Ellagitannins in Cancer Chemoprevention and Therapy
Abstract
:1. Introduction
2. Dietary Sources, Types, and Occurrence
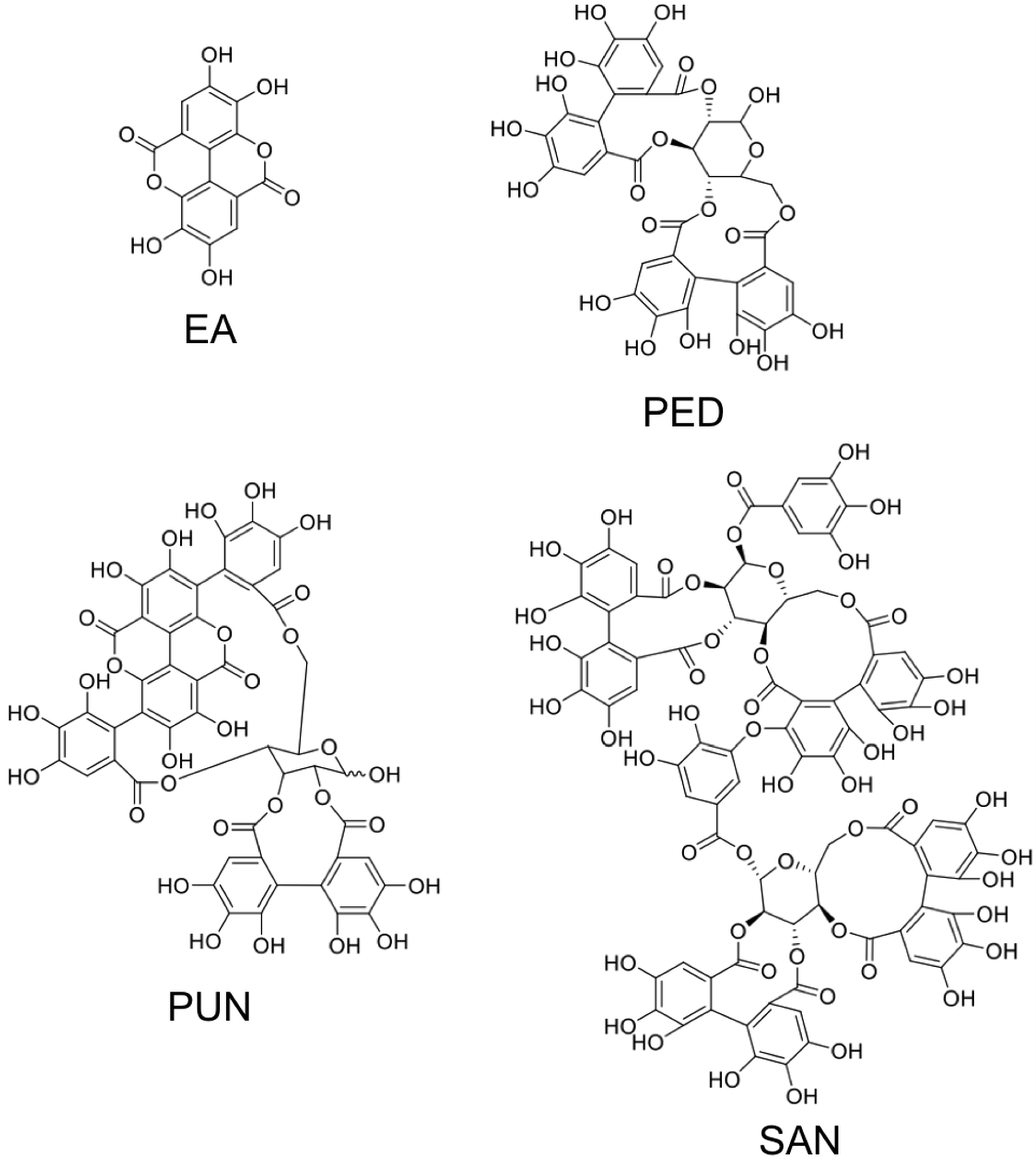
3. Ellagitannins—Classification and Chemistry
3.1. Simple Ellagitannins
3.2. Glycosidic Ellagitannins
4. Ellagitannins Pharmacokinetics
5. Ellagitannins for Tumor Chemoprevention and Therapy
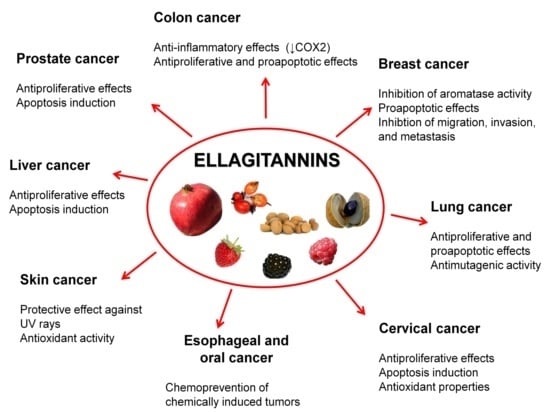
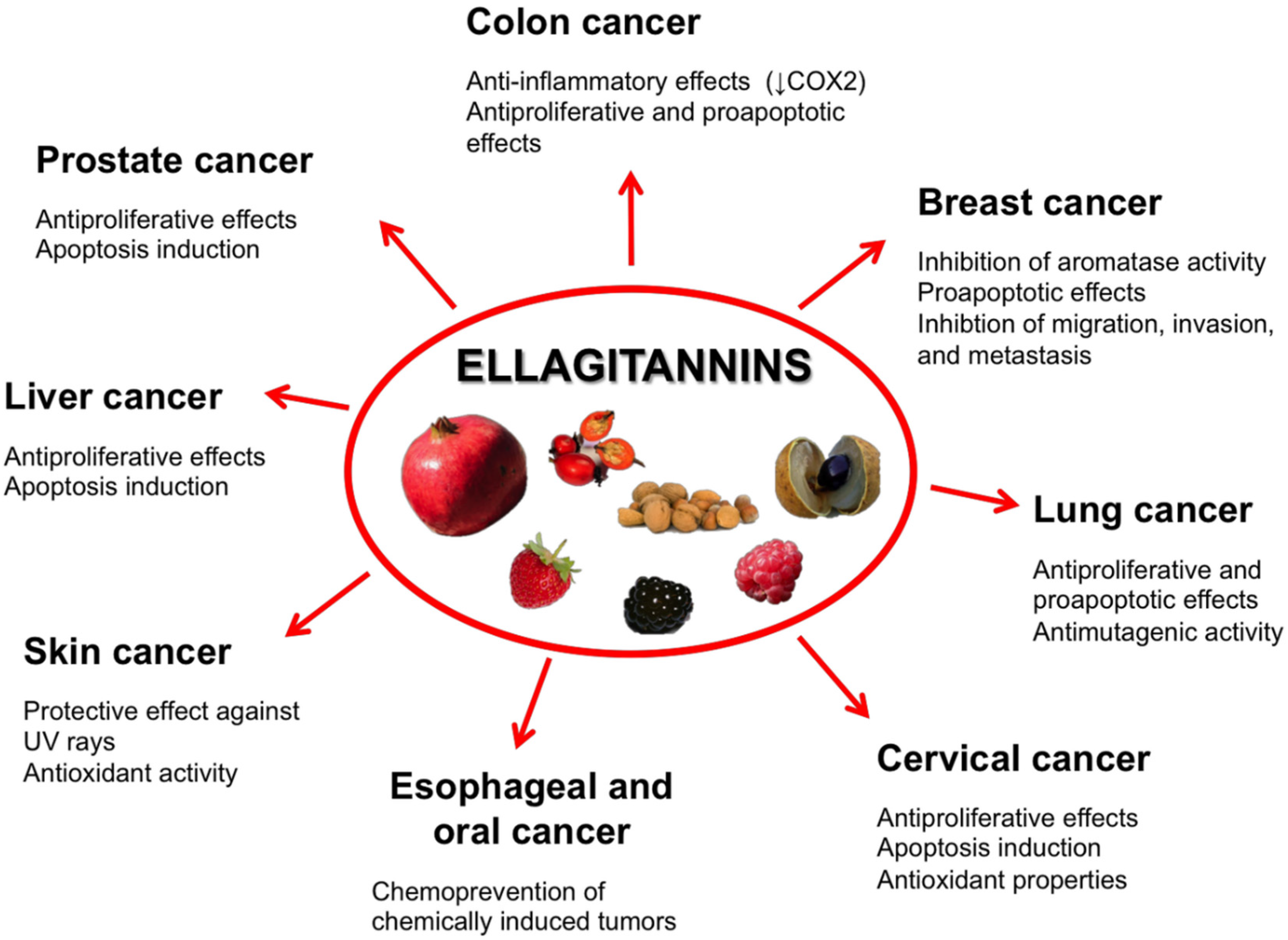
5.1. Prostate Cancer
5.2. Colon Cancer
5.3. Breast Cancer
5.4. Oral, Esophageal, and Gastric Cancers
5.5. Liver Cancer
5.6. Cervical Cancer
5.7. Lung Cancer
5.8. Skin Cancer
6. Risks and Safe Consumption Levels
7. Concluding Remarks
Author Contributions
Conflicts of Interest
References
- Siegel, R.; Ward, E.; Brawley, O.; Jemal, A. The impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J. Clin. 2011, 61, 212–236. [Google Scholar]
- Durko, L.; Malecka-Panas, E. Lifestyle modifications and colorectal cancer. Curr. Colorectal. Cancer Rep. 2014, 10, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouysegu, L. Plant polyphenols: Chemical properties, biological activities, and synthesis. Angew. Chem. Int. Ed. Engl. 2011, 50, 586–621. [Google Scholar] [CrossRef] [PubMed]
- Ismail, T.; Sestili, P.; Akhtar, S. Pomegranate peel and fruit extracts: A review of potential anti-inflammatory and anti-infective effects. J. Ethnopharmacol. 2012, 143, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Landete, J. Ellagitannins, ellagic acid and their derived metabolites: A review about source, metabolism, functions and health. Food Res. Int. 2011, 44, 1150–1160. [Google Scholar] [CrossRef]
- Koponen, J.M.; Happonen, A.M.; Mattila, P.H.; Torronen, A.R. Contents of anthocyanins and ellagitannins in selected foods consumed in Finland. J. Agric. Food Chem. 2007, 55, 1612–1619. [Google Scholar] [CrossRef] [PubMed]
- Mullen, W.; McGinn, J.; Lean, M.E.; MacLean, M.R.; Gardner, P.; Duthie, G.G.; Yokota, T.; Crozier, A. Ellagitannins, flavonoids, and other phenolics in red raspberries and their contribution to antioxidant capacity and vasorelaxation properties. J. Agric. Food Chem. 2002, 50, 5191–5196. [Google Scholar] [CrossRef] [PubMed]
- Mullen, W.; Stewart, A.J.; Lean, M.E.; Gardner, P.; Duthie, G.G.; Crozier, A. Effect of freezing and storage on the phenolics, ellagitannins, flavonoids, and antioxidant capacity of red raspberries. J. Agric. Food Chem. 2002, 50, 5197–5201. [Google Scholar] [CrossRef] [PubMed]
- Gancel, A.L.; Feneuil, A.; Acosta, O.; Perez, A.M.; Vaillant, F. Impact of industrial processing and storage on major polyphenols and the antioxidant capacity of tropical highland blackberry (Rubus adenotrichus). Food Res. Int. 2011, 44, 2243–2251. [Google Scholar] [CrossRef]
- Hager, T.J.; Howard, L.R.; Liyanage, R.; Lay, J.O.; Prior, R.L. Ellagitannin composition of blackberry as determined by HPLC-ESI-MS and MALDI-TOF-MS. J. Agric. Food Chem. 2008, 56, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Mertz, C.; Cheynier, V.; Gunata, Z.; Brat, P. Analysis of phenolic compounds in two blackberry species (Rubus glaucus and Rubus adenotrichus) by high-performance liquid chromatography with diode array detection and electrospray ion trap mass spectrometry. J. Agric. Food Chem. 2007, 55, 8616–8624. [Google Scholar] [CrossRef] [PubMed]
- Lansky, E.P.; Newman, R.A. Punica granatum (pomegranate) and its potential for prevention and treatment of inflammation and cancer. J. Ethnopharmacol. 2007, 109, 177–206. [Google Scholar] [CrossRef] [PubMed]
- Beekwilder, J.; Jonker, H.; Meesters, P.; Hall, R.D.; van der Meer, I.M.; de Vos, C.H.R. Antioxidants in raspberry: On-line analysis links antioxidant activity to a diversity of individual metabolites. J. Agric. Food Chem. 2005, 53, 3313–3320. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Johnson, J.V.; Talcott, S.T. Identification of ellagic acid conjugates and other polyphenolics in muscadine grapes by HPLC-ESI-MS. J. Agric. Food Chem. 2005, 53, 6003–6010. [Google Scholar] [CrossRef] [PubMed]
- Maatta-Riihinen, K.R.; Kamal-Eldin, A.; Torronen, A.R. Identification and quantification of phenolic compounds in berries of Fragaria and Rubus species (family Rosaceae). J. Agric. Food Chem. 2004, 52, 6178–6187. [Google Scholar] [CrossRef] [PubMed]
- Mullen, W.; Yokota, T.; Lean, M.E.; Crozier, A. Analysis of ellagitannins and conjugates of ellagic acid and quercetin in raspberry fruits by LC-MSn. Phytochemistry 2003, 64, 617–624. [Google Scholar] [CrossRef]
- Rangkadilok, N.; Worasuttayangkurn, L.; Bennett, R.N.; Satayavivad, J. Identification and quantification of polyphenolic compounds in longan (Euphoria longana Lam.) fruit. J. Agric. Food Chem. 2005, 53, 1387–1392. [Google Scholar] [CrossRef] [PubMed]
- Seeram, N.P.; Lee, R.; Heber, D. Bioavailability of ellagic acid in human plasma after consumption of ellagitannins from pomegranate (Punica granatum L.) juice. Clin. Chim. Acta 2004, 348, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Abe, L.T.; Lajolo, F.M.; Genovese, M.I. Comparison of phenol content and antioxidant capacity of nuts. Food Sci. Technol. (Camp.) 2010, 30, 254–259. [Google Scholar] [CrossRef]
- Kaume, L.; Howard, L.R.; Devareddy, L. The blackberry fruit: A review on its composition and chemistry, metabolism and bioavailability, and health benefits. J. Agric. Food Chem. 2012, 60, 5716–5727. [Google Scholar] [CrossRef] [PubMed]
- Shi, N.; Clinton, S.K.; Liu, Z.; Wang, Y.; Riedl, K.M.; Schwartz, S.J.; Zhang, X.; Pan, Z.; Chen, T. Strawberry phytochemicals inhibit azoxymethane/dextran sodium sulfate-induced colorectal carcinogenesis in Crj: CD-1 mice. Nutrients 2015, 7, 1696–1715. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Ito, H.; Hatano, T.; Kurata, M.; Nakanishi, T.; Inada, A.; Murata, H.; Inatomi, Y.; Matsuura, N.; Ono, K.; et al. New hydrolyzable tannins, Shephagenins A and B, from shepherdia argentea as HIV-1 reverse transcriptase inhibitors. Chem. Pharm. Bull. 1996, 44, 1436–1439. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Hatano, T.; Ito, H. Chemistry and function of vegetable polyphenols with high molecular weights. BioFactors 2000, 13, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Harborne, J.B. Plant Phenolics; Academic Press Ltd.: Chicago, IL, USA, 1983; Volume 1. [Google Scholar]
- Haslam, E. Plant Polyphenols: Vegetable Tannins Revisited; CUP Archive: Cambridge, UK, 1989. [Google Scholar]
- Haslam, E.; Cai, Y. Plant polyphenols (vegetable tannins): Gallic acid metabolism. Nat. Prod. Rep. 1994, 11, 41–66. [Google Scholar] [CrossRef] [PubMed]
- Khanbabaee, K.; van Ree, T. Tannins: Classification and definition. Nat. Prod. Rep. 2001, 18, 641–649. [Google Scholar]
- Salminen, J.P.; Karonen, M. Chemical ecology of tannins and other phenolics: We need a change in approach. Funct. Ecol. 2011, 25, 325–338. [Google Scholar] [CrossRef]
- Gross, G.G. From lignins to tannins: Forty years of enzyme studies on the biosynthesis of phenolic compounds. Phytochemistry 2008, 69, 3018–3031. [Google Scholar] [CrossRef] [PubMed]
- Larrosa, M.; García-Conesa, M.T.; Espín, J.C.; Tomás-Barberán, F.A. Bioavailability and Metabolism of Ellagic Acid and Ellagitannins; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Larrosa, M.; Tomas-Barberan, F.A.; Espin, J.C. The dietary hydrolysable tannin punicalagin releases ellagic acid that induces apoptosis in human colon adenocarcinoma Caco-2 cells by using the mitochondrial pathway. J. Nutr. Biochem. 2006, 17, 611–625. [Google Scholar] [CrossRef] [PubMed]
- Kilkowski, W.J.; Gross, G.G. Color reaction of hydrolyzable tannins with bradford reagent, coomassie brilliant blue. Phytochemistry 1999, 51, 363–366. [Google Scholar] [CrossRef]
- Barbehenn, R.V.; Jones, C.P.; Hagerman, A.E.; Karonen, M.; Salminen, J.P. Ellagitannins have greater oxidative activities than condensed tannins and galloyl glucoses at high pH: Potential impact on caterpillars. J. Chem. Ecol. 2006, 32, 2253–2267. [Google Scholar] [CrossRef] [PubMed]
- Barbehenn, R.V.; Jones, C.P.; Karonen, M.; Salminen, J.P. Tannin composition affects the oxidative activities of tree leaves. J. Chem. Ecol. 2006, 32, 2235–2251. [Google Scholar] [CrossRef] [PubMed]
- Quideau, S. Chemistry and Biology of Ellagitannins: An Underestimated Class of Bioactive Plant Polyphenols; World Scientific: Hackensack, NJ, USA, 2009. [Google Scholar]
- Okuda, T.; Yoshida, T.; Hatano, T. Hydrolyzable tannins and related polyphenols. In Fortschritte der Chemie Organischer Naturstoffe/Progress in the Chemistry of Organic Natural Products; Springer: Vienna, Austria, 1995; pp. 1–117. [Google Scholar]
- Okuda, T.; Yoshida, T.; Hatano, T. Correlation of oxidative transformations of hydrolyzable tannins and plant evolution. Phytochemistry 2000, 55, 513–529. [Google Scholar] [CrossRef]
- Okuda, T.; Yoshida, T.; Ashida, M.; Yazaki, K. Tannis of Casuarina and Stachyurus species. Part 1. Structures of pendunculagin, casuarictin, strictinin, casuarinin, casuariin, and stachyurin. J. Chem. Soc. Perkin Trans. 1983, 1, 1765–1772. [Google Scholar] [CrossRef]
- Okuda, T.; Yoshida, T.; Kuwahara, M.; Memon, M.U.; Shingu, T. Agrimoniin and potentillin, an ellagitannin dimer and monomer having an α-glucose core. J. Chem. Soc. Chem. Commun. 1982, 163–164. [Google Scholar] [CrossRef]
- Okuda, T.; Yoshida, T.; Kuwahara, M.; Memon, M.U.; Shingu, T. Tannins of rosaceous medicinal plants. I. Structures of potentillin, agrimonic acids A and B, and agrimoniin, a dimeric ellagitannin. Chem. Pharm. Bull. 1984, 32, 2165–2173. [Google Scholar] [CrossRef]
- Lipińska, L.; Klewicka, E.; Sójka, M. Structure, occurrence and biological activity of ellagitannins: A general review. Acta Sci. Pol. Technol. Aliment. 2014, 13, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Kaneshima, T.; Myoda, T.; Nakata, M.; Fujimori, T.; Toeda, K.; Nishizawa, M. Antioxidant activity of C-glycosidic ellagitannins from the seeds and peel of camu-camu (Myrciaria dubia). LWT Food Sci. Technol. 2016, 69, 76–81. [Google Scholar] [CrossRef]
- Tanaka, T.; Ueda, N.; Shinohara, H.; Nonaka, G.-I.; Kouno, I. Four new C-glycosidic ellagitannins, castacrenins DG, from Japanese chestnut wood (castanea crenata SIEB. Et ZUCC.). Chem. Pharm. Bull. 1997, 45, 1751–1755. [Google Scholar] [CrossRef]
- Omar, M.; Matsuo, Y.; Maeda, H.; Saito, Y.; Tanaka, T. New metabolites of C-glycosidic ellagitannin from Japanese oak sapwood. Org Lett. 2014, 16, 1378–1381. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.-H.; Tanaka, T.; Kouno, I. Three novel C-glycosidic ellagitannins, Rhoipteleanins H, I, and J, from Rhoiptelea c hiliantha. J. Nat. Prod. 1999, 62, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Quideau, S.; Jourdes, M.; Lefeuvre, D.; Montaudon, D.; Saucier, C.; Glories, Y.; Pardon, P.; Pourquier, P. The chemistry of wine polyphenolic C-glycosidic ellagitannins targeting human topoisomerase II. Chemistry 2005, 11, 6503–6513. [Google Scholar] [CrossRef] [PubMed]
- Clifford, M.N.; Scalbert, A. Ellagitannins—Nature, occurrence and dietary burden. J. Sci. Food Agric. 2000, 80, 1118–1125. [Google Scholar] [CrossRef]
- Garcia-Munoz, C.; Vaillant, F. Metabolic fate of ellagitannins: Implications for health, and research perspectives for innovative functional foods. Crit. Rev. Food Sci. Nutr. 2014, 54, 1584–1598. [Google Scholar] [CrossRef] [PubMed]
- Bialonska, D.; Kasimsetty, S.G.; Khan, S.I.; Ferreira, D. Urolithins, intestinal microbial metabolites of pomegranate ellagitannins, exhibit potent antioxidant activity in a cell-based assay. J. Agric. Food Chem. 2009, 57, 10181–10186. [Google Scholar] [CrossRef] [PubMed]
- González-Barrio, R.O.; Borges, G.; Mullen, W.; Crozier, A. Bioavailability of anthocyanins and ellagitannins following consumption of raspberries by healthy humans and subjects with an ileostomy. J. Agric. Food Chem. 2010, 58, 3933–3939. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Munoz, C.; Hernàndez, L.; Pèrez, A.; Vaillant, F. Diversity of urinary excretion patterns of main ellagitannins’ colonic metabolites after ingestion of tropical highland blackberry (Rubus adenotrichus) juice. Food Res. Int. 2014, 55, 161–169. [Google Scholar] [CrossRef]
- Seeram, N.P.; Lee, R.; Scheuller, H.S.; Heber, D. Identification of phenolic compounds in strawberries by liquid chromatography electrospray ionization mass spectroscopy. Food Chem. 2006, 97, 1–11. [Google Scholar] [CrossRef]
- Cerdá, B.; Espín, J.C.; Parra, S.; Martínez, P.; Tomás-Barberán, F.A. The potent in vitro antioxidant ellagitannins from pomegranate juice are metabolised into bioavailable but poor antioxidant hydroxy-6H-dibenzopyran-6-one derivatives by the colonic microflora of healthy humans. Eur. J. Nutr. 2004, 43, 205–220. [Google Scholar] [CrossRef] [PubMed]
- Larrosa, M.; Garcia-Conesa, M.T.; Espin, J.C.; Tomas-Barberan, F.A. Ellagitannins, ellagic acid and vascular health. Mol. Asp. Med. 2010, 31, 513–539. [Google Scholar] [CrossRef] [PubMed]
- Heber, D. Multitargeted therapy of cancer by ellagitannins. Cancer Lett. 2008, 269, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Tomás-Barberán, F.A.; García-Villalba, R.; González-Sarrías, A.; Selma, M.V.; Espín, J.C. Ellagic acid metabolism by human gut microbiota: Consistent observation of three urolithin phenotypes in intervention trials, independent of food source, age, and health status. J. Agric. Food Chem. 2014, 62, 6535–6538. [Google Scholar] [CrossRef] [PubMed]
- Nicoli, M.; Anese, M.; Parpinel, M. Influence of processing on the antioxidant properties of fruit and vegetables. Trends Food Sci. Technol. 1999, 10, 94–100. [Google Scholar] [CrossRef]
- Balkwill, F.; Coussens, L.M. Cancer: An inflammatory link. Nature 2004, 431, 405–406. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, B.A.; Geoffroy, O.; Willingham, M.C.; Re, G.G.; Nixon, D.W. p53/p21(WAF1/CIP1) expression and its possible role in G1 arrest and apoptosis in ellagic acid treated cancer cells. Cancer Lett. 1999, 136, 215–221. [Google Scholar] [CrossRef]
- Vanella, L.; di Giacomo, C.; Acquaviva, R.; Barbagallo, I.; Cardile, V.; Kim, D.H.; Abraham, N.G.; Sorrenti, V. Apoptotic markers in a prostate cancer cell line: Effect of ellagic acid. Oncol. Rep. 2013, 30, 2804–2810. [Google Scholar] [PubMed]
- Vicinanza, R.; Zhang, Y.; Henning, S.M.; Heber, D. Pomegranate juice metabolites, ellagic acid and urolithin a, synergistically inhibit androgen-independent prostate cancer cell growth via distinct effects on cell cycle control and apoptosis. Evid. Based Complement. Altern. Med. 2013, 2013, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-S.; Bai, M.-H.; Zhang, T.; Li, G.-D.; Liu, M. Ellagic acid induces cell cycle arrest and apoptosis through TGF-β/Smad3 signaling pathway in human breast cancer MCF-7 cells. Int. J. Oncol. 2015, 46, 1730–1738. [Google Scholar] [CrossRef]
- Wen, X.Y.; Wu, S.Y.; Li, Z.Q.; Liu, Z.Q.; Zhang, J.J.; Wang, G.F.; Jiang, Z.H.; Wu, S.G. Ellagitannin (BJA3121), an anti-proliferative natural polyphenol compound, can regulate the expression of miRNAs in HepG2 cancer cells. Phytother. Res. 2009, 23, 778–784. [Google Scholar] [CrossRef] [PubMed]
- Seeram, N.P.; Adams, L.S.; Henning, S.M.; Niu, Y.; Zhang, Y.; Nair, M.G.; Heber, D. In vitro antiproliferative, apoptotic and antioxidant activities of punicalagin, ellagic acid and a total pomegranate tannin extract are enhanced in combination with other polyphenols as found in pomegranate juice. J. Nutr. Biochem. 2005, 16, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Sartippour, M.R.; Seeram, N.P.; Rao, J.Y.; Moro, A.; Harris, D.M.; Henning, S.M.; Firouzi, A.; Rettig, M.B.; Aronson, W.J.; Pantuck, A.J. Ellagitannin-rich pomegranate extract inhibits angiogenesis in prostate cancer in vitro and in vivo. Int. J. Oncol. 2008, 32, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-J.; Lee, H.-K. Sanguiin H-6 blocks endothelial cell growth through inhibition of VEGF binding to VEGF receptor. Arch. Pharmacal. Res. 2005, 28, 1270–1274. [Google Scholar] [CrossRef]
- Gambari, R.; Hau, D.K.P.; Wong, W.Y.; Chui, C.H. Sensitization of Hep3B hepatoma cells to cisplatin and doxorubicin by corilagin. Phytotherapy Res. 2014, 28, 781–783. [Google Scholar] [CrossRef] [PubMed]
- CDC. 2012 Top Ten Cancers. Available online: https://nccd.cdc.gov/uscs/toptencancers.aspx (accessed on 29 January 2016).
- Masko, E.M.; Allott, E.H.; Freedland, S.J. The relationship between nutrition and prostate cancer: Is more always better? Eur. Urol. 2013, 63, 810–820. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.H.; Kristal, A.R.; Stanford, J.L. Fruit and vegetable intakes and prostate cancer risk. J. Natl. Cancer Inst. 2000, 92, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Kolonel, L.N.; Hankin, J.H.; Whittemore, A.S.; Wu, A.H.; Gallagher, R.P.; Wilkens, L.R.; John, E.M.; Howe, G.R.; Dreon, D.M.; West, D.W.; et al. Vegetables, fruits, legumes and prostate cancer: A multiethnic case-control study. Cancer Epidemiol. Biomark. Prev. 2000, 9, 795–804. [Google Scholar]
- Seeram, N.P.; Aronson, W.J.; Zhang, Y.; Henning, S.M.; Moro, A.; Lee, R.-P.; Sartippour, M.; Harris, D.M.; Rettig, M.; Suchard, M.A. Pomegranate ellagitannin-derived metabolites inhibit prostate cancer growth and localize to the mouse prostate gland. J. Agric. Food Chem. 2007, 55, 7732–7737. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, M.; Jiang, W.; Kumi-Diaka, J.; Lansky, E.P.; Gommersall, L.M.; Patel, A.; Mansel, R.E.; Neeman, I.; Geldof, A.A.; Campbell, M.J. Pomegranate extracts potently suppress proliferation, xenograft growth, and invasion of human prostate cancer cells. J. Med. Food 2004, 7, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.; Afaq, F.; Sarfaraz, S.; Adhami, V.M.; Syed, D.N.; Mukhtar, H. Pomegranate fruit juice for chemoprevention and chemotherapy of prostatesystemic antioxidant propo cancer. Proc. Natl. Acad. Sci. USA 2005, 102, 14813–14818. [Google Scholar] [CrossRef] [PubMed]
- Stolarczyk, M.; Piwowarski, J.P.; Granica, S.; Stefanska, J.; Naruszewicz, M.; Kiss, A.K. Extracts from Epilobium sp. Herbs, their components and gut microbiota metabolites of epilobium ellagitannins, urolithins, inhibit hormone-dependent prostate cancer cells-(lNCaP) proliferation and PSA secretion. Phytother. Res. 2013, 27, 1842–1848. [Google Scholar] [CrossRef] [PubMed]
- Stolarczyk, M.; Naruszewicz, M.; Kiss, A.K. Extracts from Epilobium sp. Herbs induce apoptosis in human hormone-dependent prostate cancer cells by activating the mitochondrial pathway. J. Pharm. Pharmacol. 2013, 65, 1044–1054. [Google Scholar] [CrossRef] [PubMed]
- Walia, H.; Arora, S. Terminalia chebula—A pharmacognistic account. J. Med. Plant Res. 2013, 7, 1351–1361. [Google Scholar]
- Saleem, A.; Husheem, M.; Harkonen, P.; Pihlaja, K. Inhibition of cancer cell growth by crude extract and the phenolics of Terminalia chebula retz. Fruit. J. Ethnopharmacol. 2002, 81, 327–336. [Google Scholar] [CrossRef]
- Calixto, J.B. Twenty-five years of research on medicinal plants in Latin America: A personal view. J. Ethnopharmacol. 2005, 100, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Eberhart, C.E.; Coffey, R.J.; Radhika, A.; Giardiello, F.M.; Ferrenbach, S.; Dubois, R.N. Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas. Gastroenterology 1994, 107, 1183–1188. [Google Scholar] [PubMed]
- Fajardo, A.M.; Piazza, G.A. Chemoprevention in gastrointestinal physiology and disease. Anti-inflammatory approaches for colorectal cancer chemoprevention. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 309, G59–G70. [Google Scholar] [CrossRef] [PubMed]
- Madka, V.; Rao, C.V. Anti-inflammatory phytochemicals for chemoprevention of colon cancer. Curr. Cancer Drug Targets 2013, 13, 542–557. [Google Scholar] [CrossRef] [PubMed]
- Adams, L.S.; Seeram, N.P.; Aggarwal, B.B.; Takada, Y.; Sand, D.; Heber, D. Pomegranate juice, total pomegranate ellagitannins, and punicalagin suppress inflammatory cell signaling in colon cancer cells. J. Agric. Food Chem. 2006, 54, 980–985. [Google Scholar] [CrossRef] [PubMed]
- Kasimsetty, S.G.; Bialonska, D.; Reddy, M.K.; Ma, G.; Khan, S.I.; Ferreira, D. Colon cancer chemopreventive activities of pomegranate ellagitannins and urolithins. J. Agric. Food Chem. 2010, 58, 2180–2187. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Li, L.; Celver, J.; Killian, C.; Kovoor, A.; Seeram, N.P. Effects of fruit ellagitannin extracts, ellagic acid, and their colonic metabolite, urolithin a, on Wnt signaling. J. Agric. Food Chem. 2009, 58, 3965–3969. [Google Scholar] [CrossRef] [PubMed]
- CDC. Breast Cancer Statistics. Available online: http://www.cdc.gov/cancer/breast/statistics/ (accessed on 2 February 2016).
- Russo, I.H.; Russo, J. Role of hormones in mammary cancer initiation and progression. J. Mammary Gland Biol. Neoplasia 1998, 3, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Gebre-Medhin, M.; Kindblom, L.-G.; Wennbo, H.; Törnell, J.; Meis-Kindblom, J.M. Growth hormone receptor is expressed in human breast cancer. Am. J. Pathol. 2001, 158, 1217–1222. [Google Scholar] [CrossRef]
- Chen, Z.; Gu, K.; Zheng, Y.; Zheng, W.; Lu, W.; Shu, X.O. The use of complementary and alternative medicine among Chinese women with breast cancer. J. Altern. Complement. Med. 2008, 14, 1049–1055. [Google Scholar] [CrossRef] [PubMed]
- Brodie, A.; Sabnis, G.; Jelovac, D. Aromatase and breast cancer. J. Steroid Biochem. Mol. Biol. 2006, 102, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Chen, S. Aromatase and breast cancer. Front. Biosci. 1998, 3, d922–d933. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.D.; Mehta, R.; Yu, W.; Neeman, I.; Livney, T.; Amichay, A.; Poirier, D.; Nicholls, P.; Kirby, A.; Jiang, W. Chemopreventive and adjuvant therapeutic potential of pomegranate (Punica granatum) for human breast cancer. Breast Cancer Res. Treat. 2002, 71, 203–217. [Google Scholar] [CrossRef] [PubMed]
- Aqil, F.; Gupta, A.; Munagala, R.; Jeyabalan, J.; Kausar, H.; Sharma, R.J.; Singh, I.P.; Gupta, R.C. Antioxidant and antiproliferative activities of anthocyanin/ellagitannin-enriched extracts from Syzygium cumini L. (Jamun, the Indian Blackberry). Nutr. Cancer 2012, 64, 428–438. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Adams, L.S.; Chen, S.; Killian, C.; Ahmed, A.; Seeram, N.P. Eugenia jambolana lam. Berry extract inhibits growth and induces apoptosis of human breast cancer but not non-tumorigenic breast cells. J. Agric. Food Chem. 2009, 57, 826–831. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Gao, X.; Li, X.; Jiang, N.; Luo, F.; Gu, C.; Chen, M.; Cheng, H.; Liu, P. Ellagic acid enhances the efficacy of PI3K inhibitor GDC-0941 in breast cancer cells. Curr. Mol. Med. 2015, 15, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Barrajón-Catalán, E.; Fernández-Arroyo, S.; Saura, D.; Guillén, E.; Fernández-Gutiérrez, A.; Segura-Carretero, A.; Micol, V. Cistaceae aqueous extracts containing ellagitannins show antioxidant and antimicrobial capacity, and cytotoxic activity against human cancer cells. Food Chem. Toxicol. 2010, 48, 2273–2282. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, K.I.; Nomura, M.; Sasakura, M.; Matsui, E.; Koshiura, R.; Murayama, T.; Furukawa, T.; Hatano, T.; Yoshida, T.; Okuda, T. Antitumor activity of oenothein B, a unique macrocyclic ellagitannin. Jpn. J. Cancer Res. Gann 1993, 84, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Enzinger, P.C.; Mayer, R.J. Esophageal cancer. N. Engl. J. Med. 2003, 349, 2241–2252. [Google Scholar] [CrossRef] [PubMed]
- De Stefani, E.; Barrios, E.; Fierro, L. Black (air-cured) and blond (flue-cured) tobacco and cancer risk. III: Oesophageal cancer. Eur. J. Cancer 1993, 29A, 763–766. [Google Scholar] [CrossRef]
- Stoner, G.D.; Chen, T.; Kresty, L.A.; Aziz, R.M.; Reinemann, T.; Nines, R. Protection against esophageal cancer in rodents with lyophilized berries: Potential mechanisms. Nutr. Cancer 2006, 54, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Kresty, L.A.; Morse, M.A.; Morgan, C.; Carlton, P.S.; Lu, J.; Gupta, A.; Blackwood, M.; Stoner, G.D. Chemoprevention of esophageal tumorigenesis by dietary administration of lyophilized black raspberries. Cancer Res. 2001, 61, 6112–6119. [Google Scholar] [PubMed]
- Bishayee, A.; Haskell, Y.; Do, C.; Siveen, K.S.; Mohandas, N.; Sethi, G.; Stoner, G.D. Potential benefits of edible berries in the management of aerodigestive and gastrointestinal tract cancers: Preclinical and clinical evidence. Crit. Rev. Food Sci. Nutr. 2015, in press. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Stoner, G.D. Inhibition of N-nitrosobenzylmethylamine-induced esophageal tumorigenesis in rats by ellagic acid. Carcinogenesis 1990, 11, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Daniel, E.M.; Stoner, G.D. The effects of ellagic acid and 13-cis-retinoic acid on N-nitrosobenzylmethylamine-induced esophageal tumorigenesis in rats. Cancer Lett. 1991, 56, 117–124. [Google Scholar] [CrossRef]
- Stoner, G.D.; Morse, M.A. Isothiocyanates and plant polyphenols as inhibitors of lung and esophageal cancer. Cancer Lett. 1997, 114, 113–119. [Google Scholar] [CrossRef]
- Wang, L.S.; Dombkowski, A.A.; Seguin, C.; Rocha, C.; Cukovic, D.; Mukundan, A.; Henry, C.; Stoner, G.D. Mechanistic basis for the chemopreventive effects of black raspberries at a late stage of rat esophageal carcinogenesis. Mol. Carcinog. 2011, 50, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.S.; Hecht, S.; Carmella, S.; Seguin, C.; Rocha, C.; Yu, N.; Stoner, K.; Chiu, S.; Stoner, G. Berry ellagitannins may not be sufficient for prevention of tumors in the rodent esophagus. J. Agric. Food Chem. 2010, 58, 3992–3995. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, J.; Zhao, W.; Bao, L.; Song, X.; Xia, Y.; Wang, X.; Zhang, C.; Wang, X.; Yao, X. Fatty acid synthase inhibitors from Geum japonicum Thunb. var. chinense. Chem. Biodivers. 2009, 6, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Seeram, N.P.; Lee, R.; Feng, L.; Heber, D. Isolation and identification of strawberry phenolics with antioxidant and human cancer cell antiproliferative properties. J. Agric. Food Chem. 2008, 56, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Weisburg, J.H.; Schuck, A.G.; Reiss, S.E.; Wolf, B.J.; Fertel, S.R.; Zuckerbraun, H.L.; Babich, H. Ellagic acid, a dietary polyphenol, selectively cytotoxic to HSC-2 oral carcinoma cells. Anticancer Res. 2013, 33, 1829–1836. [Google Scholar] [PubMed]
- Zhu, X.; Xiong, L.; Zhang, X.; Shi, N.; Zhang, Y.; Ke, J.; Sun, Z.; Chen, T. Lyophilized strawberries prevent 7, 12-dimethylbenz [α] anthracene (DMBA)-induced oral squamous cell carcinogenesis in hamsters. J. Funct. Foods 2015, 15, 476–486. [Google Scholar] [CrossRef]
- Casto, B.C.; Knobloch, T.J.; Galioto, R.L.; Yu, Z.; Accurso, B.T.; Warner, B.M. Chemoprevention of oral cancer by lyophilized strawberries. Anticancer Res. 2013, 33, 4757–4766. [Google Scholar] [PubMed]
- Priyadarsini, R.V.; Kumar, N.; Khan, I.; Thiyagarajan, P.; Kondaiah, P.; Nagini, S. Gene expression signature of DMBA-induced hamster buccal pouch carcinomas: Modulation by chlorophyllin and ellagic acid. PLoS ONE 2012, 7, e34628. [Google Scholar] [CrossRef] [PubMed]
- Anitha, P.; Priyadarsini, R.V.; Kavitha, K.; Thiyagarajan, P.; Nagini, S. Ellagic acid coordinately attenuates Wnt/β-catenin and NF-κb signaling pathways to induce intrinsic apoptosis in an animal model of oral oncogenesis. Eur. J. Nutr. 2013, 52, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Kowshik, J.; Giri, H.; Kranthi Kiran Kishore, T.; Kesavan, R.; Naik Vankudavath, R.; Bhanuprakash Reddy, G.; Dixit, M.; Nagini, S. Ellagic acid inhibits VEGF/VEGFR2, PI3K/Akt and MAPK signaling cascades in the hamster cheek pouch carcinogenesis model. Anti-Cancer Agents Med. Chem. 2014, 14, 1249–1260. [Google Scholar] [CrossRef]
- Ding, Y.; Yao, H.; Yao, Y.; Fai, L.Y.; Zhang, Z. Protection of dietary polyphenols against oral cancer. Nutrients 2013, 5, 2173–2191. [Google Scholar] [CrossRef] [PubMed]
- Naghavi, M.; Wang, H.; Lozano, R.; Davis, A.; Liang, X.; Zhou, M.; Vollset, S.E.; Ozgoren, A.A.; Abdalla, S.; Abd-Allah, F. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: A systematic analysis for the global burden of disease study 2013. Lancet 2015, 385, 117–171. [Google Scholar]
- Oh, G.-S.; Pae, H.-O.; Oh, H.; Hong, S.-G.; Kim, I.-K.; Chai, K.-Y.; Yun, Y.-G.; Kwon, T.-O.; Chung, H.-T. In vitro anti-proliferative effect of 1,2,3,4,6-penta-O-galloyl-beta-d-glucose on human hepatocellular carcinoma cell line, SK-HEP-1 cells. Cancer Lett. 2001, 174, 17–24. [Google Scholar] [CrossRef]
- Yin, S.; Dong, Y.; Li, J.; Lü, J.; Hu, H. Penta-1,2,3,4,6-O-galloyl-beta-d-glucose induces senescence-like terminal S-phase arrest in human hepatoma and breast cancer cells. Mol. Carcinog. 2011, 50, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Yin, S.; Jiang, C.; Luo, X.; Guo, X.; Zhao, C.; Fan, L.; Meng, Y.; Lu, J.; Song, X. Involvement of autophagy induction in penta-1,2,3,4,6-O-galloyl-β-d-glucose-induced senescence-like growth arrest in human cancer cells. Autophagy 2014, 10, 296–310. [Google Scholar] [CrossRef] [PubMed]
- Hau, D.K.-P.; Zhu, G.-Y.; Leung, A.K.-M.; Wong, R.S.-M.; Cheng, G.Y.-M.; Lai, P.B.; Tong, S.-W.; Lau, F.-Y.; Chan, K.-W.; Wong, W.-Y.; et al. In vivo anti-tumour activity of corilagin on Hep3B hepatocellular carcinoma. Phytomedicine 2010, 18, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Ming, Y.; Zheng, Z.; Chen, L.; Zheng, G.; Liu, S.; Yu, Y.; Tong, Q. Corilagin inhibits hepatocellular carcinoma cell proliferation by inducing G2/M phase arrest. Cell Biol. Int. 2013, 37, 1046–1054. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.-T.; Yang, L.; Jiang, J.-G. Effects of thonningianin A in natural foods on apoptosis and cell cycle arrest of HepG-2 human hepatocellular carcinoma cells. Food Funct. 2015, 6, 2588–2597. [Google Scholar] [CrossRef] [PubMed]
- CDC. Cervical Cancer Statistics. Available online: http://www.cdc.gov/cancer/cervical/statistics/ (accessed on 21 March 2016).
- Bosch, F.X.; Manos, M.M.; Munoz, N.; Sherman, M.; Jansen, A.M.; Peto, J.; Schiffman, M.H.; Moreno, V.; Kurman, R.; Shah, K.V.; et al. Prevalence of human papillomavirus in cervical cancer: A worldwide perspective. J. Natl. Cancer Inst. 1995, 87, 796–802. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, S.; Abdul Wahab, N.; Zainal Abidin, N.; Manickam, S.; Zakaria, Z. Growth inhibition of human gynecologic and colon cancer cells by phyllanthus watsonii through apoptosis induction. PLoS ONE 2012, 7, e34793. [Google Scholar]
- Ross, H.A.; McDougall, G.J.; Stewart, D. Antiproliferative activity is predominantly associated with ellagitannins in raspberry extracts. Phytochemistry 2007, 68, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Yi, Z.C.; Liu, Y.Z.; Li, H.X.; Yin, Y.; Zhuang, F.Y.; Fan, Y.B.; Wang, Z. Tellimagrandin I enhances gap junctional communication and attenuates the tumor phenotype of human cervical carcinoma HeLa cells in vitro. Cancer Lett. 2006, 242, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Chen, L.G.; Yang, L.L. Camelliin B induced apoptosis in HeLa cell line. Toxicology 2001, 168, 231–240. [Google Scholar] [CrossRef]
- Le, V.; Esposito, D.; Grace, M.H.; Ha, D.; Pham, A.; Bortolazzo, A.; Bevens, Z.; Kim, J.; Okuda, R.; Komarnytsky, S.; et al. Cytotoxic effects of ellagitannins isolated from walnuts in human cancer cells. Nutr. Cancer 2014, 66, 1304–1314. [Google Scholar] [CrossRef] [PubMed]
- Moktar, A.; Ravoori, S.; Vadhanam, M.V.; Gairola, C.G.; Gupta, R.C. Cigarette smoke-induced DNA damage and repair detected by the comet assay in HPV-transformed cervical cells. Int. J. Oncol. 2009, 35, 1297–1304. [Google Scholar] [PubMed]
- Khan, N.; Afaq, F.; Kweon, M.-H.; Kim, K.; Mukhtar, H. Oral consumption of pomegranate fruit extract inhibits growth and progression of primary lung tumors in mice. Cancer Res. 2007, 67, 3475–3482. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Hadi, N.; Afaq, F.; Syed, D.N.; Kweon, M.H.; Mukhtar, H. Pomegranate fruit extract inhibits prosurvival pathways in human A549 lung carcinoma cells and tumor growth in athymic nude mice. Carcinogenesis 2007, 28, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Zahin, M.; Ahmad, I.; Gupta, R.C.; Aqil, F. Punicalagin and ellagic acid demonstrate antimutagenic activity and inhibition of benzo [a] pyrene induced DNA adducts. BioMed Res. Int. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.P.; Mahal, H.; Kapoor, S.; Aradhya, S. In vitro studies on the binding, antioxidant, and cytotoxic actions of punicalagin. J. Agric. Food chem. 2007, 55, 1491–1500. [Google Scholar] [CrossRef] [PubMed]
- Kuo, P.-L.; Hsu, Y.-L.; Lin, T.-C.; Lin, L.-T.; Chang, J.-K.; Lin, C.-C. Casuarinin from the bark of Terminalia arjuna induces apoptosis and cell cycle arrest in human breast adenocarcinoma MCF-7 cells. Planta Med. 2005, 71, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, M.; Watanabe, Y.; Kasai, K.; Yamakoshi, J.; Koga, T. Inhibitory effect of an ellagic acid-rich pomegranate extract on tyrosinase activity and ultraviolet-induced pigmentation. Biosci. Biotechnol. Biochem. 2005, 69, 2368–2373. [Google Scholar] [CrossRef] [PubMed]
- Afaq, F.; Zaid, M.A.; Khan, N.; Dreher, M.; Mukhtar, H. Protective effect of pomegranate-derived products on UVB-mediated damage in human reconstituted skin. Exp. Dermatol. 2009, 18, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Afaq, F.; Zaid, M.; Khan, N.; Syed, D.; Yun, J.-M.; Sarfaraz, S.; Suh, Y.; Mukhtar, H. Inhibitory effect of oral feeding of pomegranate fruit extract on UVB-induced skin carcinogenesis in SKH-1 hairless mice. In Proceedings of the 99th AACR Annual Meeting, San Diego, CA, USA, 12–16 April 2008; AACR Publications: Philadelphia, PA, USA; San Diego, CA, USA; p. 1246.
- Chung, K.-T.; Wei, C.-I.; Johnson, M.G. Are tannins a double-edged sword in biology and health? Trends Food Sci. Technol. 1998, 9, 168–175. [Google Scholar] [CrossRef]
- Mennen, L.I.; Walker, R.; Bennetau-Pelissero, C.; Scalbert, A. Risks and safety of polyphenol consumption. Am. J. Clin. Nutr. 2005, 81, 326S–329S. [Google Scholar] [PubMed]
- Sánchez-Lamar, A.; Fonseca, G.; Fuentes, J.L.; Cozzi, R.; Cundari, E.; Fiore, M.; Ricordy, R.; Perticone, P.; Degrassi, F.; de Salvia, R. Assessment of the genotoxic risk of Punica granatum L.(Punicaceae) whole fruit extracts. J. Ethnopharmacol. 2008, 115, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Labieniec, M.; Gabryelak, T. Effects of tannins on Chinese hamster cell line B14. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2003, 539, 127–135. [Google Scholar] [CrossRef]
- Chen, S.C.; Chung, K.T. Mutagenicity and antimutagenicity studies of tannic acid and its related compounds. Food Chem. Toxicol. 2000, 38, 1–5. [Google Scholar] [CrossRef]
- Filippich, L.J.; Zhu, J.; Oelrichs, P.; Alsalami, M.T.; Doig, A.J.; Cao, G.R.; English, P.B. Hepatotoxic and nephrotoxic principles in terminalia oblongata. Res. Vet. Sci. 1991, 50, 170–177. [Google Scholar] [CrossRef]
- Cerdá, B.; Cerón, J.J.; Tomás-Barberán, F.A.; Espín, J.C. Repeated oral administration of high doses of the pomegranate ellagitannin punicalagin to rats for 37 days is not toxic. J. Agric. Food Chem. 2003, 51, 3493–3501. [Google Scholar] [CrossRef] [PubMed]
- McDougall, G.J.; Shpiro, F.; Dobson, P.; Smith, P.; Blake, A.; Stewart, D. Different polyphenolic components of soft fruits inhibit α-amylase and α-glucosidase. J. Agric. Food Chem. 2005, 53, 2760–2766. [Google Scholar] [CrossRef] [PubMed]
- Godbout, A.; Chiasson, J.L. Who should benefit from the use of alpha-glucosidase inhibitors? Curr. Diabetes Rep. 2007, 7, 333–339. [Google Scholar] [CrossRef]
- Li, H.; Tanaka, T.; Zhang, Y.-J.; Yang, C.-R.; Kouno, I. Rubusuaviins A–F, monomeric and oligomeric ellagitannins from Chinese sweet tea and their α-amylase inhibitory activity. Chem. Pharm. Bull. 2007, 55, 1325–1331. [Google Scholar] [CrossRef] [PubMed]
- Santos-Buelga, C.; Scalbert, A. Proanthocyanidins and tannin-like compounds—Nature, occurrence, dietary intake and effects on nutrition and health. J. Sci. Food Agric. 2000, 80, 1094–1117. [Google Scholar] [CrossRef]
- Frutos, P.; Raso, M.; Hervás, G.; Mantecón, Á.R.; Pérez, V.; Giráldez, F.J. Is there any detrimental effect when a chestnut hydrolysable tannin extract is included in the diet of finishing lambs? Anim. Res. 2004, 53, 127–136. [Google Scholar] [CrossRef]
- Tasaki, M.; Umemura, T.; Maeda, M.; Ishii, Y.; Okamura, T.; Inoue, T.; Kuroiwa, Y.; Hirose, M.; Nishikawa, A. Safety assessment of ellagic acid, a food additive, in a subchronic toxicity study using F344 rats. Food Chem. Toxicol. 2008, 46, 1119–1124. [Google Scholar] [CrossRef] [PubMed]
- Patel, C.; Dadhaniya, P.; Hingorani, L.; Soni, M. Safety assessment of pomegranate fruit extract: Acute and subchronic toxicity studies. Food Chem. Toxicol. 2008, 46, 2728–2735. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.M.; Barraj, L.M.; Spungen, J.H.; Herman, D.R.; Randolph, R.K. Global assessment of select phytonutrient intakes by level of fruit and vegetable consumption. Br. J. Nutr. 2014, 112, 1004–1018. [Google Scholar] [CrossRef] [PubMed]
- Ovaskainen, M.-L.; Törrönen, R.; Koponen, J.M.; Sinkko, H.; Hellström, J.; Reinivuo, H.; Mattila, P. Dietary intake and major food sources of polyphenols in Finnish adults. J. Nutr. 2008, 138, 562–566. [Google Scholar] [PubMed]
- Radtke, J.; Linseisen, J.; Wolfram, G. Phenolic acid intake of adults in a Bavarian subgroup of the national food consumption survey. Z. Ernahrungswiss. 1998, 37, 190–197. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ismail, T.; Calcabrini, C.; Diaz, A.R.; Fimognari, C.; Turrini, E.; Catanzaro, E.; Akhtar, S.; Sestili, P. Ellagitannins in Cancer Chemoprevention and Therapy. Toxins 2016, 8, 151. https://doi.org/10.3390/toxins8050151
Ismail T, Calcabrini C, Diaz AR, Fimognari C, Turrini E, Catanzaro E, Akhtar S, Sestili P. Ellagitannins in Cancer Chemoprevention and Therapy. Toxins. 2016; 8(5):151. https://doi.org/10.3390/toxins8050151
Chicago/Turabian StyleIsmail, Tariq, Cinzia Calcabrini, Anna Rita Diaz, Carmela Fimognari, Eleonora Turrini, Elena Catanzaro, Saeed Akhtar, and Piero Sestili. 2016. "Ellagitannins in Cancer Chemoprevention and Therapy" Toxins 8, no. 5: 151. https://doi.org/10.3390/toxins8050151
APA StyleIsmail, T., Calcabrini, C., Diaz, A. R., Fimognari, C., Turrini, E., Catanzaro, E., Akhtar, S., & Sestili, P. (2016). Ellagitannins in Cancer Chemoprevention and Therapy. Toxins, 8(5), 151. https://doi.org/10.3390/toxins8050151