Impact of Indoxyl Sulfate on Progenitor Cell-Related Neovascularization of Peripheral Arterial Disease and Post-Angioplasty Thrombosis of Dialysis Vascular Access
Abstract
:1. Chronic Kidney Disease and Vascular Disease
1.1. Chronic Kidney Disease and Vascular Disease
1.2. Peripheral Arterial Disease in Patients with Chronic Kidney Disease
1.3. Vascular Access Dysfunction in Patients on Hemodialysis
2. Vascular Toxicity of Indoxyl Sulfate
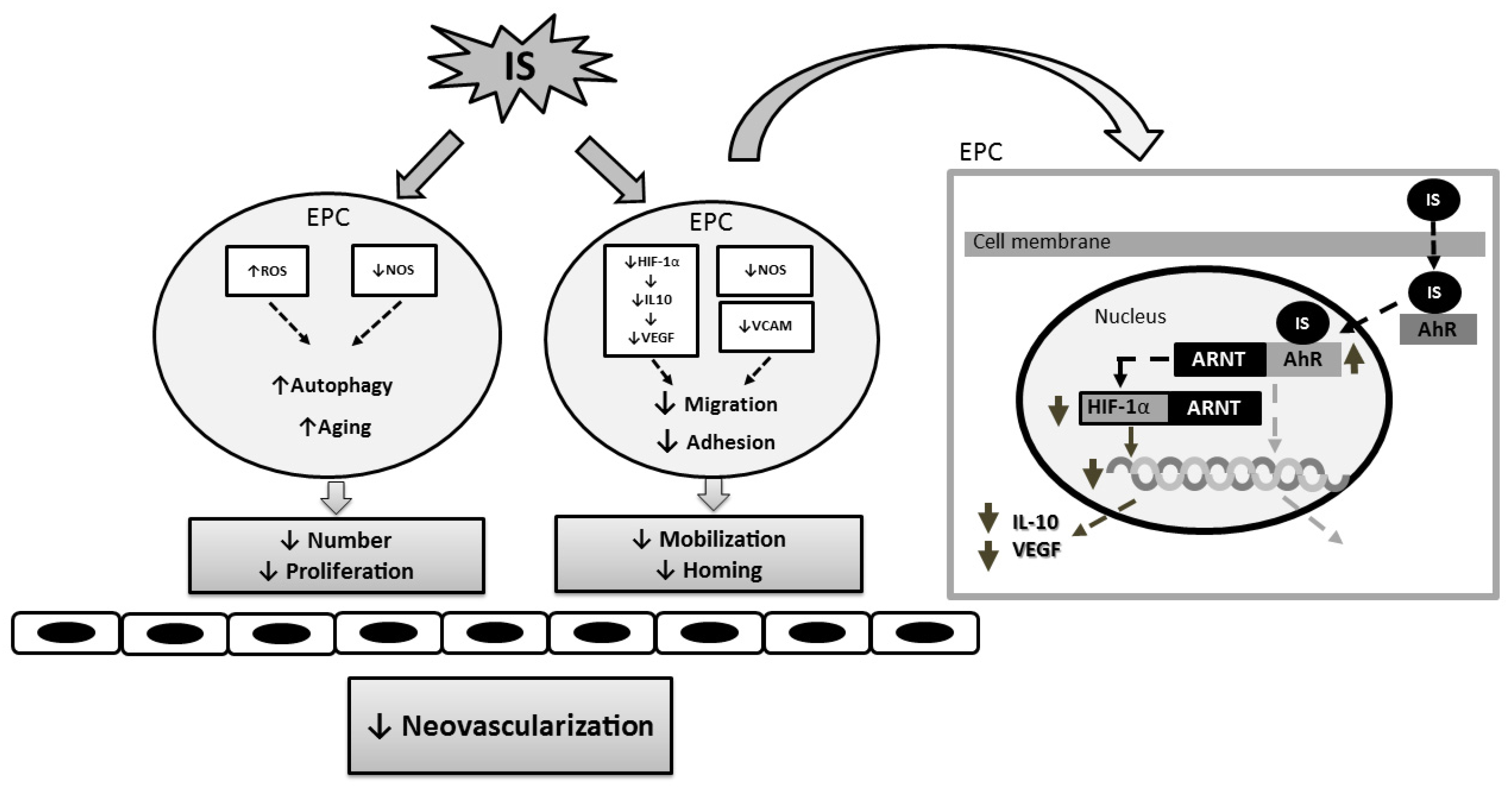
3. Indoxyl Sulfate and Progenitor Cell-Related Neovascularization
3.1. Angiogenesis and Peripheral Arterial Disease in Chronic Kidney Disease
3.2. Mechanisms Underlying the Effect of Indoxyl Sulfate on Neovascularization
4. Indoxyl Sulfate and Thrombosis of Dialysis Vascular Accesses
4.1. Thrombosis of Dialysis Vascular Accesses
4.2. Mechanisms Underlying the Effect of Indoxyl Sulfate on Thrombosis
5. Overall Summary
Acknowledgments
Conflicts of Interest
References
- Ocak, G.; Vossen, C.Y.; Rotmans, J.I.; Lijfering, W.M.; Rosendaal, F.R.; Parlevliet, K.J.; Krediet, R.T.; Boeschoten, E.W.; Dekker, F.W.; Verduijn, M. Venous and arterial thrombosis in dialysis patients. Thromb. Haemost. 2011, 106, 1046–1052. [Google Scholar] [CrossRef] [PubMed]
- Casserly, L.F.; Dember, L.M. Thrombosis in end-stage renal disease. Semin. Dial. 2003, 16, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Ocak, G.; van Stralen, K.J.; Rosendaal, F.R.; Verduijn, M.; Ravani, P.; Palsson, R.; Leivestad, T.; Hoitsma, A.J.; Ferrer-Alamar, M.; Finne, P.; et al. Mortality due to pulmonary embolism, myocardial infarction, and stroke among incident dialysis patients. J. Thromb. Haemost. 2012, 10, 2484–2493. [Google Scholar] [CrossRef] [PubMed]
- Findlay, M.D.; Thomson, P.C.; Fulton, R.L.; Solbu, M.D.; Jardine, A.G.; Patel, R.K.; Stevens, K.K.; Geddes, C.C.; Dawson, J.; Mark, P.B. Risk factors of ischemic stroke and subsequent outcome in patients receiving hemodialysis. Stroke 2015, 46, 2477–2481. [Google Scholar] [CrossRef] [PubMed]
- Trespalacios, F.C.; Taylor, A.J.; Agodoa, L.Y.; Abbott, K.C. Incident acute coronary syndromes in chronic dialysis patients in the united states1. Kidney Int. 2002, 62, 1799–1805. [Google Scholar] [CrossRef] [PubMed]
- Rajagopalan, S.; Dellegrottaglie, S.; Furniss, A.L.; Gillespie, B.W.; Satayathum, S.; Lameire, N.; Saito, A.; Akiba, T.; Jadoul, M.; Ginsberg, N.; et al. Peripheral arterial disease in patients with end-stage renal disease: Observations from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Circulation 2006, 114, 1914–1922. [Google Scholar] [CrossRef] [PubMed]
- Garimella, P.S.; Hart, P.D.; O’Hare, A.; DeLoach, S.; Herzog, C.A.; Hirsch, A.T. Peripheral artery disease and CKD: A focus on peripheral artery disease as a critical component of CKD care. Am. J. Kidney Dis. 2012, 60, 641–654. [Google Scholar] [CrossRef] [PubMed]
- Bethesda, M. US Renal Data System, Usrds 2010 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Rena Disease in the United States; National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2010.
- Neuen, B.L.; Gunnarsson, R.; Webster, A.C.; Baer, R.A.; Golledge, J.; Mantha, M.L. Predictors of patency after balloon angioplasty in hemodialysis fistulas: A systematic review. J. Vasc. Interv. Radiol. 2014, 25, 917–924. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, A.; Montes, R.; Muñoz-Terol, J.; Gil-Peralta, A.; Toro, J.; Naranjo, M.; González-Pérez, P.; Martín-Herrera, C.; Ruiz-Fernández, A. Peripheral arterial disease in patients with stages IV and V chronic renal failure. Nephrol. Dial. Transplant. 2006, 21, 3525–3531. [Google Scholar] [CrossRef] [PubMed]
- Schwab, S.; Besarab, A.; Beathard, G.; Brouwer, D.; Etheredge, E.; Hartigan, M.; Levine, M.; McCann, R.; Sherman, R.; Trerotola, S. National kidney foundation-dialysis outcomes quality initiative. NKF-DOQI clinical practice guidelines for vascular access. Am. J. Kidney Dis. 1997, 30, S150–S191. [Google Scholar]
- Neuen, B.L.; Gunnarsson, R.; Baer, R.A.; Tosenovsky, P.; Green, S.J.; Golledge, J.; Mantha, M.L. Factors associated with patency following angioplasty of hemodialysis fistulae. J. Vasc. Interv. Radiol. 2014, 25, 1419–1426. [Google Scholar] [CrossRef] [PubMed]
- Lilly, R.Z.; Carlton, D.; Barker, J.; Saddekni, S.; Hamrick, K.; Oser, R.; Westfall, A.O.; Allon, M. Predictors of arteriovenous graft patency after radiologic intervention in hemodialysis patients. Am. J. Kidney Dis. 2001, 37, 945–953. [Google Scholar] [CrossRef]
- Barreto, F.C.; Barreto, D.V.; Liabeuf, S.; Meert, N.; Glorieux, G.; Temmar, M.; Choukroun, G.; Vanholder, R.; Massy, Z.A. Serum indoxyl sulfate is associated with vascular disease and mortality in chronic kidney disease patients. Clin. J. Am. Soc. Nephrol. 2009, 4, 1551–1558. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-J.; Wu, V.; Wu, P.-C.; Wu, C.-J. Meta-analysis of the associations of p-cresyl sulfate (PCS) and indoxyl sulfate (IS) with cardiovascular events and all-cause mortality in patients with chronic renal failure. PLoS ONE 2015, 10, e0132589. [Google Scholar] [CrossRef] [PubMed]
- Taki, K.; Tsuruta, Y.; Niwa, T. Indoxyl sulfate and atherosclerotic risk factors in hemodialysis patients. Am. J. Nephrol. 2007, 27, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Kim, Y.J.; Kang, D.-H. Indoxyl sulfate–induced endothelial dysfunction in patients with chronic kidney disease via an induction of oxidative stress. Clin. J. Am. Soc. Nephrol. 2011, 6, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.J.; Pan, C.F.; Liu, H.L.; Chuang, C.K.; Jayakumar, T.; Wang, T.J.; Chen, H.H.; Wu, C.J. The role of protein-bound uremic toxins on peripheral artery disease and vascular access failure in patients on hemodialysis. Atherosclerosis 2012, 225, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Jourde-Chiche, N.; Dou, L.; Sabatier, F.; Calaf, R.; Cerini, C.; Robert, S.; Camoin-Jau, L.; Charpiot, P.; Argiles, A.; Dignat-George, F.; et al. Levels of circulating endothelial progenitor cells are related to uremic toxins and vascular injury in hemodialysis patients. J. Thromb. Haemost. 2009, 7, 1576–1584. [Google Scholar] [CrossRef] [PubMed]
- Tumur, Z.; Shimizu, H.; Enomoto, A.; Miyazaki, H.; Niwa, T. Indoxyl sulfate upregulates expression of ICAM-1 and MCP-1 by oxidative stress-induced NF-kappaβ activation. Am. J. Nephrol. 2010, 31, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Muteliefu, G.; Enomoto, A.; Jiang, P.; Takahashi, M.; Niwa, T. Indoxyl sulphate induces oxidative stress and the expression of osteoblast-specific proteins in vascular smooth muscle cells. Nephrol. Dial. Transplant. 2009, 24, 2051–2058. [Google Scholar] [CrossRef] [PubMed]
- Adijiang, A.; Goto, S.; Uramoto, S.; Nishijima, F.; Niwa, T. Indoxyl sulphate promotes aortic calcification with expression of osteoblast-specific proteins in hypertensive rats. Nephrol. Dial. Transplant. 2008, 23, 1892–1901. [Google Scholar] [CrossRef] [PubMed]
- Muteliefu, G.; Enomoto, A.; Niwa, T. Indoxyl sulfate promotes proliferation of human aortic smooth muscle cells by inducing oxidative stress. J. Ren. Nutr. 2009, 19, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Tsuruoka, S.; Ioka, T.; Ando, H.; Ito, C.; Akimoto, T.; Fujimura, A.; Asano, Y.; Kusano, E. Indoxyl sulfate stimulates proliferation of rat vascular smooth muscle cells. Kidney Int. 2006, 69, 1780–1785. [Google Scholar] [CrossRef] [PubMed]
- Dou, L.; Bertrand, E.; Cerini, C.; Faure, V.; Sampol, J.; Vanholder, R.; Berland, Y.; Brunet, P. The uremic solutes p-cresol and indoxyl sulfate inhibit endothelial proliferation and wound repair. Kidney Int. 2004, 65, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Dou, L.; Jourde-Chiche, N.; Faure, V.; Cerini, C.; Berland, Y.; Dignat-George, F.; Brunet, P. The uremic solute indoxyl sulfate induces oxidative stress in endothelial cells. J. Thromb. Haemost. 2007, 5, 1302–1308. [Google Scholar] [CrossRef] [PubMed]
- Itoh, Y.; Ezawa, A.; Kikuchi, K.; Tsuruta, Y.; Niwa, T. Protein-bound uremic toxins in hemodialysis patients measured by liquid chromatography/tandem mass spectrometry and their effects on endothelial ROS production. Anal. Bioanal. Chem. 2012, 403, 1841–1850. [Google Scholar] [CrossRef] [PubMed]
- Faure, V.; Dou, L.; Sabatier, F.; Cerini, C.; Sampol, J.; Berland, Y.; Brunet, P.; Dignat-George, F. Elevation of circulating endothelial microparticles in patients with chronic renal failure. J. Thromb. Haemost. 2006, 4, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Gondouin, B.; Cerini, C.; Dou, L.; Sallee, M.; Duval-Sabatier, A.; Pletinck, A.; Calaf, R.; Lacroix, R.; Jourde-Chiche, N.; Poitevin, S.; et al. Indolic uremic solutes increase tissue factor production in endothelial cells by the aryl hydrocarbon receptor pathway. Kidney Int. 2013, 84, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Kharait, S.; Haddad, D.J.; Springer, M.L. Nitric oxide counters the inhibitory effects of uremic toxin indoxyl sulfate on endothelial cells by governing ERK MAP kinase and myosin light chain activation. Biochem. Biophys. Res. Commun. 2011, 409, 758–763. [Google Scholar] [CrossRef] [PubMed]
- Mozar, A.; Louvet, L.; Morliere, P.; Godin, C.; Boudot, C.; Kamel, S.; Drueke, T.B.; Massy, Z.A. Uremic toxin indoxyl sulfate inhibits human vascular smooth muscle cell proliferation. Ther. Apher. Dial. 2011, 15, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Chitalia, V.C.; Shivanna, S.; Martorell, J.; Balcells, M.; Bosch, I.; Kolandaivelu, K.; Edelman, E.R. Uremic serum and solutes increase post–vascular interventional thrombotic risk through altered stability of smooth muscle cell tissue factor. Circulation 2013, 127, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Osaka, M.; Higuchi, Y.; Nishijima, F.; Ishii, H.; Yoshida, M. Indoxyl sulfate induces leukocyte-endothelial interactions through up-regulation of E-selectin. J. Biol. Chem. 2010, 285, 38869–38875. [Google Scholar] [CrossRef] [PubMed]
- Pletinck, A.; Glorieux, G.; Schepers, E.; Cohen, G.; Gondouin, B.; Van Landschoot, M.; Eloot, S.; Rops, A.; Van de Voorde, J.; De Vriese, A.; et al. Protein-bound uremic toxins stimulate crosstalk between leukocytes and vessel wall. J. Am. Soc. Nephrol. 2013, 24, 1981–1994. [Google Scholar] [CrossRef] [PubMed]
- Lekawanvijit, S.; Adrahtas, A.; Kelly, D.J.; Kompa, A.R.; Wang, B.H.; Krum, H. Does indoxyl sulfate, a uraemic toxin, have direct effects on cardiac fibroblasts and myocytes? Eur. Heart J. 2010, 31, 1771–1779. [Google Scholar] [CrossRef] [PubMed]
- Wu, V.C.; Young, G.H.; Huang, P.H.; Lo, S.C.; Wang, K.C.; Sun, C.Y.; Liang, C.J.; Huang, T.M.; Chen, J.H.; Chang, F.C.; et al. In acute kidney injury, indoxyl sulfate impairs human endothelial progenitor cells: Modulation by statin. Angiogenesis 2013, 16, 609–624. [Google Scholar] [CrossRef] [PubMed]
- Hung, S.C.; Kuo, K.L.; Huang, H.L.; Lin, C.C.; Tsai, T.H.; Wang, C.H.; Chen, J.W.; Lin, S.J.; Huang, P.H.; Tarng, D.C. Indoxyl sulfate suppresses endothelial progenitor cell-mediated neovascularization. Kidney Int. 2016, 89, 574–585. [Google Scholar] [CrossRef] [PubMed]
- Tumur, Z.; Niwa, T. Indoxyl sulfate inhibits nitric oxide production and cell viability by inducing oxidative stress in vascular endothelial cells. Am. J. Nephrol. 2009, 29, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Eggers, P.W.; Gohdes, D.; Pugh, J. Nontraumatic lower extremity amputations in the medicare end-stage renal disease population. Kidney Int. 1999, 56, 1524–1533. [Google Scholar] [CrossRef] [PubMed]
- Jacobi, J.; Porst, M.; Cordasic, N.; Namer, B.; Schmieder, R.E.; Eckardt, K.U.; Hilgers, K.F. Subtotal nephrectomy impairs ischemia-induced angiogenesis and hindlimb re-perfusion in rats. Kidney Int. 2006, 69, 2013–2021. [Google Scholar] [CrossRef] [PubMed]
- Yancopoulos, G.D.; Davis, S.; Gale, N.W.; Rudge, J.S.; Wiegand, S.J.; Holash, J. Vascular-specific growth factors and blood vessel formation. Nature 2000, 407, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Murasawa, S.; Asahara, T. Endothelial progenitor cells for vasculogenesis. Physiology (Bethesda) 2005, 20, 36–42. [Google Scholar] [CrossRef] [PubMed]
- De Groot, K.; Bahlmann, F.H.; Sowa, J.; Koenig, J.; Menne, J.; Haller, H.; Fliser, D. Uremia causes endothelial progenitor cell deficiency. Kidney Int. 2004, 66, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Shivanna, S.; Kolandaivelu, K.; Shashar, M.; Belghasim, M.; Al-Rabadi, L.; Balcells, M.; Zhang, A.; Weinberg, J.; Francis, J.; Pollastri, M.P.; et al. The aryl hydrocarbon receptor is a critical regulator of tissue factor stability and an antithrombotic target in uremia. J. Am. Soc. Nephrol. 2016, 27, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Sallee, M.; Dou, L.; Cerini, C.; Poitevin, S.; Brunet, P.; Burtey, S. The aryl hydrocarbon receptor-activating effect of uremic toxins from tryptophan metabolism: A new concept to understand cardiovascular complications of chronic kidney disease. Toxins (Basel) 2014, 6, 934–949. [Google Scholar] [CrossRef] [PubMed]
- Schwab, S.J.; Harrington, J.T.; Singh, A.; Roher, R.; Shohaib, S.A.; Perrone, R.D.; Meyer, K.; Beasley, D. Vascular access for hemodialysis. Kidney Int. 1999, 55, 2078–2090. [Google Scholar] [CrossRef] [PubMed]
- Crowther, M.A.; Kelton, J.G. Congenital thrombophilic states associated with venous thrombosis: A qualitative overview and proposed classification system. Ann. Intern. Med. 2003, 138, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.I.; Paik, J.; Greene, T.; Desai, M.; Bech, F.; Cheung, A.K.; Chertow, G.M. Intradialytic hypotension and vascular access thrombosis. J. Am. Soc. Nephrol. 2011, 22, 1526–1533. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, O.; Assadian, A.; Frank, H.; Moessmer, G.; Heemann, U.; Eckstein, H.H. Hereditary and acquired thrombophilic disorders complicating vascular access in haemodialysisl. NDT Plus 2010, 3, 393–396. [Google Scholar] [PubMed]
- Baskin, E.; Duman, O.; Besbas, N.; Ozen, S. Hypercoagulopathy in a hemodialysis patient: Are elevations in factors VII and VIII effective? Nephron 1999, 83, 180. [Google Scholar] [CrossRef] [PubMed]
- Segarra, A.; Chacon, P.; Martinez-Eyarre, C.; Argelaguer, X.; Vila, J.; Ruiz, P.; Fort, J.; Bartolome, J.; Camps, J.; Moliner, E.; et al. Circulating levels of plasminogen activator inhibitor type-1, tissue plasminogen activator, and thrombomodulin in hemodialysis patients: Biochemical correlations and role as independent predictors of coronary artery stenosis. J. Am. Soc. Nephrol. 2001, 12, 1255–1263. [Google Scholar] [PubMed]
- Malyszko, J.; Malyszko, J.S.; Hryszko, T.; Mysliwiec, M. Thrombin activatable fibrinolysis inhibitor (TAFI) and markers of endothelial cell injury in dialyzed patients with diabetic nephropathy. Thromb. Haemost. 2004, 91, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Mercier, E.; Branger, B.; Vecina, F.; Al-Sabadani, B.; Berlan, J.; Dauzat, M.; Fourcade, J.; Gris, J.C. Tissue factor coagulation pathway and blood cells activation state in renal insufficiency. Hematol. J. 2001, 2, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Knoll, G.A.; Wells, P.S.; Young, D.; Perkins, S.L.; Pilkey, R.M.; Clinch, J.J.; Rodger, M.A. Thrombophilia and the risk for hemodialysis vascular access thrombosis. J. Am. Soc. Nephrol. 2005, 16, 1108–1114. [Google Scholar] [CrossRef] [PubMed]
- Salmela, B.; Hartman, J.; Peltonen, S.; Albäck, A.; Lassila, R. Thrombophilia and arteriovenous fistula survival in ESRD. Clin. J. Am. Soc. Nephrol. 2013, 8, 962–968. [Google Scholar] [CrossRef] [PubMed]
- Nampoory, M.R.; Das, K.C.; Johny, K.V.; Al-Hilali, N.; Abraham, M.; Easow, S.; Saed, T.; Al-Muzeirei, I.A.; Sugathan, T.N.; Al Mousawi, M. Hypercoagulability, a serious problem in patients with ESRD on maintenance hemodialysis, and its correction after kidney transplantation. Am. J. Kidney Dis. 2003, 42, 797–805. [Google Scholar] [CrossRef]
- Wu, C.C.; Hsieh, M.Y.; Hung, S.C.; Kuo, K.L.; Tsai, T.H.; Lai, C.L.; Chen, J.W.; Lin, S.J.; Huang, P.H.; Tarng, D.C. Serum indoxyl sulfate associates with postangioplasty thrombosis of dialysis grafts. J. Am. Soc. Nephrol. 2016, 27, 1254–1264. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, T.; Ise, M.; Hirata, M.; Endo, K.; Ito, Y.; Seo, H.; Niwa, T. Indoxyl sulfate stimulates renal synthesis of transforming growth factor-beta 1 and progression of renal failure. Kidney Int. Suppl. 1997, 63, S211–S214. [Google Scholar] [PubMed]
- Yisireyili, M.; Saito, S.; Abudureyimu, S.; Adelibieke, Y.; Ng, H.-Y.; Nishijima, F.; Takeshita, K.; Murohara, T.; Niwa, T. Indoxyl sulfate-induced activation of (pro)renin receptor promotes cell proliferation and tissue factor expression in vascular smooth muscle cells. PLoS ONE 2014, 9, e109268. [Google Scholar] [CrossRef] [PubMed]
- Muteliefu, G.; Shimizu, H.; Enomoto, A.; Nishijima, F.; Takahashi, M.; Niwa, T. Indoxyl sulfate promotes vascular smooth muscle cell senescence with upregulation of p53, p21, and prelamin a through oxidative stress. Am. J. Physiol. Cell Physiol. 2012, 303, C126–C134. [Google Scholar] [CrossRef] [PubMed]
- Sata, M.; Saiura, A.; Kunisato, A.; Tojo, A.; Okada, S.; Tokuhisa, T.; Hirai, H.; Makuuchi, M.; Hirata, Y.; Nagai, R. Hematopoietic stem cells differentiate into vascular cells that participate in the pathogenesis of atherosclerosis. Nat. Med. 2002, 8, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.C.; Huang, P.H.; Lai, C.L.; Leu, H.B.; Chen, J.W.; Lin, S.J. The impact of endothelial progenitor cells on restenosis after percutaneous angioplasty of hemodialysis vascular access. PLoS ONE 2014, 9, e101058. [Google Scholar] [CrossRef] [PubMed]
- Lev, E.I.; Leshem-Lev, D.; Mager, A.; Vaknin-Assa, H.; Harel, N.; Zimra, Y.; Bental, T.; Greenberg, G.; Dvir, D.; Solodky, A.; et al. Circulating endothelial progenitor cell levels and function in patients who experienced late coronary stent thrombosis. Eur. Heart J. 2010, 31, 2625–2632. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.Y.; Lin, T.T.; Hsieh, M.Y.; Lin, L.; Yang, C.W.; Chuang, S.Y.; Huang, P.H.; Wu, C.C. Circulating progenitor cells affect thrombosis of dialysis arteriovenous fistulas. Am. J. Nephrol. 2016, 44, 428–438. [Google Scholar] [CrossRef] [PubMed]

| Cells | Primary Effect | Reference |
|---|---|---|
| Endothelial cell | Induction of ROS | Dou [26], Yu [17], Itoh [27] |
| Inhibit endothelial NO production | Yu [17] | |
| Increase endothelial microparticle release | Faure [28] | |
| Increase tissue factor production | Gondouin [29] | |
| Inhibit endothelial cell proliferation | Dou, Yu [17] | |
| Inhibit endothelial cell migration | Kharait [30] | |
| Smooth muscle cell | Increase proliferation | Yamamoto [24] |
| Inhibit proliferation | Mozar [31] | |
| Reduce tissue factor breakdown | Chitalia [32] | |
| Leukocyte | Increase leukocyte adhesion | Ito [33], Tumur [20], Pletinck [34] |
| Increase inflammatory cytokine expression | Lekawanvijit [35] | |
| Progenitor cell | Induction of ROS | Wu [36] |
| Inhibit NO production | Wu [36] | |
| Inhibit HIF/IL-10/VEGF pathway | Hung [37] | |
| Decrease in number and function | Hung [37], Wu [36] |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.-C.; Hung, S.-C.; Kuo, K.-L.; Tarng, D.-C. Impact of Indoxyl Sulfate on Progenitor Cell-Related Neovascularization of Peripheral Arterial Disease and Post-Angioplasty Thrombosis of Dialysis Vascular Access. Toxins 2017, 9, 25. https://doi.org/10.3390/toxins9010025
Wu C-C, Hung S-C, Kuo K-L, Tarng D-C. Impact of Indoxyl Sulfate on Progenitor Cell-Related Neovascularization of Peripheral Arterial Disease and Post-Angioplasty Thrombosis of Dialysis Vascular Access. Toxins. 2017; 9(1):25. https://doi.org/10.3390/toxins9010025
Chicago/Turabian StyleWu, Chih-Cheng, Szu-Chun Hung, Ko-Lin Kuo, and Der-Cherng Tarng. 2017. "Impact of Indoxyl Sulfate on Progenitor Cell-Related Neovascularization of Peripheral Arterial Disease and Post-Angioplasty Thrombosis of Dialysis Vascular Access" Toxins 9, no. 1: 25. https://doi.org/10.3390/toxins9010025
APA StyleWu, C.-C., Hung, S.-C., Kuo, K.-L., & Tarng, D.-C. (2017). Impact of Indoxyl Sulfate on Progenitor Cell-Related Neovascularization of Peripheral Arterial Disease and Post-Angioplasty Thrombosis of Dialysis Vascular Access. Toxins, 9(1), 25. https://doi.org/10.3390/toxins9010025







