The European AntibotABE Framework Program and Its Update: Development of Innovative Botulinum Antibodies
Abstract
:1. Introduction
1.1. Background
1.2. Botulinum Neurotoxins (BoNTs)
1.3. Antitoxin Treatment
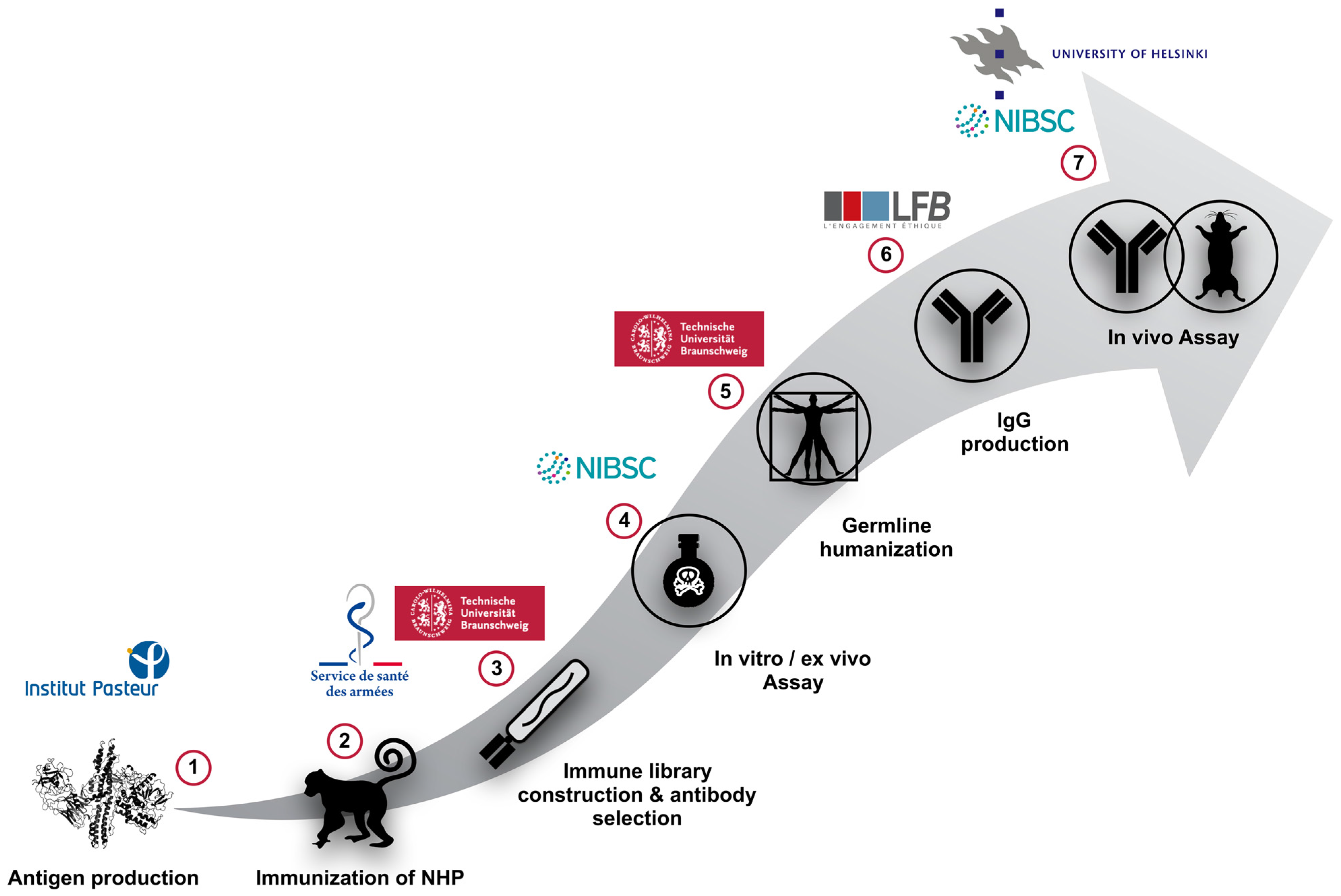
2. Animal Immunization and Antibody Phage-Display Library Construction
2.1. Immunization of Macaques (Macaca fascicularis) Using the Non-Toxic and Recombinant HC or LC of BoNT/A, B or E
2.2. Phage-Displayed Immune Libraries Construction
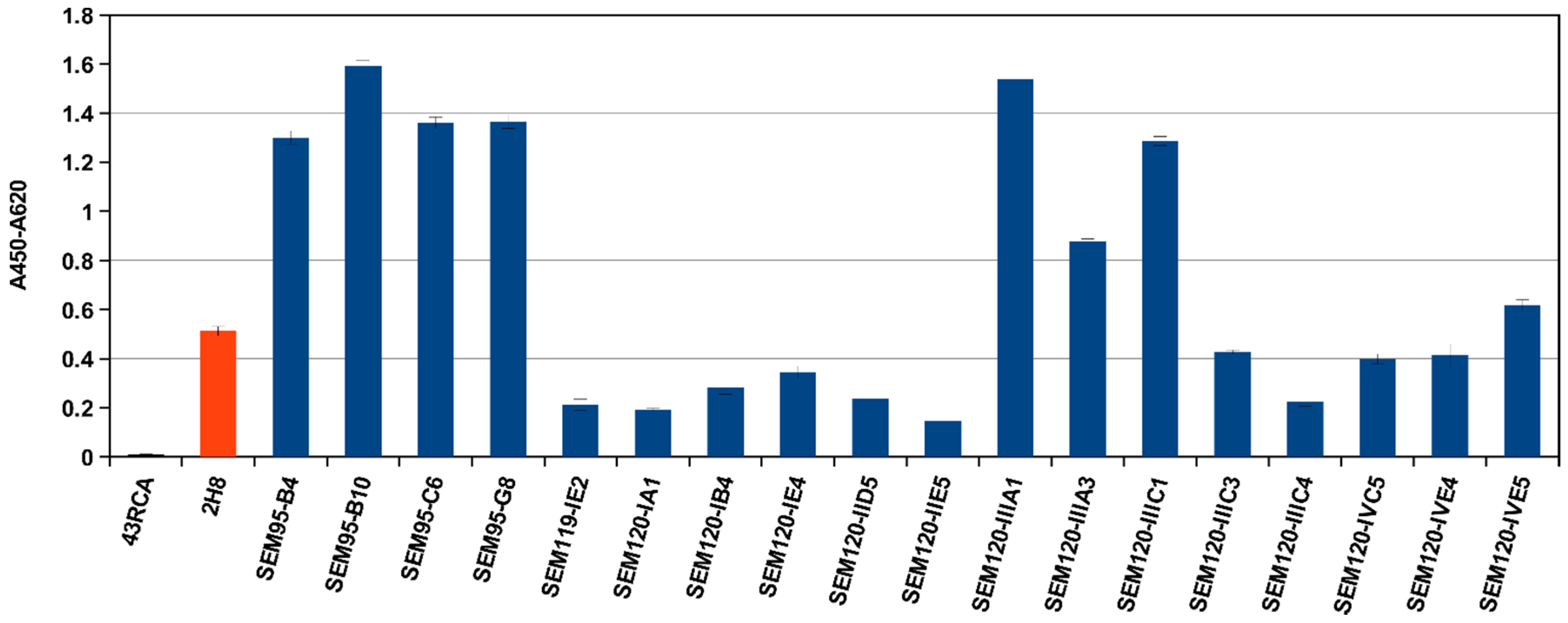
2.3. Selection of scFv Directed against BoNT/A1 and A2, or B1 and B2 or E3 Subtypes/Serotypes by Multi-Step Panning and Screening by ELISA
2.4. Further Characterization by Affinity Measurements
3. In Vitro Evaluations and Ex Vivo Neutralizing Properties of High Affinity scFvs and scFv-Fcs
3.1. In Vitro Screening of Anti-BoNT LC Antibodies as scFv or scFv-Fc by Endopeptidase Inhibition Assays
3.2. Ex Vivo Screening of Anti-BoNT HC and Anti-BoNT LC Antibodies as scFv or scFv-Fc in the Mouse Phrenic Nerve Hemidiaphragm Paralysis Assay
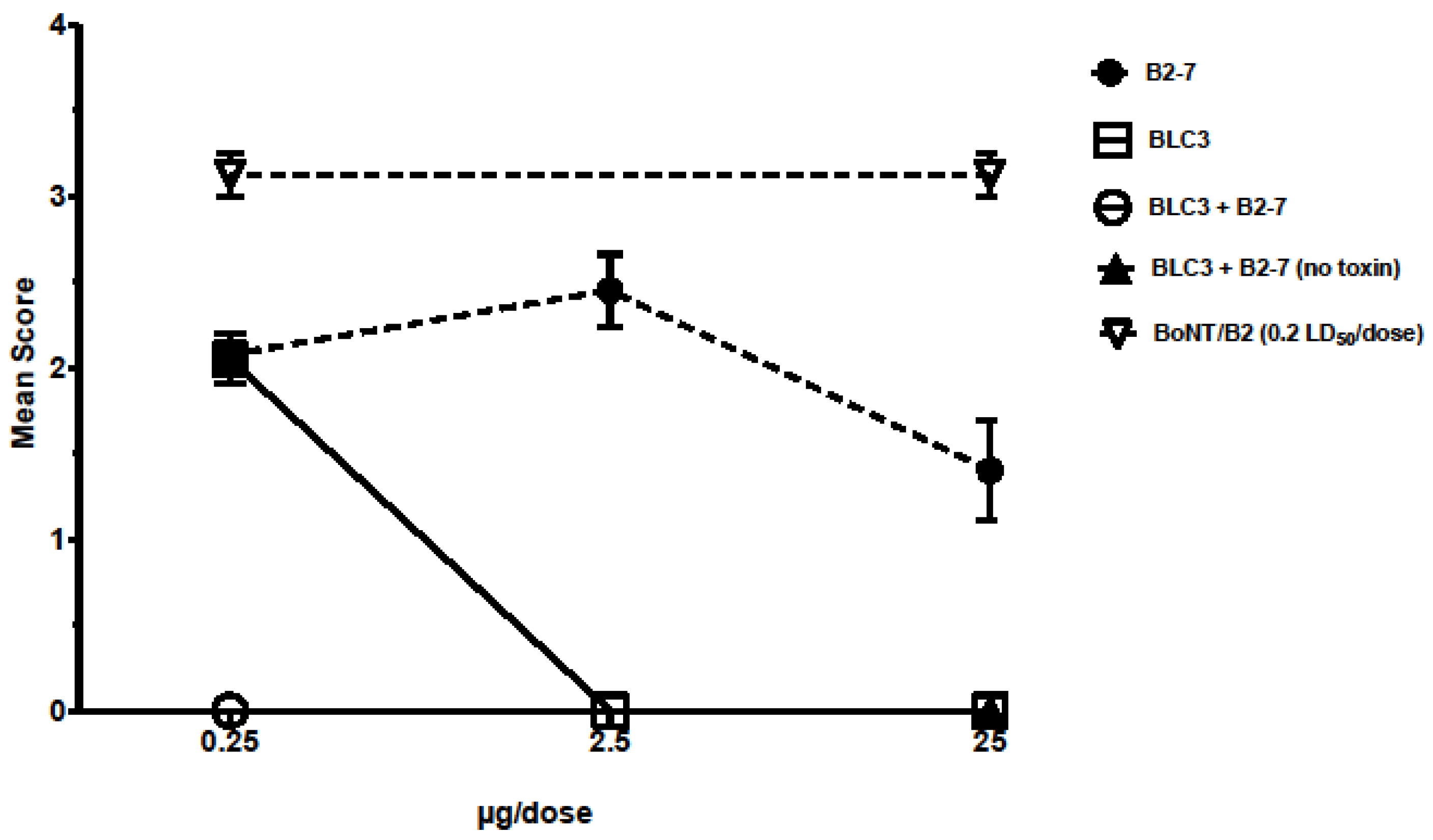
3.3. Isolation of the Most Promising Antibody Combinations (Anti-BoNT/HC and Anti-BoNT/LC Directed against the Same Serotype) Using the In Vivo Flaccid Paralysis Assay, Synergistic Protection Assessment, Epitope Characterization and Affinities Measurements
4. Germline-Humanization and In Vivo Characterization of the Selected Antibodies
4.1. Generation of Variants of the Germline Humanized Antibodies and Identification of the Most Promising Germline-Humanized Variant for Each Library
4.2. Expression of the Selected Germline-Humanized Variants as Full-Lengths IgGs
4.3. Assessment of the Protection Induced by Germline-Humanized IgGs in Lethal and Non-Lethal In Vivo Assays, Individually and in Combination
5. Output of the AntiBotABE Project
6. Dissemination of the AntiBotABE Project
- The first aim was to promote knowledge of our results among the potentials users of our antibodies, the governmental structures involved in bioterrorism preparedness, and the general public.
- In a next step, we addressed potential stakeholders for the further clinical and regulatory development of the AntiBotABE antibody cocktail (oligoclonal antibody). In order to draw the attention of European institutions, national governments, regional authorities and other public and private funding sources to the needs and benefits of our antibodies; and the need to stockpile them in advance: several presentations to scientific and decision-makers fora such as the European Defense Agency (EDA) and the North Atlantic Treaty Organization (NATO) have been made.
- Enhancing the reputation of participants at local, national and international levels and attracting the interest of correspondents, including the public; Aid the search for financial backers, licensees or industrial implementers to exploit the results and maintain market demand for the developed products or services. To this aim, regular up-dates of the AntiBotABE progress have been presented as oral presentations and posters at international meetings by the Consortium members.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pelat, T.; Hust, M.; Hale, M.; Lefranc, M.P.; Dübel, S.; Thullier, P. Isolation of a human-like antibody fragment (scFv) that neutralizes ricin biological activity. BMC Biotechnol. 2009, 9, 60. [Google Scholar] [CrossRef] [PubMed]
- Pelat, T.; Hust, M.; Laffly, E.; Condemine, F.; Bottex, C.; Vidal, D.; Lefranc, M.-P.; Dübel, S.; Thullier, P. High-affinity, human antibody-like antibody fragment (single-chain variable fragment) neutralizing the lethal factor (LF) of Bacillus anthracis by inhibiting protective antigen-LF complex formation. Antimicrob. Agents Chemother. 2007, 51, 2758–2764. [Google Scholar] [CrossRef] [PubMed]
- Laffly, E.; Danjou, L.; Condemine, F.; Vidal, D.; Drouet, E.; Lefranc, M.P.; Bottex, C.; Thullier, P. Selection of a macaque Fab with framework regions like those in humans, high affinity, and ability to neutralize the protective antigen (PA) of Bacillus anthracis by binding to the segment of PA between residues 686 and 694. Antimicrob. Agents Chemother. 2005, 49, 3414–3420. [Google Scholar] [CrossRef] [PubMed]
- Rülker, T.; Voß, L.; Thullier, P.; O’ Brien, L.M.; Pelat, T.; Perkins, S.D.; Langermann, C.; Schirrmann, T.; Dübel, S.; Marschall, H.-J.; et al. Isolation and characterisation of a human-like antibody fragment (scFv) that inactivates VEEV in vitro and in vivo. PLoS ONE 2012, 7, e37242. [Google Scholar] [CrossRef] [PubMed]
- Hülseweh, B.; Rülker, T.; Pelat, T.; Langermann, C.; Frenzel, A.; Schirrmann, T.; Dübel, S.; Thullier, P.; Hust, M. Human-like antibodies neutralizing Western equine encephalitis virus. MAbs 2014, 6, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Froude, J.W.; Pelat, T.; Miethe, S.; Zak, S.E.; Wec, A.Z.; Chandran, K.; Brannan, J.M.; Bakken, R.R.; Hust, M.; Thullier, P.; et al. Generation and characterization of protective antibodies to Marburg virus. MAbs 2017, 9, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Sobel, J. Botulism. Clin. Infect. Dis. 2005, 41, 1167–1173. [Google Scholar] [CrossRef] [PubMed]
- Fujinaga, Y.; Sugawara, Y.; Matsumura, T. Uptake of botulinum neurotoxin in the intestine. Curr. Top. Microbiol. Immunol. 2013, 364, 45–59. [Google Scholar] [PubMed]
- Hibbs, R.G.; Weber, J.T.; Corwin, A.; Allos, B.M.; el Rehim, M.S.A.; Sharkawy, S.E.; Sarn, J.E.; McKee, K.T., Jr. Experience with the use of an investigational F(ab’)2 heptavalent botulism immune globulin of equine origin during an outbreak of type E botulism in Egypt. Clin. Infect. Dis. 1996, 23, 337–340. [Google Scholar] [CrossRef] [PubMed]
- Jalava, K.; Selby, K.; Pihlajasaari, A.; Kolho, E.; Dahlsten, E.; Forss, N.; Bäcklund, T.; Korkeala, H.; Honkanen-Buzalski, T.; Hulkko, T.; et al. Two cases of food-borne botulism in Finland caused by conserved olives, October 2011. Euro Surveill. 2011, 16, 20034. [Google Scholar] [PubMed]
- Pingeon, J.M.; Vanbockstael, C.; Popoff, M.R.; King, L.A.; Deschamps, B.; Pradel, G.; Dupont, H.; Spanjaard, A.; Houdard, A.; Mazuet, C.; et al. Two outbreaks of botulism associated with consumption of green olive paste, France, September 2011. Euro Surveill. 2011, 16, 20035. [Google Scholar] [PubMed]
- Barash, J.R.; Arnon, S.S. A Novel Strain of Clostridium botulinum That Produces Type B and Type H Botulinum Toxins. J. Infect. Dis. 2014, 209, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Dover, N.; Barash, J.R.; Hill, K.K.; Xie, G.; Arnon, S.S. Molecular characterization of a novel botulinum neurotoxin type H gene. J. Infect. Dis. 2014, 209, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Maslanka, S.E.; Lúquez, C.; Dykes, J.K.; Tepp, W.H.; Pier, C.L.; Pellett, S.; Raphael, B.H.; Kalb, S.R.; Barr, J.R.; Rao, A.; et al. A Novel Botulinum Neurotoxin, Previously Reported as Serotype, H.; has a Hybrid-Like Structure With Regions of Similarity to the Structures of Serotypes A and F and Is Neutralized With Serotype A Antitoxin. J. Infect. Dis. 2016, 213, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Masuyer, G.; Zhang, J.; Shen, Y.; Lundin, D.; Henriksson, L.; Miyashita, S.I.; Martínez-Carranza, M.; Dong, M.; Stenmark, P. Identification and characterization of a novel botulinum neurotoxin. Nat. Commun. 2017, 8, 14130. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, M.J.; Adams, J.B.; Doxey, A.C. Botulinum neurotoxin homologs in non-Clostridium species. FEBS Lett. 2015, 589, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Gill, D.M. Bacterial toxins: A table of lethal amounts. Microbiol. Rev. 1982, 46, 86–94. [Google Scholar] [PubMed]
- Fischer, A.; Garcia-Rodriguez, C.; Geren, I.; Lou, J.; Marks, J.D.; Nakagawa, T.; Montal, M. Molecular architecture of botulinum neurotoxin E revealed by single particle electron microscopy. J. Biol. Chem. 2008, 283, 3997–4003. [Google Scholar] [CrossRef] [PubMed]
- Arnon, S.S.; Schechter, R.; Inglesby, T.V.; Henderson, D.A.; Bartlett, J.G.; Ascher, M.S.; Eitzen, E.; Fine, A.D.; Hauer, J.; Layton, M.; et al. Botulinum toxin as a biological weapon: Medical and public health management. JAMA 2001, 285, 1059–1070. [Google Scholar] [CrossRef] [PubMed]
- Froude, J.W.; Stiles, B.; Pelat, T.; Thullier, P. Antibodies for biodefense. MAbs 2011, 3, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Bozheyeva, G.; Kunakbayev, Y.; Yeleukenov, D. Former Soviet Biological Weapons Facilities in Kazakhstan: Past, Present, and Future; Occasional Paper No. 1; Monterey Institute of International Studies: Monterey, CA, USA, 1999. [Google Scholar]
- Broad, W.J. Sowing Death: A Special Report; How Japan Germ Terror Alerted World. The New York Times. 26 May 1998. Available online: http://www.nytimes.com/1998/05/26/world/sowing-death-a-special-report-how-japan-germ-terror-alerted-world.html (accessed on 22 September 2014).
- Wein, L.M.; Liu, Y. Analyzing a bioterror attack on the food supply: The case of botulinum toxin in milk. Proc. Natl. Acad. Sci. USA 2005, 102, 9984–9989. [Google Scholar] [CrossRef] [PubMed]
- Montecucco, C. How do tetanus and botulinum toxins bind to neuronal membranes? Trends Biochem. Sci. 1986, 11, 314–317. [Google Scholar] [CrossRef]
- Montal Botulinum Neurotoxin: A Marvel of Protein Design. Ann. Rev. Biochem. 2010, 79, 591–617.
- Verderio, C.; Rossetto, O.; Grumelli, C.; Frassoni, C.; Montecucco, C.; Matteoli, M. Entering neurons: Botulinum toxins and synaptic vesicle recycling. EMBO Rep. 2006, 7, 995–999. [Google Scholar] [CrossRef] [PubMed]
- Rossetto, O.; Pirazzini, M.; Montecucco, C. Botulinum neurotoxins: Genetic, structural and mechanistic insights. Nat. Rev. Microbiol. 2014, 12, 535–549. [Google Scholar] [CrossRef] [PubMed]
- Pirazzini, M.; Rossetto, O.; Eleopra, R.; Montecucco, C. Botulinum neurotoxins: Biology, pharmacology, and toxicology. Pharmacol. Rev. 2017, 69, 200–235. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, T.; Jin, Y.; Kabumoto, Y.; Takegahara, Y.; Oguma, K.; Lencer, W.I.; Fujinaga, Y. The HA proteins of botulinum toxin disrupt intestinal epithelial intercellular junctions to increase toxin absorption. Cell Microbiol. 2008, 10, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Takegahara, Y.; Sugawara, Y.; Matsumura, T.; Fujinaga, Y. Disruption of the epithelial barrier by botulinum haemagglutinin (HA) proteins—Differences in cell tropism and the mechanism of action between HA proteins of types A or B, and HA proteins of type, C. Microbiology 2009, 155, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Zhong, X.; Gu, S.; Kruel, A.M.; Dorner, M.B.; Perry, K.; Rummel, A.; Dong, M.; Jin, R. Molecular basis for disruption of E-cadherin adhesion by botulinum neurotoxin A complex. Science 2014, 344, 1405–1410. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, Y.; Matsumura, T.; Takegahara, Y.; Jin, Y.; Tsukasaki, Y.; Takeichi, M.; Fujinaga, Y. Botulinum hemagglutinin disrupts the intercellular epithelial barrier by directly binding E-cadherin. J. Cell Biol. 2010, 189, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, T.; Sugawara, Y.; Yutani, M.; Amatsu, S.; Yagita, H.; Kohda, T.; Fukuoka, S.; Nakamura, Y.; Fukuda, S.; Hase, K.; et al. Botulinum toxin A complex exploits intestinal M cells to enter the host and exert neurotoxicity. Nat. Commun. 2015, 6, 6255. [Google Scholar] [CrossRef] [PubMed]
- Connan, C.; Varela-Chavez, C.; Mazuet, C.; Molgó, J.; Haustant, G.M.; Disson, O.; Lecuit, M.; Vandewalle, A.; Popoff, M.R. Translocation and dissemination to target neurons of botulinum neurotoxin type B in the mouse intestinal wall. Cell. Microbiol. 2016, 18, 282–301. [Google Scholar] [CrossRef] [PubMed]
- Pirazzini, M.; Rossetto, O. Challenges in searching for therapeutics against Botulinum Neurotoxins. Expert Opin. Drug Discov. 2017, 12, 497–510. [Google Scholar] [CrossRef] [PubMed]
- Arnon, S.S.; Schechter, R.; Maslanka, S.E.; Jewell, N.P.; Hatheway, C.L. Human botulism immune globulin for the treatment of infant botulism. N. Engl. J. Med. 2006, 354, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Black, R.E.; Gunn, R.A. Hypersensitivity reactions associated with botulinal antitoxin. Am. J. Med. 1980, 69, 567–570. [Google Scholar] [CrossRef]
- Mayers, C.; Veall, S.; Bedford, R.; Holley, J. Anti-immunoglobulin responses to IgG, F(ab’)2, and Fab botulinum antitoxins in mice. Immunopharmacol. Immunotoxicol. 2003, 25, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Foster, K.A. Engineered toxins: New therapeutics. Toxicon 2009, 54, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Fagan, R.P.; Neil, K.P.; Sasich, R.; Luquez, C.; Asaad, H.; Maslanka, S.; Khalil, W. Initial recovery and rebound of type F intestinal colonization botulism after administration of investigational heptavalent botulinum antitoxin. Clin. Infect. Dis. 2011, 53, e125–e128. [Google Scholar] [CrossRef] [PubMed]
- Rusnak, J.M.; Smith, L.A. Botulinum neurotoxin vaccines: Past history and recent developments. Hum. Vaccines 2009, 5, 794–805. [Google Scholar] [CrossRef]
- Smith, L.A. Botulism and vaccines for its prevention. Vaccine 2009, 27 (Suppl. 4), D33–D39. [Google Scholar] [CrossRef] [PubMed]
- Kaji, R. New and emerging indications of botulinum toxin therapy. Park. Relat. Disord. 2011, 17 (Suppl. 1), S25–S27. [Google Scholar] [CrossRef] [PubMed]
- Dolly, J.O.; Lawrence, G.W.; Meng, J.; Wang, J.; Ovsepian, S.V. Neuro-exocytosis: Botulinum toxins as inhibitory probes and versatile therapeutics. Curr. Opin. Pharmacol. 2009, 9, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Dressler, D.; Paus, S.; Seitzinger, A.; Gebhardt, B.; Kupsch, A. Long-term efficacy and safety of incobotulinumtoxinA injections in patients with cervical dystonia. J. Neurol. Neurosurg. Psychiatry 2013, 84, 1014–1019. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.J.; Lou, J.; Geren, I.N.; Forsyth, C.M.; Tsai, R.; Laporte, S.L.; Tepp, W.H.; Bradshaw, M.; Johnson, E.A.; Smith, L.A.; et al. Sequence variation within botulinum neurotoxin serotypes impacts antibody binding and neutralization. Infect Immun. 2005, 73, 5450–5457. [Google Scholar] [CrossRef] [PubMed]
- Avril, A.; Froude, J.; Mathieu, J.; Pelat, T.; Thullier, P. Isolation of antibodies from non-human primates for clinical use. Curr. Drug Discov. Technol. 2014, 11, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Pelat, T.; Hust, M.; Thullier, P. Obtention and engineering of non-human primate (NHP) antibodies for therapeutics. Mini. Rev. Med. Chem. 2009, 9, 1633–1638. [Google Scholar] [CrossRef] [PubMed]
- Adekar, S.P.; Takahashi, T.; Jones, R.M.; Al-Saleem, F.H.; Ancharski, D.M.; Root, M.J.; Kapadnis, B.P.; Simpson, L.L.; Dessain, S.K. Neutralization of botulinum neurotoxin by a human monoclonal antibody specific for the catalytic light chain. PLoS ONE 2008, 3, e3023. [Google Scholar] [CrossRef] [PubMed]
- Chahboun, S.; Hust, M.; Liu, Y.; Pelat, T.; Miethe, S.; Helmsing, S.; Jones, R.G.; Sesardic, D.; Thullier, P. Isolation of a nanomolar scFv inhibiting the endopeptidase activity of botulinum toxin A, by single-round panning of an immune phage-displayed library of macaque origin. BMC Biotechnol. 2011, 11, 113. [Google Scholar] [CrossRef] [PubMed]
- Miethe, S.; Rasetti-Escargueil, C.; Liu, Y.; Chahboun, S.; Pelat, T.; Avril, A.; Frenzel, A.; Schirrmann, T.; Thullier, P.; Sesardic, D.; et al. Development of neutralizing scFv-Fc against botulinum neurotoxin A light chain from a macaque immune library. mAbs 2014, 6, 446–459. [Google Scholar] [CrossRef] [PubMed]
- Kalb, S.R.; Lou, J.; Garcia-Rodriguez, C.; Geren, I.N.; Smith, T.J.; Moura, H.; Marks, J.D.; Smith, L.A.; Pirkle, J.L.; Barr, J.R. Extraction and inhibition of enzymatic activity of botulinum neurotoxins/A1, /A2, and /A3 by a panel of monoclonal anti-BoNT/A antibodies. PLoS ONE 2009, 4, e5355. [Google Scholar] [CrossRef] [PubMed]
- Nowakowski, A.; Wang, C.; Powers, D.B.; Amersdorfer, P.; Smith, T.J.; Montgomery, V.A.; Sheridan, R.; Blake, R.; Smith, L.A.; Marks, J.D. Potent neutralization of botulinum neurotoxin by recombinant oligoclonal antibody. Proc. Natl. Acad. Sci. USA 2002, 99, 11346–11350. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.W.; Stanker, L.H.; Henderson, T.D., 2nd; Lou, J.; Marks, J.D. Antibody protection against botulinum neurotoxin intoxication in mice. Infect. Immun. 2009, 77, 4305–4313. [Google Scholar] [CrossRef] [PubMed]
- Conway, J.O.; Sherwood, L.J.; Collazo, M.T.; Garza, J.A.; Hayhurst, A. Llama single domain antibodies specific for the 7 botulinum neurotoxin serotypes as heptaplex immunoreagents. PLoS ONE 2010, 5, e881. [Google Scholar] [CrossRef] [PubMed]
- Dolimbek, B.Z.; Aoki, K.R.; Steward, L.E.; Jankovic, J.; Atassi, M.Z. Mapping of the regions on the heavy chain of botulinum neurotoxin A (BoNT/A) recognized by antibodies of cervical dystonia patients with immunoresistance to BoNT/A. Mol. Immunol. 2007, 44, 1029–1041. [Google Scholar] [CrossRef] [PubMed]
- Amersdorfer, P.; Wong, C.; Chen, S.; Smith, T.; Deshpande, S.; Sheridan, R.; Finnern, R.; Marks, J.D. Molecular characterization of murine humoral immune response to botulinum neurotoxin type A binding domain as assessed by using phage antibody libraries. Infect. Immun. 1997, 65, 3743–3752. [Google Scholar] [PubMed]
- Razai, A.; Garcia-Rodriguez, C.; Lou, J.; Geren, I.N.; Forsyth, C.M.; Robles, Y.; Tsai, R.; Smith, T.J.; Smith, L.A.; Siegel, R.W.; et al. Molecular evolution of antibody affinity for sensitive detection of botulinum neurotoxin type, A. J. Mol. Biol. 2005, 351, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Amersdorfer, P.; Wong, C.; Smith, T.; Chen, S.; Deshpande, S.; Sheridan, R.; Marks, J.D. Genetic and immunological comparison of anti-botulinum type A antibodies from immune and nonimmune human phage libraries. Vaccine 2002, 20, 1640–1648. [Google Scholar] [CrossRef]
- Dong, J.; Thompson, A.A.; Fan, Y.; Lou, J.; Conrad, F.; Ho, M.; Pires-Alves, M.; Wilson, B.A.; Stevens, R.C.; Marks, J.D. A single-domain llama antibody potently inhibits the enzymatic activity of botulinum neurotoxin by binding to the non-catalytic alpha-exosite binding region. J. Mol. Biol. 2010, 397, 1106–1118. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Joshi, S.G.; Al-Saleem, F.; Ancharski, D.; Singh, A.; Nasser, Z.; Simpson, L.L. Localization of the sites and characterization of the mechanisms by which anti-light chain antibodies neutralize the actions of the botulinum holotoxin. Vaccine 2009, 27, 2616–2624. [Google Scholar] [CrossRef] [PubMed]
- Kalb, S.R.; Santana, W.I.; Geren, I.N.; Garcia-Rodriguez, C.; Lou, J.; Smith, T.J.; Marks, J.D.; Smith, L.A.; Pirkle, J.L.; Barr, J.R. Extraction and inhibition of enzymatic activity of botulinum neurotoxins /B1, /B2, /B3, /B4, and /B5 by a panel of monoclonal anti-BoNT/B antibodies. BMC Biochem. 2011, 12, 58. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Rodriguez, C.; Geren, I.N.; Lou, J.; Conrad, F.; Forsyth, C.; Wen, W.; Chakraborti, S.; Zao, H.; Manzanarez, G.; Smith, T.J.; et al. Neutralizing human monoclonal antibodies binding multiple serotypes of botulinum neurotoxin. Protein Eng. Des. Sel. 2011, 24, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Kubota, T.; Watanabe, T.; Yokosawa, N.; Tsuzuki, K.; Indoh, T.; Moriishi, K.; Sanda, K.; Maki, Y.; Inoue, K.; Fujii, N. Epitope regions in the heavy chain of Clostridium botulinum type E neurotoxin recognized by monoclonal antibodies. Appl. Environ. Microbiol. 1997, 63, 1214–1218. [Google Scholar] [PubMed]
- Schütte, M.; Thullier, P.; Pelat, T.; Wezler, X.; Rosenstock, P.; Hinz, D.; Kirsch, M.I.; Hasenberg, M.; Frank, R.; Schirrmann, T.; et al. Identification of a putative Crf splice variant and generation of recombinant antibodies for the specific detection of Aspergillus fumigatus. PLoS ONE 2009, 4, e6625. [Google Scholar] [CrossRef] [PubMed]
- Chassagne, S.; Laffly, E.; Drouet, E.; Hérodin, F.; Lefranc, M.-P.; Thullier, P. A high-affinity macaque antibody Fab with human-like framework regions obtained from a small phage display immune library. Mol. Immunol. 2004, 41, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Laffly, E.; Pelat, T.; Cédrone, F.; Blésa, S.; Bedouelle, H.; Thullier, P. Improvement of an antibody neutralizing the anthrax toxin by simultaneous mutagenesis of its six hypervariable loops. J. Mol. Biol. 2008, 378, 1094–1103. [Google Scholar] [CrossRef] [PubMed]
- Kuwata, T.; Katsumata, Y.; Takaki, K.; Miura, T.; Igarashi, T. Isolation of potent neutralizing monoclonal antibodies from an SIV-Infected rhesus macaque by phage display. AIDS Res. Hum. Retrovir. 2011, 27, 487–500. [Google Scholar] [CrossRef] [PubMed]
- Rasetti-Escargueil, R.G.A.; Jones, Y.; Liu, D. Sesardic, Measurement of botulinum types A, B and E neurotoxicity using the phrenic nerve-hemidiaphragm: Improved precision with in-bred mice. Toxicon 2009, 53, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Rasetti-Escargueil, C.; Liu, Y.; Rigsby, P.; Jones, R.G.A.; Sesardic, D. Phrenic nerve hemidiaphragm as a highly sensitive replacement assay for determination of functional botulinum toxin antibodies. Toxicon 2011, 57, 1008–1016. [Google Scholar] [CrossRef] [PubMed]
- Thullier, P.; Chahboun, S.; Pelat, T. A comparison of human and macaque (Macaca mulatta) immunoglobulin germline V regions and its implications for antibody engineering. MAbs 2010, 2, 528–538. [Google Scholar] [CrossRef] [PubMed]
- Thullier, P.; Huish, O.; Pelat, T.; Martin, A.C. The humanness of macaque antibody sequences. J. Mol. Biol. 2010, 396, 1439–1450. [Google Scholar] [CrossRef] [PubMed]
- Avril, A.; Miethe, S.; Popoff, M.R.; Mazuet, C.; Chahboun, S.; Rasetti-Escargueil, C.; Sesardic, D.; Thullier, P.; Hust, M.; Pelat, T. Isolation of nanomolar scFvs of non-human primate origin, cross-neutralizing botulinum neurotoxins A1 and A2 by targeting their heavy chain. BMC Biotechnol. 2015, 15, 86. [Google Scholar] [CrossRef] [PubMed]
- Rasetti-Escargueil, C.; Avril, A.; Chahboun, S.; Tierney, R.; Bak, N.; Miethe, S.; Mazuet, C.; Popoff, M.R.; Thullier, P.; Hust, M.; et al. Development of human-like scFv-Fc antibodies neutralizing Botulinum toxin serotype B. MAbs 2015, 7, 1161–1177. [Google Scholar] [CrossRef] [PubMed]
- Miethe, S.; Rasetti-Escargueil, C.; Avril, A.; Liu, Y.; Chahboun, S.; Pelat, T.; Thullier, P.; Sesardic, D.; Hust, M. Development of human-like scFv-Fc neutralizing botulinum neurotoxin E. PLoS ONE 2015, 10, e0139905. [Google Scholar] [CrossRef] [PubMed]
- Derman, Y.; Selby, K.; Miethe, S.; Frenzel, A.; Liu, Y.; Rasetti-Escargueil, C.; Avril, A.; Pelat, T.; Urbain, R.; Fontayne, A.; et al. Neutralization of Botulinum Neurotoxin Type E by a Humanized Antibody. Toxins (Basel) 2016, 8, 257. [Google Scholar] [CrossRef] [PubMed]
- Ahmadzadeh, V.; Farajnia, S.; Feizi, M.A.H.; Nejad, R.A.K. Antibody humanization methods for development of therapeutic applications. Monoclon. Antib. Immunodiagn. Immunother. 2014, 33, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Pelat, T.; Thullier, P. Non-human primate immune libraries combined with germline humanization: An (almost) new, and powerful approach for the isolation of therapeutic antibodies. MAbs 2009, 1, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Tan, P.; Mitchell, D.A.; Buss, T.N.; Holmes, M.A.; Anasetti, C.; Foote, J. “Superhumanized” antibodies: Reduction of immunogenic potential by complementarity-determining region grafting with human germline sequences: Application to an anti-CD28. J. Immunol. 2002, 169, 1119–1125. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.T.; Jolly, C.J.; Köhler, J.; Neuberger, M.S. The contribution of somatic hypermutation to the diversity of serum immunoglobulin: Dramatic increase with age. Immunity 2000, 13, 409–417. [Google Scholar] [CrossRef]
- Pommié, C.; Levadoux, S.; Sabatier, R.; Lefranc, G.; Lefranc, M.-P. IMGT standardized criteria for statistical analysis of immunoglobulin V-REGION amino acid properties. J. Mol. Recognit. 2004, 17, 17–32. [Google Scholar] [CrossRef] [PubMed]
- IMGT/V-QUEST Online Tool. Available online: http://www.imgt.org (accessed on 28 September 2017).
- Hust, M.; Meyer, T.; Voedisch, B.; Rülker, T.; Thie, H.; El-Ghezal, A.; Kirsch, M.I.; Schütte, M.; Helmsing, S.; Meier, D.; et al. A human scFv antibody generation pipeline for proteome research. J. Biotechnol. 2011, 152, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Pathan, N.I.; Zou, A.; Chu, P.; Wynne, D.; Leigh, B.; Hanna, N. Lumiliximab (IDEC-152), an anti-CD23 antibody, induces apoptosis in vitro and in vivo in CLL cells. Proc. Am. Assoc. Cancer Res. 2004, 45. [Google Scholar]
- Rosenwasser, L.J.; Busse, W.W.; Lizambri, R.G.; Olejnik, T.A.; Totoritis, M.C. Allergic asthma and an anti-CD23 mAb (IDEC-152): Results of a phase, I.; single-dose, dose-escalating clinical trial. J. Allergy Clin. Immunol. 2003, 112, 563–570. [Google Scholar] [CrossRef]
- Whitelegg, N.R.; Rees, A.R. WAM: An improved algorithm for modelling antibodies on the WEB. Protein Eng. 2000, 13, 819–824. [Google Scholar] [CrossRef] [PubMed]
- Miethe, S.; Mazuet, C.; Liu, Y.; Tierney, R.; Rasetti-Escargueil, C.; Avril, A.; Frenzel, A.; Thullier, P.; Pelat, T.; Urbain, R.; et al. Development of Germline-Humanized Antibodies Neutralizing Botulinum Neurotoxin A and B. PLoS ONE 2016, 11, e0161446. [Google Scholar] [CrossRef] [PubMed]
- Diamant, E.; Lachmi, B.-E.; Keren, A.; Barnea, A.; Marcuk, H.; Cohen, S. Evaluating the synergistic neutralizing effect of anti-botulinum oligoclonal antibody preparations. PLoS ONE 2014, 9, e87089. [Google Scholar] [CrossRef] [PubMed]
- Bakherad, H.; Gargari, S.L.M.; Rasooli, I.; Rajabibazl, M.; Mohammadi, M.; Ebrahimizadeh, W.; Ardakani, L.S.; Zare, H. In vivo neutralization of botulinum neurotoxins serotype E with heavy-chain camelid antibodies (VHH). Mol. Biotechnol. 2013, 55, 159–167. [Google Scholar] [CrossRef] [PubMed]
- HBAT Package Insert. Available online: https://www.fda.gov/downloads/.../UCM345147.pdf (accessed on 28 September 2017).
| Antigen | Antibody | Format | In Vitro Inhibition: IC 50 (nM) | Ex Vivo Neutralization: Neutralizing Concentration 50% (nM) | Affinity (KD, nM) |
|---|---|---|---|---|---|
| BoNT/A LC | SEM120-IIIC1 | scFv-Fc | 10 | 1000 | 0.82 |
| BoNT/A HC | AHC38 | scFv | n/a | 33 | 1.9 |
| BoNT/B LC | BLC3 | scFv-Fc | 66 | 66 | 0.4 |
| BoNT/B HC | B2-7 | scFv | n/a | >1000 | 4.8 |
| BoNT/E LC | ELC18 | scFv | 112 | 3.3 | 0.58 |
| BoNT/E HC | - | n/a | n/a | n/a | n/a |
| Antigen | Antibody | Format | Germinality Index (GI %) |
|---|---|---|---|
| BoNT/A LC | SEM120-IIIC1 | scFv-Fc | 86.8 (VH)–87.6 (VL) |
| BoNT/A HC | AHC38 | scFv | 86.5 (VH)–84.4 (VL) |
| BoNT/B LC | BLC3 | scFv-Fc | 85.7 (VH)–85.7 (VL) |
| BoNT/B HC | B2-7 | scFv | 85.7 (VH)–77.2 (VL) |
| BoNT/E LC | ELC18 | scFv | 85.7 (VH)–89.9 (VL) |
| BoNT/E HC | - | n/a | n/a |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rasetti-Escargueil, C.; Avril, A.; Miethe, S.; Mazuet, C.; Derman, Y.; Selby, K.; Thullier, P.; Pelat, T.; Urbain, R.; Fontayne, A.; et al. The European AntibotABE Framework Program and Its Update: Development of Innovative Botulinum Antibodies. Toxins 2017, 9, 309. https://doi.org/10.3390/toxins9100309
Rasetti-Escargueil C, Avril A, Miethe S, Mazuet C, Derman Y, Selby K, Thullier P, Pelat T, Urbain R, Fontayne A, et al. The European AntibotABE Framework Program and Its Update: Development of Innovative Botulinum Antibodies. Toxins. 2017; 9(10):309. https://doi.org/10.3390/toxins9100309
Chicago/Turabian StyleRasetti-Escargueil, Christine, Arnaud Avril, Sebastian Miethe, Christelle Mazuet, Yagmur Derman, Katja Selby, Philippe Thullier, Thibaut Pelat, Remi Urbain, Alexandre Fontayne, and et al. 2017. "The European AntibotABE Framework Program and Its Update: Development of Innovative Botulinum Antibodies" Toxins 9, no. 10: 309. https://doi.org/10.3390/toxins9100309
APA StyleRasetti-Escargueil, C., Avril, A., Miethe, S., Mazuet, C., Derman, Y., Selby, K., Thullier, P., Pelat, T., Urbain, R., Fontayne, A., Korkeala, H., Sesardic, D., Hust, M., & Popoff, M. R. (2017). The European AntibotABE Framework Program and Its Update: Development of Innovative Botulinum Antibodies. Toxins, 9(10), 309. https://doi.org/10.3390/toxins9100309







