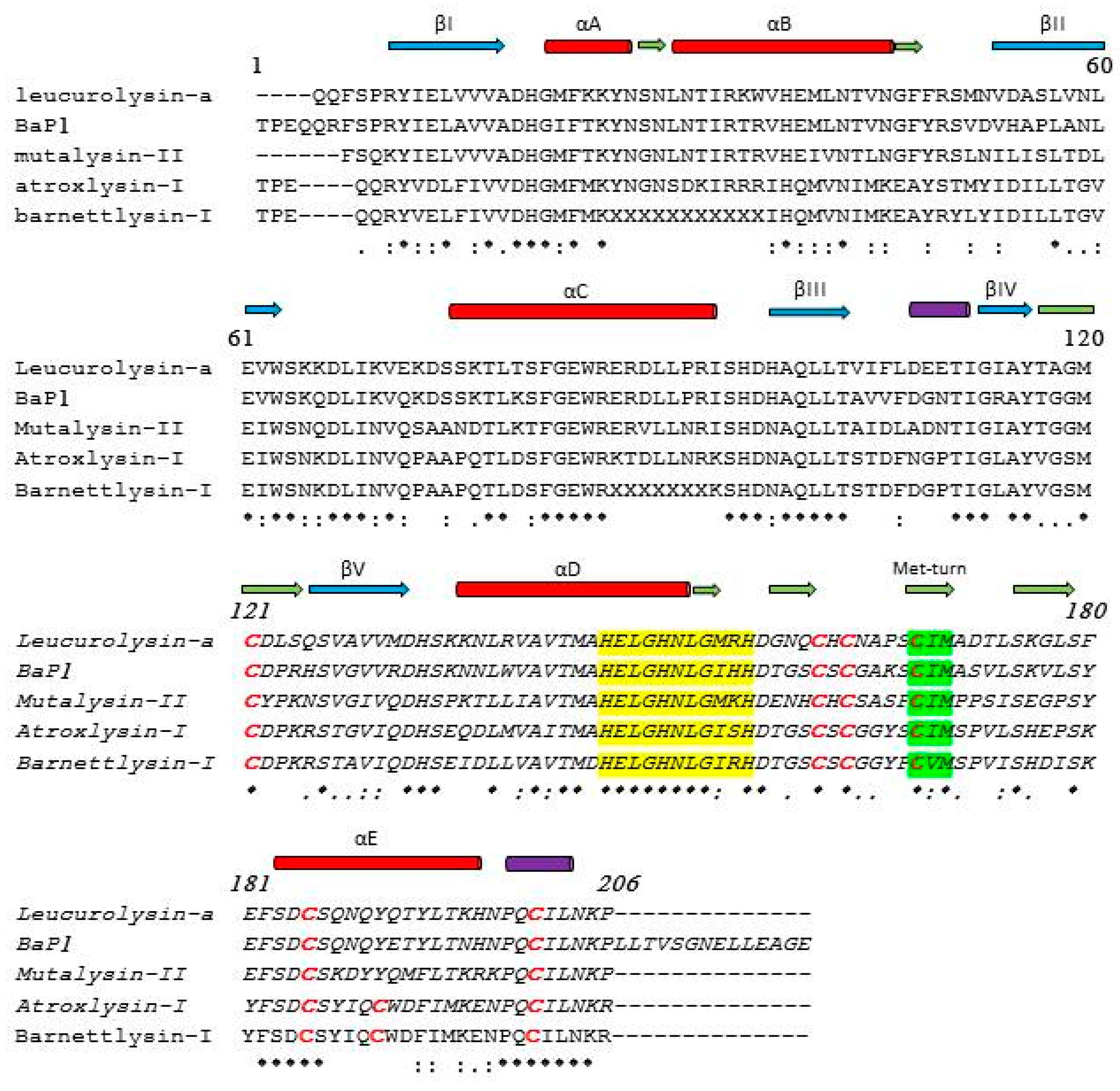
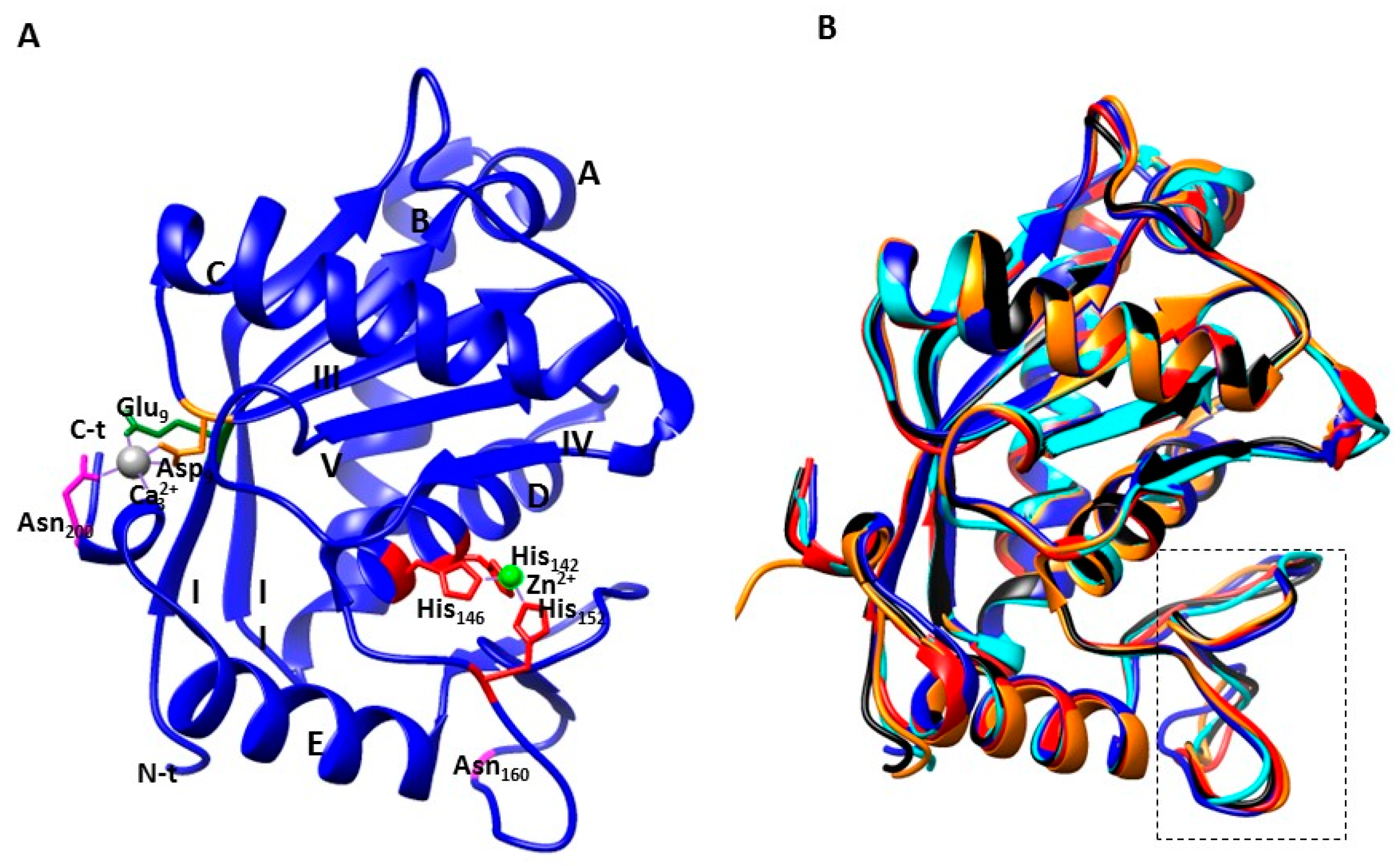
3. Three-Dimensional Structures of P-I Class SVMPs
4. Action on Some Plasma and ECM Protein Substrates
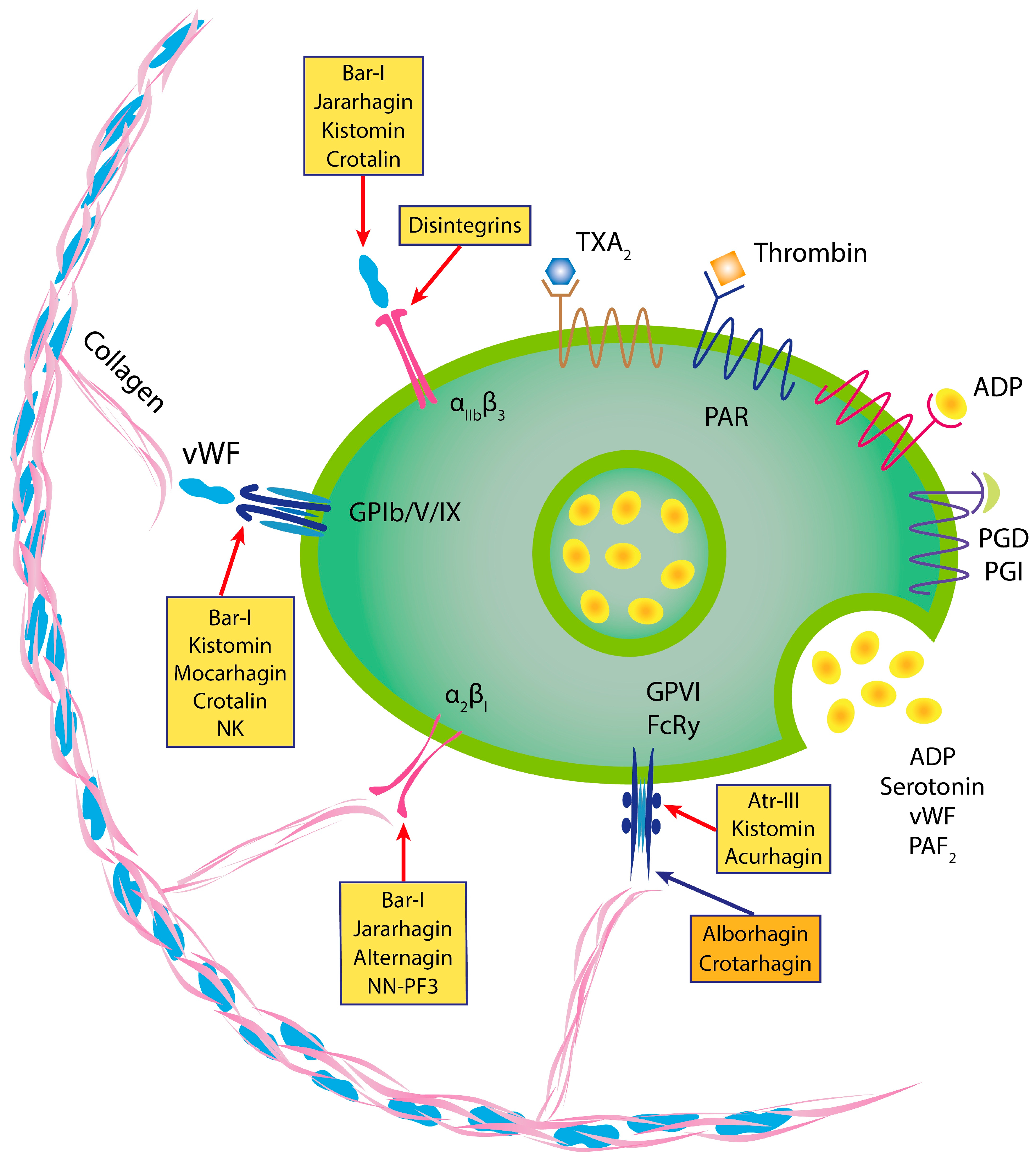
5. Antiplatelet Properties of P-I SVMPs
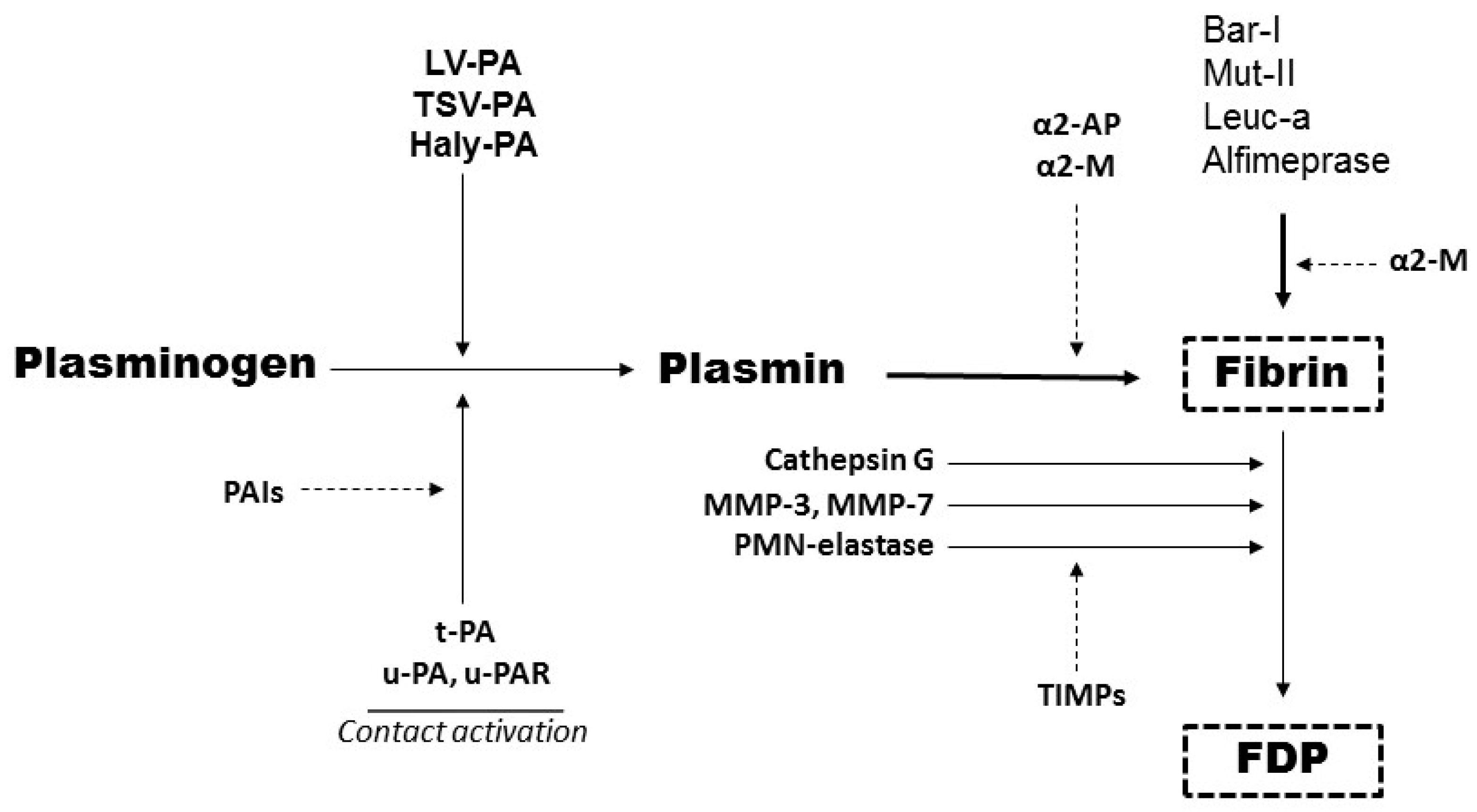
6. Biochemical Advantages of P-I SVMPs in Comparison to Plasminogen Activators (PAs)
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
| Proteinase | Bonds Cleaved | Reference |
|---|---|---|
| Oxidized Insulin B chain | ||
| leuc-a | Ala14-Leu15, Tyr16-Leu17 | [29] |
| atr-I | Ala14-Leu15, Tyr16-Leu17 | [28] |
| BaP1 | Ala14-Leu15, Tyr16-Leu17 | [36] |
| mut-II | His5-Leu6, His11-Leu11, Ala14-Leu15, Phe24-Phe25 | [78] |
| Human α2-M (bait region) | ||
| leuc-a | Arg696-Leu697 | [105] |
| atr-I | Arg696-Leu697 | [unpublished] |
| mut-II | Arg696-Leu697 | [unpublished] |
| bar-I | Arg696-Leu697 | [34] |
| Human fibrinogen Aα-chain | ||
| leuc-a | Lys413-Leu414 | [105] |
| atr-I | Lys413-Leu414 | [unpublished] |
| mut-II | Lys413-Leu414 | [unpublished] |
| bar-I | Lys413-Leu414 | [34] |
References
- Casewell, N.R.; Wüster, W.; Vonk, F.J.; Harrison, R.A.; Fry, B.G. Complex cocktails: The evolutionary novelty of venoms. Trends Ecol. Evol. 2013, 28, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Fry, B.G. The structural and functional diversification of the Toxicofera reptile venom system. Toxicon 2012, 60, 434–448. [Google Scholar] [CrossRef] [PubMed]
- King, G.F. Venoms as platform for human drug: Translating toxins into therapeutics. Expert Opin. Biol. Ther. 2011, 11, 1469–1484. [Google Scholar] [CrossRef] [PubMed]
- Markland, F.S. Snake venoms and the hemostatic system. Toxicon 1998, 36, 1749–1800. [Google Scholar] [CrossRef]
- Kang, T.S.; Georgieva, D.; Genov, N.; Murakami, M.T.; Sinha, M.; Kumar, R.P.; Kaur, P.; Kurmar, W.S.; Dey, S.; Sharma, S.; et al. Enzymatic toxins from snake venoms: Structural characterization and mechanism of catalysis. FEBS J. 2011, 278, 4544–4576. [Google Scholar] [CrossRef] [PubMed]
- Sajevic, T.; Leonardi, A.; Krizaj, I. Haemostatically active proteins in snake venoms. Toxicon 2011, 57, 627–645. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Clemetson, J.M.; Clemetson, K.J. Snake venoms and hemostasis. J. Thromb. Haemost. 2005, 3, 1791–1799. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, Y.; Morita, T. Snake venom components affecting blood coagulation and the vascular system: Structural similarities and marked diversity. Curr. Pharm. Des. 2007, 13, 2927–2934. [Google Scholar] [CrossRef]
- Fox, J.W.; Serrano, S.M.T. Approaching the golden age of natural product pharmaceuticals from venom libraries: An overview of toxins and toxin-derivatives currently involved in therapeutic or diagnostic applications. Curr. Pharm. Des. 2007, 13, 2927–2934. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.S. Natural products to drugs: Natural product derived compounds in clinical trials. Nat. Prod. Rep. 2005, 22, 162–195. [Google Scholar] [CrossRef] [PubMed]
- Paterson, I.; Anderson, E.A. The renaissance of natural products as drug candidates. Science 2005, 310, 451–453. [Google Scholar] [CrossRef] [PubMed]
- White, J. Snake venom and coagulopathy. Toxicon 2005, 45, 951–967. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, J.M.; Rucavado, A.; Escalante, T.; Dias, C. Hemorrhage induced by snake venom metalloproteinases: Biochemical and biophysical mechanisms involved in microvessel damage. Toxicon 2005, 45, 997–1011. [Google Scholar] [CrossRef] [PubMed]
- Takeda, S.; Takeya, H.; Iwanaga, S. Snake venom metalloproteinases: Structure, function and relevance to the mammalian ADAM/ADAMTS family proteins. Biochim. Biophys. Acta 2012, 1824, 164–176. [Google Scholar] [CrossRef] [PubMed]
- Kohlhoff, M.; Borges, M.H.; Yarleque, A.; Cabezas, C.; Richardson, M.; Sanchez, E.F. Exploring the proteomes of the venoms of the Peruvian pit vipers. J. Proteom. 2012, 75, 2181–2195. [Google Scholar] [CrossRef] [PubMed]
- Calvete, J.J. Venomics: Integrative venom proteomics and beyond. Biochem. J. 2017, 474, 611–634. [Google Scholar] [CrossRef] [PubMed]
- Casewell, N.R.; Wagstaff, S.C.; Harrison, R.A.; Renjifo, C.; Wüster, W. Domain loss facilitates accelerated evolution and neofunctionalization of duplicate snake venom metalloproteinase toxin genes. Mol. Biol. Evol. 2011, 28, 2637–2649. [Google Scholar] [CrossRef] [PubMed]
- Takeda, S.; Igarashi, T.; Mori, H.; Araki, S. Crystal structures of VAP1 reveal ADAMs’ MDC domain architecture and its unique C-shaped scaffold. EMBO J. 2006, 25, 2388–2396. [Google Scholar] [CrossRef] [PubMed]
- Gomis-Ruth, F.X. Structural aspects of the metzinzin clan of metalloendopeptidases. Mol. Biotechnol. 2003, 24, 157–202. [Google Scholar] [CrossRef]
- Takeda, S. ADAM and ADAMTS family proteins and snake venom metalloproteinases: A structural overview. Toxins 2016, 8, 155. [Google Scholar] [CrossRef] [PubMed]
- Gomis-Rüth, F.X.; Kress, L.F.; Bode, W. First structure of a snake venom metalloproteinase: A prototype for matrix metalloproteinases/collagenases. EMBO J. 1993, 12, 4151–4157. [Google Scholar] [PubMed]
- Bjarnason, J.B.; Fox, J.B. Hemorrhagic metalloproteinases from snake venoms. Pharmacol. Ther. 1994, 62, 325–372. [Google Scholar] [CrossRef]
- Fox, J.W.; Serrano, S.M. Structural considerations of snake venom metalloproteinases, key members of the M12 reprolysin family of metalloproteinases. Toxicon 2005, 45, 969–985. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.W.; Serrano, S.M. Insights into and speculations about snake venom metalloproteinases (SVMP) synthesis, folding and disulfide bond formation and their contribution to venom complexity. FEBS J. 2008, 275, 3016–3030. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, E.F.; Gabriel, L.M.; Gontijo, S.; Gremski, L.H.; Veiga, S.S.; Evangelista, K.S.; Eble, J.A.; Richardson, M. Structural and functional characterization of a P-III metalloproteinase, leucurolysin-B from Bothrops leucurus venom. Arch. Biochem. Biophys. 2007, 468, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.F.; Chang, G.H.; Ouyang, C.H. Characterization of hemorrhagic principles from Trimeresurus. gramineous snake venom. Toxicon 1984, 22, 45–52. [Google Scholar] [CrossRef]
- Sanchez, E.F.; Magalhaes, A.; Diniz, C.R. Purification of a hemorrhagic factor (LHF-I) from the venom of the bushmaster snake (Lachesis muta muta). Toxicon 1987, 25, 611–619. [Google Scholar] [CrossRef]
- Sanchez, E.F.; Schneider, F.S.; Yarleque, A.; Borges, M.H.; Richardson, M.; Figueiredo, S.G.; Eble, J.A. A novel metalloproteinase atroxlysin-I from the Peruvian Bothrops atrox (jergon) snake venom acts both on ECM and platelets. Arch. Biochim. Biophys. 2010, 496, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Bello, C.; Hermogenes, A.L.; Magalhaes, A.; Veiga, S.S.; Gremski, L.H.; Richardson, M.; Sanchez, E.F. Isolation and characterization of a fibrinolytic proteinase from Bothrops leucurus (White-tailed jararaca) snake venom. Biochimie 2006, 88, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, E.F.; Diniz, C.R.; Richardson, M. The complete amino acid sequence of the haemorrhagic factor LHFII, a metalloproteinase isolated from the venom of the bushmaster snake (Lachesis muta muta). FEBS Lett. 1991, 282, 178–182. [Google Scholar] [CrossRef]
- Bode, W.; Gomis-Rüth, F.X.; Stocker, W. Astacins, serralysins, snake venom and matrix metalloproteinases exhibit identical zinc-binding environment (HEXXHXXGXXH) and Met-turn) and topologies and should be grouped into a common family, the “metzincins”. FEBS Lett. 1993, 331, 134–140. [Google Scholar] [CrossRef]
- Aguero, U.; Arantes, R.M.S.; Lazerda-Queiroz, N.; Mesquita, O.N.; Magalhaes, A.; Sanchez, E.F.; Carvalho-Tavares, J. Effect of mutalysin-II on vascular recanalization after thrombosis induction in the ear of hairless mice model. Toxicon 2007, 50, 698–707. [Google Scholar] [CrossRef] [PubMed]
- Rucavado, A.; Nuñez, J.; Gutierrez, J.M. Blister formation and skin damage induced by BaP1, a hemorrhagic metalloproteinase from the venom of the snake Bothrops asper. Int. J. Exp. Pathol. 1998, 79, 245–254. [Google Scholar] [PubMed]
- Sanchez, E.F.; Richardson, M.; Gremski, L.H.; Veiga, S.S.; Yarleque, A.; Stephan, N.; Martins-Lima, A.; Estevao-Costa, M.I.; Eble, J.A. A novel fibrinolytic metalloproteinase, barnettlysin-I from Bothrops barnetti (barnett’s pitviper) snake venom with anti-platelet properties. Biochim. Biophys. Acta 2016, 1860, 542–556. [Google Scholar] [CrossRef] [PubMed]
- Tsai, L.H.; Wang, Y.M.; Chiang, T.Y.; Chen, Y.L.; Huang, R.J. Purification, cloning and sequence analyses for pro-metalloprotease-disintegrin variants from Deinagkistrodon acutus venom and subclassification of the small venom metalloproteinases. Eur. J. Biochem. 2000, 267, 1359–1367. [Google Scholar] [CrossRef] [PubMed]
- Escalante, T.; Ortiz, N.; Rucavado, A.; Sanchez, E.F.; Richardson, M.; Fox, J.W.; Gutierrez, J.M. Role of collagens and perlecan in microvascular stability: Exploring the mechanism of capillary vessel damage by snake venom metalloproteinases. PLoS ONE 2011, 6, e28017. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, J.M.; Escalante, T.; Rucavado, A.; Herrera, C. Hemorrhage caused by snake venom metalloproteinases: A journey of discovering and understanding. Toxins 2016, 8, 93. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.F.; Holt, J.C.; Lukasiewicz, H.; Nieviaroswski, S. Trigramin, a low molecular weight peptide inhibiting fibrinogen interaction with platelet receptors expressed on glycoprotein IIb/IIIa. J. Biol. Chem. 1987, 262, 16157–16163. [Google Scholar] [PubMed]
- Marcinkiewicz, C.; Weinreb, P.H.; Calvete, J.J.; Kisiel, D.G.; Mousa, S.A.; Tuszynski, G.P.; Lobb, R.R. Obtustatin: A potent selective inhibitor of alfa1beta1 integrin in vitro and angiogenesis in vivo. Cancer Res. 2003, 63, 2020–2023. [Google Scholar] [PubMed]
- Calvete, J.J.; Marcinkiewicz, C.; Monleon, D.; Esteve, V.; Celda, B.; Juarez, P.; Sanz, L. Snake venom disintegrins: Evolution of structure and function. Toxicon 2005, 45, 1063–1074. [Google Scholar] [CrossRef] [PubMed]
- Coller, B.S. Platelet GPIIbIIIa antagonists, the first anti-integrin receptor therapeutic. J. Clin. Investig. 1997, 99, 1467–1471. [Google Scholar] [CrossRef] [PubMed]
- Michelson, A.D. Antiplatelet therapies for the treatment of cardiovascular disease. Nat. Rev. Drug Discov. 2010, 9, 154–169. [Google Scholar] [CrossRef] [PubMed]
- Clemetson, K.J. Snaclecs (snake C-type lectins) that inhibit or activate platelets by binding to receptors. Toxicon 2010, 56, 1236–1246. [Google Scholar] [CrossRef] [PubMed]
- Moura-da-Silva, A.M.; Theakston, R.D.G.; Crampton, J.M. Evolution of disintegrin cysteine-rich and mammalian matrix-degrading metalloproteinases: Gene duplication and divergence of a common ancestor rather than convergent evolution. J. Mol. Evol. 1996, 43, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.N.; Rates, B.; Richardson, M.; Guimaraes, B.G.; Sanchez, E.F.; Pimenta, A.M.; Nagem, R.A.P. Complete amino acid sequence, crystallization and preliminary X-ray diffraction studies of leucurolysin-a, a nonhemorrhagic metalloproteinase from Bothrops leucurus snake venom. Acta Cryst. 2009, F65, 798–801. [Google Scholar]
- Wallnoefer, H.G.; Lingott, T.; Gutierrez, J.M.; Merfort, I.; Liedi, K.R. Backbone flexibility controls the activity and specificity of a protein-protein interface: Specificity in snake venoms metalloproteases. J. Am. Chem. Soc. 2010, 132, 10330–10337. [Google Scholar] [CrossRef] [PubMed]
- Lingott, T.; Schleberger, C.; Gutierrez, J.M.; Merfort, I. High-resolution crystal structure of the snake venom metalloproteinase BaP1 complexed with a peptidomimetic: Insight into inhibitor binding. Biochemistry 2009, 48, 6166–6174. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Botos, I.; Gomis-Rüth, F.X.; Doll, R.; Blood, C.; Njoroge, F.G.; Fox, J.W.; Bode, W.; Meyer, E.F. Structural interaction of natural and synthetic inhibitors with the venom metalloproteinase, atrolysin C (form d). Proc. Natl. Acad. Sci. USA 1994, 91, 8447–8451. [Google Scholar] [CrossRef] [PubMed]
- Kumasaka, T.; Yamamoto, M.; Moriyama, H.; Tanaka, N.; Sato, M.; Katsube, Y.; Yamakawa, Y.; Omori-Satoh, T.; Iwanaga, S.; Ueki, T. Crystal structure of H2-proteinase from the venom of Trimeresurus flavoviridis. J. Biochem. 1996, 119, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.; Zhu, X.; Liu, S.; Teng, M.; Niu, L. Crystal structures of acutolysin A, a three-disulfide hemorrhagic zinc metalloproteinase from the snake venom of Agkistrodon acutus. J. Mol. Biol. 1998, 283, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.Y.; Teng, M.K.; Niu, L.W. Structure of acutolysin-C, a hemorrhagic toxin from the venom of Agkistrodon acutus, providing further evidence for the mechanism of the pH-dependent proteolytic reaction of zinc metalloproteinases. Acta Crystallogr. D Biol. Crystallogr. 1999, 55, 1834–1841. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.F.; Chiou, S.H.; Ko, T.P.; Yuann, J.M.; Wang, A.H. The 1.35 A structure of cadmium-substituted TM-3, a snake venom metalloproteinase from Taiwan habu: Elucidation of a TNFalfa-converting enzyme-like active-site structure with a distorted octahedral geometry of cadmium. Acta Crystallogr. D. 2002, 58, 1118–1128. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, L.; Shannon, J.D.; Valente, R.H.; Rucavado, A.; Alape-Giron, A.; Theakston, R.D.; Fox, J.W.; Gutierrez, J.M.; Arni, R.K. Amino acid sequence and crystal structure of BaP1, a metalloproteinase from Bothrops asper snake venom that exerts multiple tissue-damaging activities. Protein Sci. 2003, 12, 2273–2281. [Google Scholar] [CrossRef] [PubMed]
- Lou, Z.; Hou, J.; Liang, X.; Chen, J.; Qiu, P.; Liu, Y.; Li, M.; Rao, Z.; Yan, G. Crystal structure of a non-hemorrhagic fibrin(ogen)olytic metalloproteinase complexed with a novel natural tri-peptide inhibitor from venom of Agkistrodon acutus. J. Struct. Biol. 2005, 152, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Akao, P.K.; Tonoli, C.C.C.; Navarro, M.S.; Cintra, A.C.O.; Neto, J.R.; Arni, R.K.; Murakami, M.T. Structural studies BmooMPalpha-I, a non-hemorrhagic metalloproteinase from Bothrops moojeni venom. Toxicon 2010, 55, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.L.; Wu, C.H.; Huang, K.F.; Wang, A.H.J. Crystal structure of a Trimeresurus mucrosquamatus venom metalloproteinase providing new insights into the inhibition by endogenous tripeptide inhibitors. Toxicon 2013, 71, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Markland, F.S.; Swenson, S. Snake venom metalloproteinases. Toxicon 2013, 62, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P.; Collen, D. Gene targeting and gene transfer studies of the biological role of the plasminogen/plasmin system. Thromb. Haemost. 1995, 74, 429–436. [Google Scholar] [PubMed]
- Novokhatny, V. Structure and activity of plasmin and other direct thrombolytic agents. Thromb. Res. 2008, 122, S3–S8. [Google Scholar] [CrossRef] [PubMed]
- Didisheim, P.; Lewis, J.H. Fibrinolytic and coagulant activities of certain snake venoms and proteases. Proc. Soc. Exp. Biol. Med. 1956, 93, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Kornalik, F.; Styblova, Z. Fibrinolytic proteases in snake venoms. Experientia 1967, 23, 999–1000. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, E.F.; Swenson, S. Proteases from South American snake venoms affecting fibrinolysis. Curr. Pharm. Anal. 2007, 3, 147–157. [Google Scholar] [CrossRef]
- Swenson, S.; Toombs, C.F.; Pena, L.; Johanson, J.; Markland, F.S. α-fibrinogenases. Curr. Drug Targets Cardiovasc. Haematol. Disord. 2004, 4, 417–434. [Google Scholar] [CrossRef] [PubMed]
- Deitcher, S.R.; Toombs, C.F. Non-clinical and clinical characterization of a novel acting thrombolytic: Alfimeprase. Pathophysiol. Haemost. Thromb. 2005, 34, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Hong, T.-T.; Huang, J.; Lucchesi, B.R. Effect of thrombolysis on myocardial injury: Recombinant tissue type plasminogen activator vs. alfimeprase. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Weaver, H.S.M.; Comerota, A.J.; Perler, B.A.; Joing, M. Efficacy and safety of alfimeprase in patients with acute peripheral arterial occlusion (PAO). J. Vasc. Surg. 2010, 51, 600–609. [Google Scholar]
- Sanchez, E.F.; Bush, L.R.; Swenson, S.; Markland, F.S. Chimeric fibrolase: Covalent attachment of an RGD-like peptide to create a potentially more effective thrombolytic agent. Thromb. Res. 1997, 87, 289–302. [Google Scholar] [CrossRef]
- Swenson, S.; Bush, L.R.; Markland, F.S. Chimeric derivative of fibrolase, a fibrinolytic enzyme from southern copperhead venom, possesses inhibitory activity on platelet aggregation. Arch. Biochem. Biophys. 2000, 384, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Koh, C.Y.; Kini, R.M. From snake venom toxins to therapeutics-Cardiovascular examples. Toxicon 2012, 59, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.R.; Scher, L. Drug evaluation: Alfimeprase, a plasminogen-independent thrombolytic. Drugs 2007, 10, 329–335. [Google Scholar]
- Mackman, N. Triggers, targets and treatments for thrombosis. Nature 2008, 451, 914–918. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.P. Arterial thrombosis-insidious, unpredictable and deadly. Nat. Med. 2011, 17, 1423–1436. [Google Scholar] [CrossRef] [PubMed]
- Marder, V.J. Thrombolytic therapy for deep vein thrombosis: Potential application of plasmin. Thromb. Res. 2009, 123, 556–561. [Google Scholar] [CrossRef]
- Marder, V.J.; Novokhatny, V. Direct fibrinolytic agents: Biochemical attributes, preclinical foundation and clinical potential. J. Thromb. Haemost. 2010, 8, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Bjarnason, J.B.; Tu, A.T. Hemorrhagic toxins from western diamondback rattlesnake (Crotalus. atrox) venom; isolation and characterization of five toxins and the role of zinc in hemorrhagic toxin e. Biochemistry 1978, 17, 395–404. [Google Scholar] [CrossRef]
- Oliveira, A.K.; Paes Leme, A.F.; Assakura, M.T.; Menezes, M.C.; Zelanis, A.; Tashima, A.K.; Lopes-Ferreira, M.; Lima, C.; Camargo, A.C.; Fox, J.W.; et al. Simplified procedure for the isolation of HF3, bothropasin, disintegrin-like/cysteine-rich protein and a novel P-I metalloproteinase from Bothrops jararaca venom. Toxicon 2009, 53, 797–801. [Google Scholar] [CrossRef] [PubMed]
- Paes Leme, A.F.; Escalante, T.; Pereira, J.G.C.; Oliveira, A.K.; Sanchez, E.F.; Gutierrez, J.M.; Serrano, S.M.T.; Fox, J.W. High resolution analysis of snake venom metalloproteinase (SVMP) peptide bond cleavage specificity using proteome based peptide library and mass spectrometry. J. Proteom. 2011, 74, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, E.F.; Cordeiro, M.; DE Oliveira, E.B.; Juliano, L.; Prado, E.S.; Diniz, C.R. Proteolytic specificity of two hemorrhagic factors, LHF-I and LHF-II from the venom of the bushmaster snake (Lachesis muta muta). Toxicon 1995, 33, 1061–1069. [Google Scholar] [CrossRef]
- Herrera, C.; Escalante, T.; Voisin, M.-B.; Rucavado, A.; Morazan, D.; Macedo, J.K.A.; Calvete, J.J.; Sanz, L.; Nourshargh, S.; Gutierrez, J.M.; et al. Tissue localization and extracellular matrix degradation by PI, PII and PIII snake venom metalloproteinases: Clues on the mechanisms of venom-induced hemorrhage. PLoS Negl. Trop. Dis. 2015, 9, e0003731. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.P.; Schoenwaelder, S.M. Antiplatelet therapy: In search of the magic bullet. Nat. Rev. Drug Discov. 2003, 2, 775–789. [Google Scholar] [CrossRef] [PubMed]
- Clemetson, K.J.; Clemetson, J.M. Platelet GPIb complex as a target for anti-thrombotic drug development. Thromb. Haemost. 2008, 99, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, E.E.; Andrews, R.K. Platelet receptor expression and shedding: Glycoprotein Ib-IX-V and glycoprotein VI. Transfus. Med. Rev. 2014, 28, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Schneider, S.W.; Nuschele, S.; Wixforth, A.; Gorzelanny, C.; Alexander-Katz, A.; Netz, R.R.; Schneider, M.F. Shear-induced unfolding triggers adhesion of von Willebrand factor fibers. Proc. Natl. Acad. Sci. USA 2007, 104, 7899–7903. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Vilaire, G.; Marcinkiewicz, C. Small molecule inhibitors of integrin α2β1. J. Med. Chem. 2007, 50, 5457–5462. [Google Scholar] [CrossRef] [PubMed]
- Clemetson, K.J.; Clemetson, J.M. Collagen receptors as potential targets for novel anti-platelet targets. Curr. Pharm. Des. 2007, 13, 2673–2683. [Google Scholar] [CrossRef] [PubMed]
- Andrews, R.K.; Arthur, J.F.; Gardiner, E.E. Targeting GPVI as a novel antithrombotic strategy. J. Blood Med. 2014, 5, 59–68. [Google Scholar] [PubMed]
- Kroll, M.H.; Hellums, J.D.; McIntire, L.V.; Schafer, A.I.; Moake, J.L. Platelets and shear stress. Blood 1996, 88, 1525–1541. [Google Scholar] [PubMed]
- Kini, M.R.; Koh, C.Y. Metalloproteinases affecting blood coagulation, fibrinolysis and platelet aggregation from snake venoms: Definition and nomenclature of interaction sites. Toxins 2016, 8, 284. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.-F.; Chang, M.-C.; Teng, C.-M. Antiplatelet protease, kistomin, selectively cleaves human platelet glycoprotein Ib. Biochim. Biopys. Acta 1993, 1158, 293–299. [Google Scholar] [CrossRef]
- Hsu, C.-C.; Wu, W.-B.; Chang, Y.-H.; Kuo, H.-L.; Huang, T.-F. Antithrombotic effect of a protein-type I class snake venom metalloproteinase, kistomin, is mediated by affecting glycoprotein Ib-von Willebrand factor interaction. Mol. Pharmacol. 2007, 72, 984–992. [Google Scholar] [CrossRef] [PubMed]
- Vanhoorebeke, K.; Ulrichts, H.; Van de Walle, G.; Fontayne, A.; Deckmyn, H. Inhibition of glycoprotein Ib and its antithrombotic potential. Curr. Pharm. Des. 2007, 13, 2684–2697. [Google Scholar] [CrossRef]
- Bonnefoy, A.; Vermylen, J.; Hoylaerts, M.F. Inhibition of von Willebrand factor-GPIb/IX/V interactions as a strategy to prevent arterial thrombosis. Expert Rev. Cardiovasc. Ther. 2003, 1, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.-B.; Peng, H.-C.; Huang, T.-F. Crotalin, a vWF and GPIb cleaving metalloproteinase from venom of Crotalus. atrox. Thromb. Haemost. 2001, 86, 1501–1511. [Google Scholar] [PubMed]
- Sanchez, E.F.; Richardson, M.; Gremski, L.H.; Veiga, S.S.; Yarleque, A.; Niland, S.; Lima, A.M.; Estevao-Costa, M.I.; Eble, J.A. Data for a direct fibrinolytic metalloproteinase, barnettlysin-I from Bothrops barnetti (barnett’s pit viper) snake venom with anti-thrombotic effect. Data Brief 2016, 7, 1609–1613. [Google Scholar] [CrossRef] [PubMed]
- Collen, D.; Lijnen, H.R. Recent developments in thrombolytic therapy. Fibrinolysis Proteolysis 2000, 14, 66–72. [Google Scholar] [CrossRef]
- Jacob-Ferreira, A.L.; Menaldo, D.L.; Bernardes, C.P.; Sartim, M.A.; de Angelis, C.D.; Tanus-Santos, J.E.; Sampaio, S.V. Evaluation of the in vivo thrombolytic activity of a metalloprotease from Botrops atrox venom using a model of venous thrombosis. Toxicon 2016, 109, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Tooms, C.F. New directions in thrombolytic therapy. Curr. Opin. Pharmacol. 2001, 1, 164–168. [Google Scholar] [CrossRef]
- Marder, V.J. Pre-clinical studies of plasmin: Superior benefit-to-risk ratio of plasmin compared to tissue plasminogen activator. Thromb. Res. 2008, 122, S9–S15. [Google Scholar] [CrossRef] [PubMed]
- Swenson, S.; Markland, F.S. Snake venom fibrin(ogen)olytic enzymes. Toxicon 2005, 45, 1021–1039. [Google Scholar] [CrossRef] [PubMed]
- Robbie, L.A.; Bennett, B.; Croll, A.M.; Brown, P.A.; Booth, N.A. Proteins of the fibrinolytic system in human thrombi. Thromb. Haemost. 1996, 75, 127–133. [Google Scholar] [PubMed]
- Marder, V.J.; Landskroner, K.; Novokhatny, V.; Zimmerman, T.P.; Kong, M.; Kanouse, J.J.; Jesmok, G. Plasmin induces local thrombolysis without causing hemorrhage: A comparison with tissue type plasminogen activator in the rabbit. J. Thromb. Haemost. 2001, 86, 739–746. [Google Scholar]
- Marder, V.J.; Jahan, R.; Gruber, T.; Goyal, A.; Arora, V. Thrombolysis with plasmin. Stroke 2010, 41, S41–S49. [Google Scholar] [CrossRef] [PubMed]
- Kral, J.B.; Schrottmaier, W.C.; Salzmann, M.; Assinger, A. Platelet Interaction with Innate Immune Cells. Transfus. Med. Hemother. 2016, 43, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Lavergne, M.; Janus-Bell, E.; Schaff, M.; Gachet, C.; Mangin, P.H. Platelet Integrins in Tumor Metastasis: Do They Represent a Therapeutic Target? Cancers 2017, 9, 133. [Google Scholar] [CrossRef] [PubMed]
- Gremski, L.H.; Veiga, S.S.; Sanchez, E.F. Leucuraolysin-A. In Handbook of Proteolytic Enzymes, 3nd ed.; Rawlings, N.D., Salvesen, G.S., Eds.; Publisher: London, UK, 2013; Volume 1, pp. 1013–1016. ISBN 978-0-12-382219-2. [Google Scholar]
| SVMP | Source | Activities | PDB ID | Year | Reference |
|---|---|---|---|---|---|
| Adamalysin II | C. adamanteus | non-hemorrhagic | 1LAG | 1993 | [21] |
| Atrolysin C | C. atrox | hemorrhagic | 1ATL, 1HTD | 1994 | [48] |
| H2 proteinase | T. Flavoviridis | non-hemorrhagic | 1WNI | 1996 | [49] |
| Acutolysin A | A. Acutus | hemorrhagic | 1BSW,1BUD | 1998 | [50] |
| Acutolysin C | A. Acutus | hemorrhagic | 1QUA | 1999 | [51] |
| TM-3 | T. Mucrosquamatus | fibrinogenolytic | 1KUF, 1KUI | 2002 | [52] |
| BaP1 | B. asper | hemorrhagic | 1ND1 | 2003 | [53] |
| FII | A. acutus | non-hemorrhagic | 1YP1 | 2005 | [54] |
| BmooMPα-I | B. moogeni | non-hemorrhagic | 3GBO | 2010 | [55] |
| TM-1 | T. mucrosquamatus | fibrinogenolytic | 4J4M | 2013 | [56] |
| Leuc-a | B. leucurus | non-hemorrhagic | 4Q1L | 2015. unpublished |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanchez, E.F.; Flores-Ortiz, R.J.; Alvarenga, V.G.; Eble, J.A. Direct Fibrinolytic Snake Venom Metalloproteinases Affecting Hemostasis: Structural, Biochemical Features and Therapeutic Potential. Toxins 2017, 9, 392. https://doi.org/10.3390/toxins9120392
Sanchez EF, Flores-Ortiz RJ, Alvarenga VG, Eble JA. Direct Fibrinolytic Snake Venom Metalloproteinases Affecting Hemostasis: Structural, Biochemical Features and Therapeutic Potential. Toxins. 2017; 9(12):392. https://doi.org/10.3390/toxins9120392
Chicago/Turabian StyleSanchez, Eladio F., Renzo J. Flores-Ortiz, Valeria G. Alvarenga, and Johannes A. Eble. 2017. "Direct Fibrinolytic Snake Venom Metalloproteinases Affecting Hemostasis: Structural, Biochemical Features and Therapeutic Potential" Toxins 9, no. 12: 392. https://doi.org/10.3390/toxins9120392
APA StyleSanchez, E. F., Flores-Ortiz, R. J., Alvarenga, V. G., & Eble, J. A. (2017). Direct Fibrinolytic Snake Venom Metalloproteinases Affecting Hemostasis: Structural, Biochemical Features and Therapeutic Potential. Toxins, 9(12), 392. https://doi.org/10.3390/toxins9120392