Comparison of Enzyme-Linked Immunosorbent Assay, Surface Plasmon Resonance and Biolayer Interferometry for Screening of Deoxynivalenol in Wheat and Wheat Dust
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Composition of Wheat Dust
2.2. Optimization of Sample Preparation
2.3. Optimization of Immunoassay Conditions
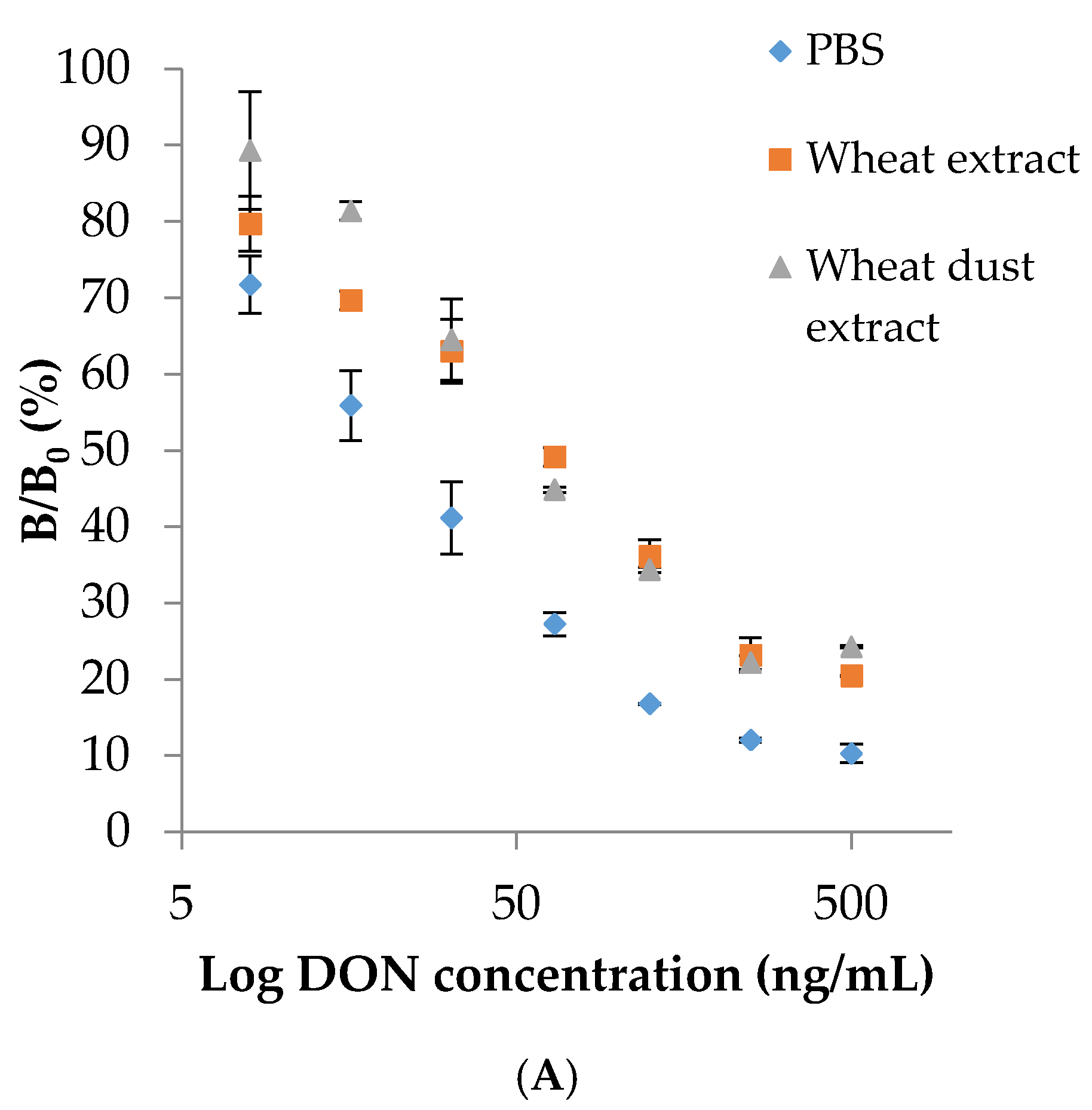
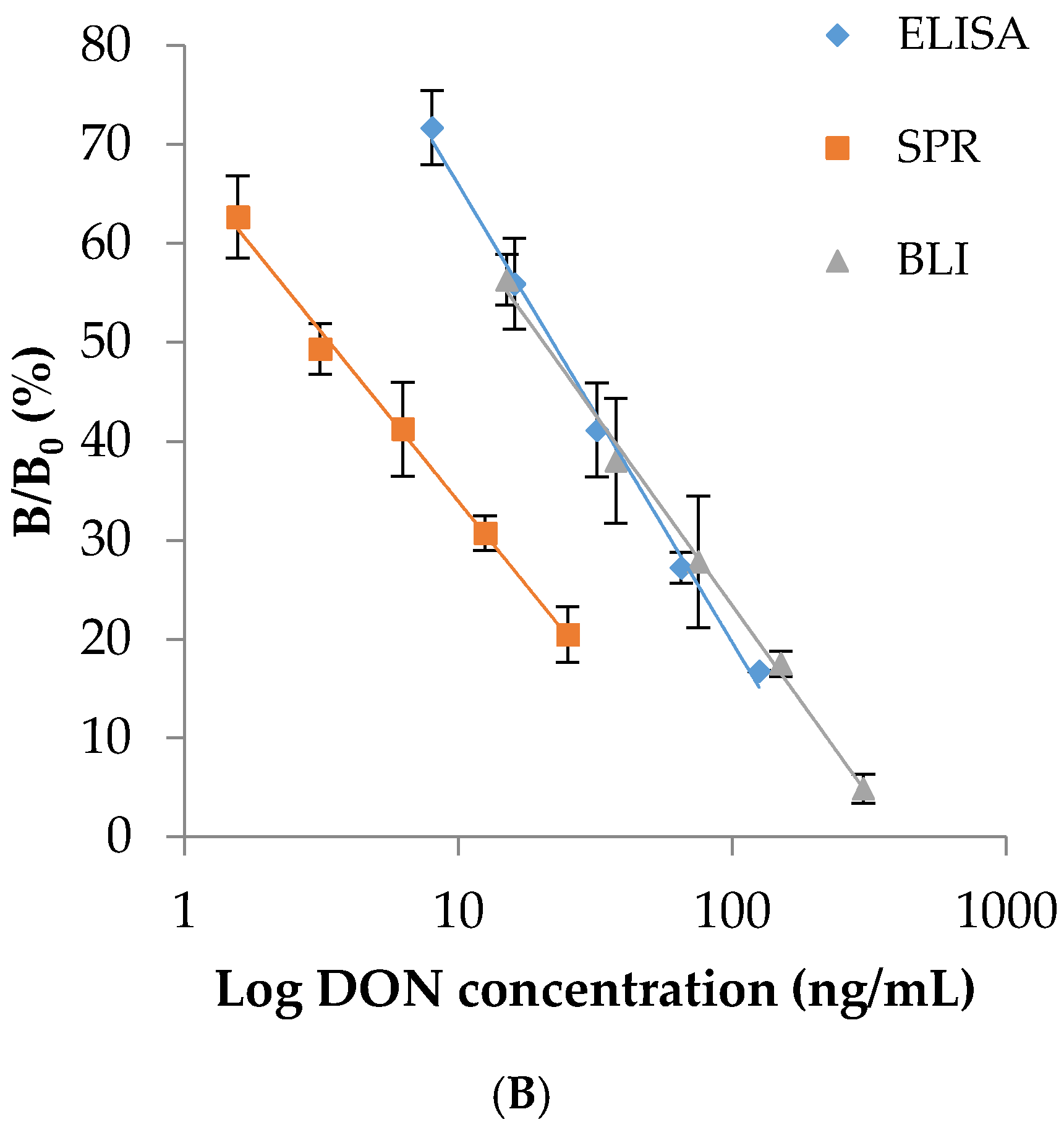
2.4. Comparison between ELISA and Sensor-Based Immunoassays
2.5. Method Validation
2.6. Analysis of Wheat and Wheat Dust Samples
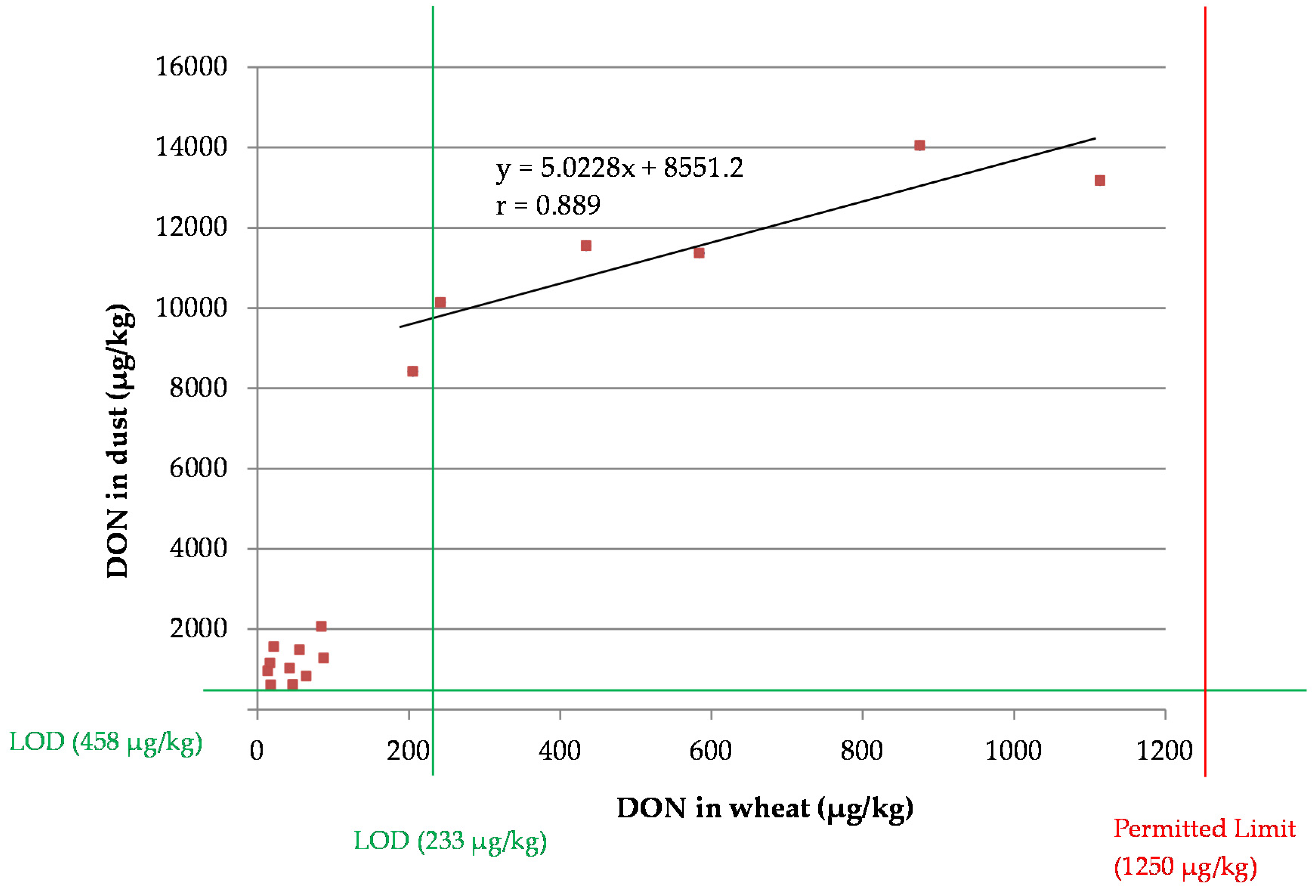
2.7. Dust Correlation Study
3. Materials and Methods
3.1. Reagents and Chemicals
3.2. Wheat Dust Collection and Chemical Composition
3.3. Sample Reparation
3.4. Analysis
3.4.1. ELISA
3.4.2. Surface Plasmon Resonance
3.4.3. Biolayer Interferometry
3.5. Method Validation
3.6. Dust Correlation Study
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Disclaimer
Abbreviations
| ACGIH | American Conference of Governmental Industrial Hygienists |
| APS | aminopropylsilane |
| BLI | biolayer interferometry |
| CBS | carbonate buffered saline |
| CDI | N,N′-carbonyldiimidazole |
| C-PBS | Casein-PBS |
| DMF | dimethylformamide |
| DON | deoxynivalenol |
| EC | European Commission |
| EDC | 1-ethyl-3-[3-dimethylaminopropyl] carbodiimide |
| ELISA | enzyme-linked immunosorbent assay |
| FPIA | fluorescence polarization immunoassay |
| GC | gas chromatography |
| Gly | glycine |
| HEPES | 2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid |
| HPLC | high performance liquid chromatography |
| HRP | horseradish peroxidase |
| IC50 | half maximal inhibitory concentration |
| ICP-AES | atomic emission spectroscopy |
| IgG | immunoglobulin G |
| KD | equilibrium dissociation constant |
| LC-MS/MS | liquid chromatography tandem mass spectrometry |
| LOD | limit of detection |
| MS | mass spectrometry |
| NHS | N-hydroxysuccinimide |
| OVA | ovalbumin |
| PB | phosphate buffer |
| PBS | phosphate buffered saline |
| PBST | phosphate buffered saline Tween 20 |
| r | correlation coefficient |
| RSD | relative standard deviation |
| RU | response units |
| SPR | surface plasmon resonance |
| STC | screening target concentration |
| TMB | 3,3′,5,5′-tetramethylbenzidine |
References
- Pestka, J. Mechanisms of deoxynivalenol-induced gene expression and apoptosis. Food Addit. Contam. A Chem. Anal. Control Expo. Risk Assess. 2008, 25, 1128–1140. [Google Scholar] [CrossRef]
- Ngundi, M.M.; Qadri, S.A.; Wallace, E.V.; Moore, M.H.; Lassman, M.E.; Shriver-Lake, L.C.; Ligler, F.S.; Taitt, C.R. Detection of deoxynivalenol in foods and indoor air using an array biosensor. Environ. Sci. Technol. 2006, 40, 2352–2356. [Google Scholar] [CrossRef] [PubMed]
- Krska, R.; Schubert-Ullrich, P.; Molinelli, A.; Sulyok, M.; Macdonald, S.; Crews, C. Mycotoxin analysis: An update. Food Addit. Contam. 2008, 25, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Vendl, O.; Berthiller, F.; Crews, C.; Krska, R. Simultaneous determination of deoxynivalenol, zearalenone, and their major masked metabolites in cereal-based food by LC-MS-MS. Anal. Bioanal. Chem. 2009, 395, 1347–1354. [Google Scholar] [CrossRef] [PubMed]
- Sulyok, M.; Krska, R.; Schuhmacher, R. Application of an LC-MS/MS based multi-mycotoxin method for the semi-quantitative determination of mycotoxins occurring in different types of food infected by moulds. Food Chem. 2010, 119, 408–416. [Google Scholar] [CrossRef]
- Maragos, C.; Busman, M. Rapid and advanced tools for mycotoxin analysis: A review. Food Addit. Contam. A Chem. Anal. Control Expo. Risk Assess. 2010, 27, 688–700. [Google Scholar] [CrossRef] [PubMed]
- Tudos, A.J.; Lucas-Van Den Bos, E.; Stigter, E.C.A. Rapid surface plasmon resonance-based inhibition assay of deoxynivalenol. J. Agric. Food Chem. 2003, 51, 5843–5848. [Google Scholar] [CrossRef] [PubMed]
- Maragos, C. Production of anti-idiotype antibodies for deoxynivalenol and their evaluation with three immunoassay platforms. Mycotoxin Res. 2014, 30, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Soelberg, S.D.; Chinowsky, T.; Geiss, G.; Spinelli, C.B.; Stevens, R.; Near, S.; Kauffman, P.; Yee, S.; Furlong, C.E. A portable surface plasmon resonance sensor system for real-time monitoring of small to large analytes. J. Ind. Microbiol. Biotechnol. 2005, 32, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Hodnik, V.; Anderluh, G. Toxin detection by surface plasmon resonance. Sensors 2009, 9, 1339–1354. [Google Scholar] [CrossRef] [PubMed]
- Di, R.; Zhao, L.; Levy, L. Detection of Quarantine Pathogens with Bio-Layer Interferometry-Based Blitz System. In Proceedings of the APS-CPS Joint Meeting, Minneapolis, MN, USA, 9–13 August 2014.
- Van Der Gaag, B.; Spath, S.; Dietrich, H.; Stigter, E.; Boonzaaijer, G.; van Osenbruggen, T.; Koopal, K. Biosensors and multiple mycotoxin analysis. Food Control 2003, 14, 251–254. [Google Scholar]
- Dorokhin, D.; Haasnoot, W.; Franssen, M.; Zuilhof, H.; Nielen, M. Imaging surface plasmon resonance for multiplex microassay sensing of mycotoxins. Anal. Bioanal. Chem. 2011, 400, 3005–3011. [Google Scholar] [CrossRef] [PubMed]
- Kadota, T.; Takezawa, Y.; Hirano, S.; Tajima, O.; Maragos, C.; Nakajima, T.; Tanake, T.; Kamata, Y.; Sugita-Konishi, Y. Rapid detection of nivalenol and deoxynivalenol in wheat using surface plasmon resonance immunoassay. Anal. Chim. Acta 2010, 673, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Maragos, C. Detection of deoxynivalenol using biolayer interferometry. Mycotoxin Res. 2011, 27, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Sanders, M.; de Boevre, M.; Dumoulin, F.; Detavernier, C.; Martens, F.; van Poucke, C.; Eeckhout, M.; de Saeger, S. Sampling of wheat dust and subsequent analysis of deoxynivalenol by LC-MS/MS. J. Agric. Food Chem. 2013, 61, 6259–6264. [Google Scholar] [CrossRef] [PubMed]
- Zukiewicz-Sobczak, W.; Cholewa, G.; Krasowska, E.; Zwolinski, J.; Sobczak, P.; Zawislak, K.; Chmielewska-Badora, J.; Piatek, J.; Wojtyla, A. Pathogenic fungi in the work environment of organic and conventional farmers. Postepy Dermatol. I Alergol. 2012, 29, 256–262. [Google Scholar] [CrossRef]
- Halstensen, A.S.; Nordby, K.C.; Elen, O.; Eduard, W. Ochratoxin A in grain dust-Estimated exposure and relations to agricultural practices in grain production. Ann. Agric. Environ. Med. 2004, 11, 245–254. [Google Scholar] [PubMed]
- Krysinska-Traczyk, E.; Kiecana, I.; Perkowski, J.; Dutkiewicz, J. Levels of fungi and mycotoxins in samples of grain and grain dust collected on farms in Eastern Poland. Ann. Agric. Environ. Med. 2001, 8, 269–274. [Google Scholar] [PubMed]
- Pettigrew, H.D.; Selmi, C.F.; Teuber, S.S.; Gershwin, M.E. Mold and human health: Separating the wheat from the chaff. Clin. Rev. Allergy Immunol. 2010, 38, 148–155. [Google Scholar] [PubMed]
- American Conference on Governmental Industrial Hygienists (ACGIH). TLVs® and BEIs®: Threshold Limit Values for Chemical Substances and Physical Agents Biological Exposure Indices; American Conference of Governmental Industrial Hygienists: Cincinnati, OH, USA, 1997. [Google Scholar]
- Martin, C.R. Characterization of grain dust properties. Trans. ASAE 1981, 24, 738–742. [Google Scholar]
- Sanders, M.; Guo, Y.; Iyer, A.; Garcia, Y.R.; Galvita, A.; Heyerick, A.; Deforce, D.; Risseeuw, M.D.; van Calenbergh, S.; Bracke, M.; et al. An immunogen synthesis strategy for the development of specific anti-deoxynivalenol monoclonal antibodies. Food Addit. Contam. A 2014, 31, 1751–1759. [Google Scholar] [CrossRef] [PubMed]
- Maragos, C.M.; McCormick, S.P. Monoclonal antibodies for the mycotoxins deoxynivalenol and 3-acetyl-deoxynivalenol. Food Agric. Immunol. 2000, 12, 181–192. [Google Scholar] [CrossRef]
- Xiao, H.; Clarke, J.R.; Marquardt, R.R.; Frohlich, A.A. Improved methods for conjugating selected mycotoxins to carrier proteins and dextran for immunoassays. J. Agric. Food Chem. 1995, 43, 2092–2097. [Google Scholar] [CrossRef]
- Guo, Y.; Sanders, M.; Galvita, A.; Heyerick, A.; Deforce, D.; Bracke, M.; Eremin, S.A.; De Saeger, S. Heterologous screening of hybridomas for the development of broad-specific monoclonal antibodies against deoxynivalenol and its analogues. World Mycotoxin J. 2014, 7, 257–265. [Google Scholar] [CrossRef]
- Dashek, W.V.; Olenchock, S.A.; Mayfield, J.E.; Wirtz, G.H.; Wolz, D.E.; Young, C.A. Carbohydrate and protein contents of grain dusts in relation to dust morphology. Environ. Health Perspect. 1986, 66, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Parnell, C.B.; Jones, D.D.; Rutherford, R.D.; Goforth, K.J. Physical-properties of 5 grain dust types. Environ. Health Perspect. 1986, 66, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Bobrovnik, S.A. Determination of antibody affinity by ELISA. Theory. J. Biochem. Biophys. Methods 2003, 57, 213–236. [Google Scholar] [CrossRef]
- Beatty, J.D.; Beatty, B.G.; Vlahos, W.G. Measurement of monoclonal antibody affinity by non-competitive enzyme immunoassay. J. Immunol. Methods 1987, 100, 173–179. [Google Scholar] [CrossRef]
| Mineral | Concentration Batch 1 (μg/g) (n = 2) | Concentration Batch 2 (μg/g) (n = 2) |
|---|---|---|
| Cu | 51 | 339 |
| Fe | 27,400 | 13,450 |
| K | 31,700 | 79,300 |
| Mg | 8700 | 44,650 |
| Mn | 768 | 2320 |
| Na | 1900 | 11,100 |
| P | 10,400 | 46,150 |
| S | 3630 | 9870 |
| Zn | 700 | 3380 |
| Spiking Level | Dilution of Extract | DON Concentration Found (μg/kg) | Recovery (%) |
|---|---|---|---|
| 4000 μg/kg | 1/10 | 2060 | 52 |
| 1/100 | 5010 | 125 | |
| 1/1000 | 52,110 | * | |
| 12,000 μg/kg | 1/10 | 3780 | 32 |
| 1/100 | 1980 | 17 | |
| 1/1000 | 100,950 | * | |
| 20,000 μg/kg | 1/10 | 7340 | 37 |
| 1/100 | 4510 | 23 | |
| 1/1000 | 23,220 | 116 |
| Validation Parameters | Wheat | Wheat Dust | ||||
|---|---|---|---|---|---|---|
| Spiking Level (μg/kg) | Results | Spiking Level (μg/kg) | Results | |||
| ELISA | BLI | ELISA | BLI | |||
| r | - | 0.998 | 0.985 | - | 0.998 | 0.991 |
| Apparent recovery | 1000 | 96% | 99% | 2000 | 96% | 83% |
| 2000 | 102% | 100% | 10,000 | 108% | 105% | |
| LOD | - | 233 μg/kg | 128 μg/kg | - | 458 μg/kg | 737 μg/kg |
| RSDr | 1500 | 3.87% | 4.00% | 2000 | 7.33% | 5.05% |
| 3000 | 7.93% | 4.11% | 8000 | 9.28% | 3.61% | |
| RSDR | 1500 | 7.50% | 7.30% | 2000 | 8.16% | 8.36% |
| 3000 | 8.73% | 5.58% | 8000 | 9.47% | 7.00% | |
| U | - | <22% | <32% | - | <29% | <34% |
| False suspect result | - | <0.01% | <0.01% | - | <0.01% | <0.01% |
| Immunoassay | Format | Determined Characteristic | |
|---|---|---|---|
| ELISA | Direct | Competitive | IC50 in buffer, wheat extract and wheat dust extract |
| Indirect | Competitive | IC50 in buffer Affinity to DON in buffer | |
| Non-competitive | Affinity to DON-OVA in buffer, wheat extract and wheat dust extract | ||
| SPR | Indirect | Competitive | IC50 in buffer |
| Non-competitive | Affinity to DON-OVA in buffer | ||
| BLI | Indirect | Competitive | IC50 in buffer, wheat extract and wheat dust extract |
| Non-competitive | Affinity to DON-OVA in buffer, wheat extract and wheat dust extract | ||
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanders, M.; McPartlin, D.; Moran, K.; Guo, Y.; Eeckhout, M.; O’Kennedy, R.; De Saeger, S.; Maragos, C. Comparison of Enzyme-Linked Immunosorbent Assay, Surface Plasmon Resonance and Biolayer Interferometry for Screening of Deoxynivalenol in Wheat and Wheat Dust. Toxins 2016, 8, 103. https://doi.org/10.3390/toxins8040103
Sanders M, McPartlin D, Moran K, Guo Y, Eeckhout M, O’Kennedy R, De Saeger S, Maragos C. Comparison of Enzyme-Linked Immunosorbent Assay, Surface Plasmon Resonance and Biolayer Interferometry for Screening of Deoxynivalenol in Wheat and Wheat Dust. Toxins. 2016; 8(4):103. https://doi.org/10.3390/toxins8040103
Chicago/Turabian StyleSanders, Melanie, Daniel McPartlin, Kara Moran, Yirong Guo, Mia Eeckhout, Richard O’Kennedy, Sarah De Saeger, and Chris Maragos. 2016. "Comparison of Enzyme-Linked Immunosorbent Assay, Surface Plasmon Resonance and Biolayer Interferometry for Screening of Deoxynivalenol in Wheat and Wheat Dust" Toxins 8, no. 4: 103. https://doi.org/10.3390/toxins8040103
APA StyleSanders, M., McPartlin, D., Moran, K., Guo, Y., Eeckhout, M., O’Kennedy, R., De Saeger, S., & Maragos, C. (2016). Comparison of Enzyme-Linked Immunosorbent Assay, Surface Plasmon Resonance and Biolayer Interferometry for Screening of Deoxynivalenol in Wheat and Wheat Dust. Toxins, 8(4), 103. https://doi.org/10.3390/toxins8040103