Respiratory Effects of Sarafotoxins from the Venom of Different Atractaspis Genus Snake Species
Abstract
:1. Introduction
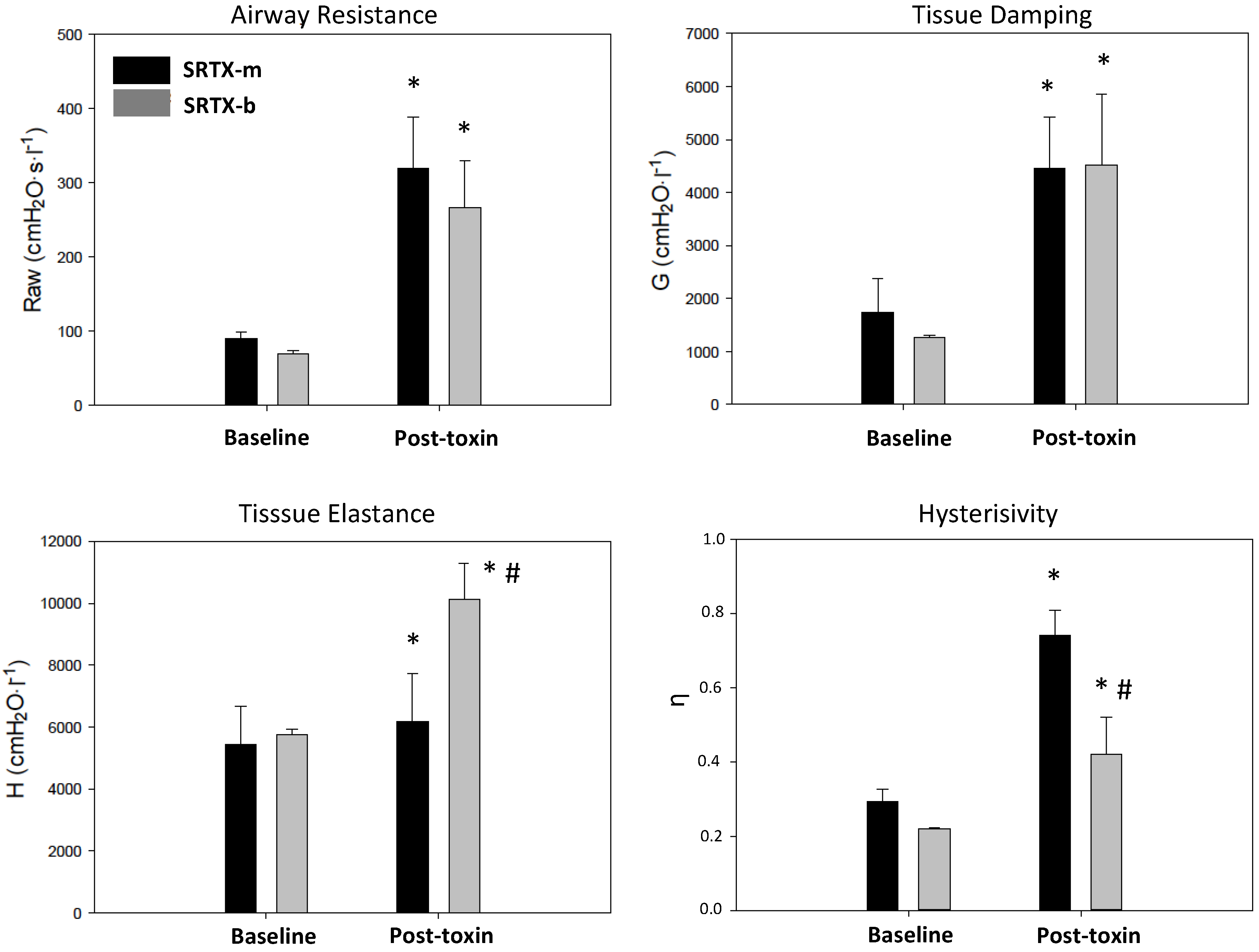
2. Results
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Peptide Synthesis
5.2. Animal Preparation
5.3. Measurement of Respiratory Mechanics
5.4 Experimental Protocol
5.5 Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| SRTXs | sarafotoxins |
| SRTX-b | sarafotoxin b extracted from the venom of Atractaspis engaddensis |
| SRTX-m | sarafotoxin m extracted from the venom of Atractaspis microlepidota microlepidota |
| ET | endothelin |
| ET-A | endothelin-receptor subtype A |
| ET-B | endothelin-receptor subtype B |
| Iaw | inertance |
| Raw | resistance |
| G | damping |
| H | elastance |
| η | hysterisivity |
References
- Yanagisawa, M.; Kurihara, H.; Kimura, S.; Tomobe, Y.; Kobayashi, M.; Mitsui, Y.; Yazaki, Y.; Goto, K.; Masaki, T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 1988, 332, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Ducancel, F. The sarafotoxins. Toxicon 2002, 40, 1541–1545. [Google Scholar] [CrossRef]
- Sokolovsky, M. Endothelins and sarafotoxins: Physiological regulation, receptor subtypes and transmembrane signaling. Trends Biochem. Sci. 1991, 16, 261–264. [Google Scholar] [CrossRef]
- Hayashi, M.A.F.; Ligny-Lemaire, C.; Wollberg, Z.; Wery, M.; Galat, A.; Ogawa, T.; Muller, B.H.; Lamthanh, H.; Doljansky, Y.; Bdolah, A.; et al. Long-sarafotoxins: Characterization of a new family of endothelin-like peptides. Peptides 2004, 25, 1243–1251. [Google Scholar] [CrossRef] [PubMed]
- Atkins, A.R.; Martin, R.C.; Smith, R. 1H NMR studies of sarafotoxin SRTb, a nonselective endothelin receptor agonist, and IRL 1620, an ETB receptor-specific agonist. Biochemistry 1995, 34, 2026–2033. [Google Scholar] [CrossRef] [PubMed]
- Mourier, G.; Hajj, M.; Cordier, F.; Zorba, A.; Gao, X.; Coskun, T.; Herbet, A.; Marcon, E.; Beau, F.; Delepierre, M.; et al. Pharmacological and structural characterization of long-sarafotoxins, a new family of endothelin-like peptides: Role of the C-terminus extension. Biochimie 2012, 94, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Mourier, G.; Dutertre, S.; Fruchart-Gaillard, C.; Ménez, A.; Servent, D. Chemical synthesis of MT1 and MT7 muscarinic toxins: Critical role of Arg-34 in their interaction with M1 muscarinic receptor. Mol. Pharmacol. 2003, 63, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Mahjoub, Y.; Malaquin, S.; Mourier, G.; Lorne, E.; Abou Arab, O.; Massy, Z.A.; Dupont, H.; Ducancel, F. Short- versus Long-Sarafotoxins: Two Structurally Related Snake Toxins with Very Different in vivo Haemodynamic Effects. PLoS ONE 2015, 10, e0132864. [Google Scholar] [CrossRef] [PubMed]
- Lal, H.; Woodward, B.; Williams, K.I. Actions of endothelins and sarafotoxin 6c in the rat isolated perfused lung. Br. J. Pharmacol. 1995, 115, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Nagase, T.; Aoki, T.; Matsui, H.; Ohga, E.; Katayama, H.; Teramoto, S.; Matsuse, T.; Fukuchi, Y.; Ouchi, Y. Airway and lung tissue behaviour during endothelin-1 induced constriction in rats: Effects of receptor antagonists. Can. J. Physiol. Pharmacol. 1997, 75, 1369–1374. [Google Scholar] [PubMed]
- Sylvin, H.; Weitzberg, E.; Alving, K. Endothelin-induced vascular and bronchial effects in pig airways: Role in acute allergic responses. J. Appl. Physiol. 2002, 93, 1608–1615. [Google Scholar] [CrossRef] [PubMed]
- White, S.R.; Hathaway, D.P.; Umans, J.G.; Tallet, J.; Abrahams, C.; Leff, A.R. Epithelial modulation of airway smooth muscle response to endothelin-1. Am. Rev. Respir. Dis. 1991, 144, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Peták, F.; Habre, W.; Hantos, Z.; Sly, P.D.; Morel, D.R. Effects of pulmonary vascular pressures and flow on airway and parenchymal mechanics in isolated rat lungs. J. Appl. Physiol. 2002, 92, 169–178. [Google Scholar]
- Filep, J.G.; Battistini, B.; Sirois, P. Pharmacological modulation of endothelin-induced contraction of guinea-pig isolated airways and thromboxane release. Br. J. Pharmacol. 1991, 103, 1633–1640. [Google Scholar] [CrossRef] [PubMed]
- Polikepahad, S.; Moore, R.M.; Venugopal, C.S. Endothelins and airways--a short review. Res. Commun. Mol. Pathol. Pharmacol. 2006, 119, 3–51. [Google Scholar] [PubMed]
- Granström, B.; Nilsson, E.; Hultkvist-Bengtsson, U.; Edvinsson, L. Analysis of ET-A and ET-B receptors using an isolated perfused rat lung preparation. Acta Physiol. Scand. 2004, 181, 259–264. [Google Scholar]
- Janosi, T.; Peták, F.; Fontao, F.; Morel, D.R.; Beghetti, M.; Habre, W. Differential roles of endothelin-1 ETA and ETB receptors and vasoactive intestinal polypeptide in regulation of the airways and the pulmonary vasculature in isolated rat lung. Exp. Physiol. 2008, 93, 1210–1219. [Google Scholar] [CrossRef] [PubMed]
- Kaczka, D.W.; Dellacá, R.L. Oscillation mechanics of the respiratory system: Applications to lung disease. Crit. Rev. Biomed. Eng. 2011, 39, 337–359. [Google Scholar] [CrossRef] [PubMed]
- Bayat, S.; Strengell, S.; Porra, L.; Janosi, T.Z.; Petak, F.; Suhonen, H.; Suortti, P.; Hantos, Z.; Sovijärvi, A.R.A.; Habre, W. Methacholine and ovalbumin challenges assessed by forced oscillations and synchrotron lung imaging. Am. J. Respir. Crit. Care Med. 2009, 180, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Barnas, G.M.; Sprung, J. Effects of mean airway pressure and tidal volume on lung and chest wall mechanics in the dog. J. Appl. Physiol. 1993, 74, 2286–2293. [Google Scholar] [PubMed]
- Lutchen, K.R.; Hantos, Z.; Peták, F.; Adamicza, A.; Suki, B. Airway inhomogeneities contribute to apparent lung tissue mechanics during constriction. J. Appl. Physiol. 1996, 80, 1841–1849. [Google Scholar] [PubMed]
- Robatto, F.M.; Simard, S.; Ludwig, M.S. How changes in the serial distribution of bronchoconstriction affect lung mechanics. J. Appl. Physiol. 1993, 74, 2838–2847. [Google Scholar] [PubMed]
- Berger, M.M.; Rozendal, C.S.; Schieber, C.; Dehler, M.; Zügel, S.; Bardenheuer, H.J.; Bärtsch, P.; Mairbäurl, H. The effect of endothelin-1 on alveolar fluid clearance and pulmonary edema formation in the rat. Anesth. Analg. 2009, 108, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Konrad, D.; Oldner, A.; Wanecek, M.; Rudehill, A.; Weitzberg, E.; Biber, B.; Johansson, G.; Häggmark, S.; Haney, M. Positive inotropic and negative lusitropic effects of endothelin receptor agonism in vivo. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H1702–H1709. [Google Scholar] [CrossRef] [PubMed]
- Trape, J.-F.; Mané, Y.; Ineich, I. Atractaspis microlepidota, A. micropholis et A. watsoni en Afrique occidentale et centrale. Bull. Soc. Herpetol. Fr. 2006, 119, 5–16. [Google Scholar]
- Pacher, P.; Mabley, J.G.; Liaudet, L.; Evgenov, O.V.; Marton, A.; Haskó, G.; Kollai, M.; Szabó, C. Left ventricular pressure-volume relationship in a rat model of advanced aging-associated heart failure. Am. J. Physiol. Heart Circ. Physiol. 2004, 287, H2132–H2137. [Google Scholar] [CrossRef] [PubMed]
- Hantos, Z.; Daróczy, B.; Suki, B.; Nagy, S.; Fredberg, J.J. Input impedance and peripheral inhomogeneity of dog lungs. J. Appl. Physiol. 1992, 72, 168–178. [Google Scholar] [PubMed]
- Bates, J.H.T.; Irvin, C.G.; Farré, R.; Hantos, Z. Oscillation mechanics of the respiratory system. Compr. Physiol. 2011, 1, 1233–1272. [Google Scholar] [PubMed]
- Franken, H.; Clément, J.; Cauberghs, M.; Van de Woestijne, K.P. Oscillating flow of a viscous compressible fluid through a rigid tube: A theoretical model. IEEE Trans. Biomed. Eng. 1981, 28, 416–420. [Google Scholar] [CrossRef] [PubMed]
- Wollberg, Z.; Shabo-Shina, R.; Intrator, N.; Bdolah, A.; Kochva, E.; Shavit, G.; Oron, Y.; Vidne, B.A.; Gitter, S. A novel cardiotoxic polypeptide from the venom of Atractaspis engaddensis (burrowing asp): Cardiac effects in mice and isolated rat and human heart preparations. Toxicon 1988, 26, 525–534. [Google Scholar] [CrossRef]
| Toxin | pH | PaO2 (mmHg) | PaCO2 (mmHg) | HCO3− (mmol·L−1) | Cl− (mmol·L−1) | AG (mmol·L−1) |
|---|---|---|---|---|---|---|
| Baseline | 7.43 ± 0.12 | 85 ± 21 | 25 ± 4 | 16 ± 5 | 113 ± 2 | 12 ± 4 |
| SRTX-m | 7.41 ± 0.14 | 65 ± 16 * | 18 ± 5 | 12 ± 2 | 119 ± 3 * | 17 ± 4 * |
| Baseline | 7.51 ± 0.04 | 100 ± 18 | 20 ± 5 | 16 ± 3 | 116 ± 2 | 12 ± 2 |
| SRTX-b | 7.49 ± 0.18 | 70 ± 25 * | 13 ± 7 * | 9 ± 2 * | 12 ± 4 * | 19 ± 3 * |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malaquin, S.; Bayat, S.; Abou Arab, O.; Mourier, G.; Lorne, E.; Kamel, S.; Dupont, H.; Ducancel, F.; Mahjoub, Y. Respiratory Effects of Sarafotoxins from the Venom of Different Atractaspis Genus Snake Species. Toxins 2016, 8, 215. https://doi.org/10.3390/toxins8070215
Malaquin S, Bayat S, Abou Arab O, Mourier G, Lorne E, Kamel S, Dupont H, Ducancel F, Mahjoub Y. Respiratory Effects of Sarafotoxins from the Venom of Different Atractaspis Genus Snake Species. Toxins. 2016; 8(7):215. https://doi.org/10.3390/toxins8070215
Chicago/Turabian StyleMalaquin, Stéphanie, Sam Bayat, Osama Abou Arab, Gilles Mourier, Emmanuel Lorne, Saïd Kamel, Hervé Dupont, Frédéric Ducancel, and Yazine Mahjoub. 2016. "Respiratory Effects of Sarafotoxins from the Venom of Different Atractaspis Genus Snake Species" Toxins 8, no. 7: 215. https://doi.org/10.3390/toxins8070215
APA StyleMalaquin, S., Bayat, S., Abou Arab, O., Mourier, G., Lorne, E., Kamel, S., Dupont, H., Ducancel, F., & Mahjoub, Y. (2016). Respiratory Effects of Sarafotoxins from the Venom of Different Atractaspis Genus Snake Species. Toxins, 8(7), 215. https://doi.org/10.3390/toxins8070215






