Differential Adsorption of Ochratoxin A and Anthocyanins by Inactivated Yeasts and Yeast Cell Walls during Simulation of Wine Aging
Abstract
:1. Introduction
2. Results
2.1. OTA-Removal by Inactivated Yeasts and Yeast Cell Walls
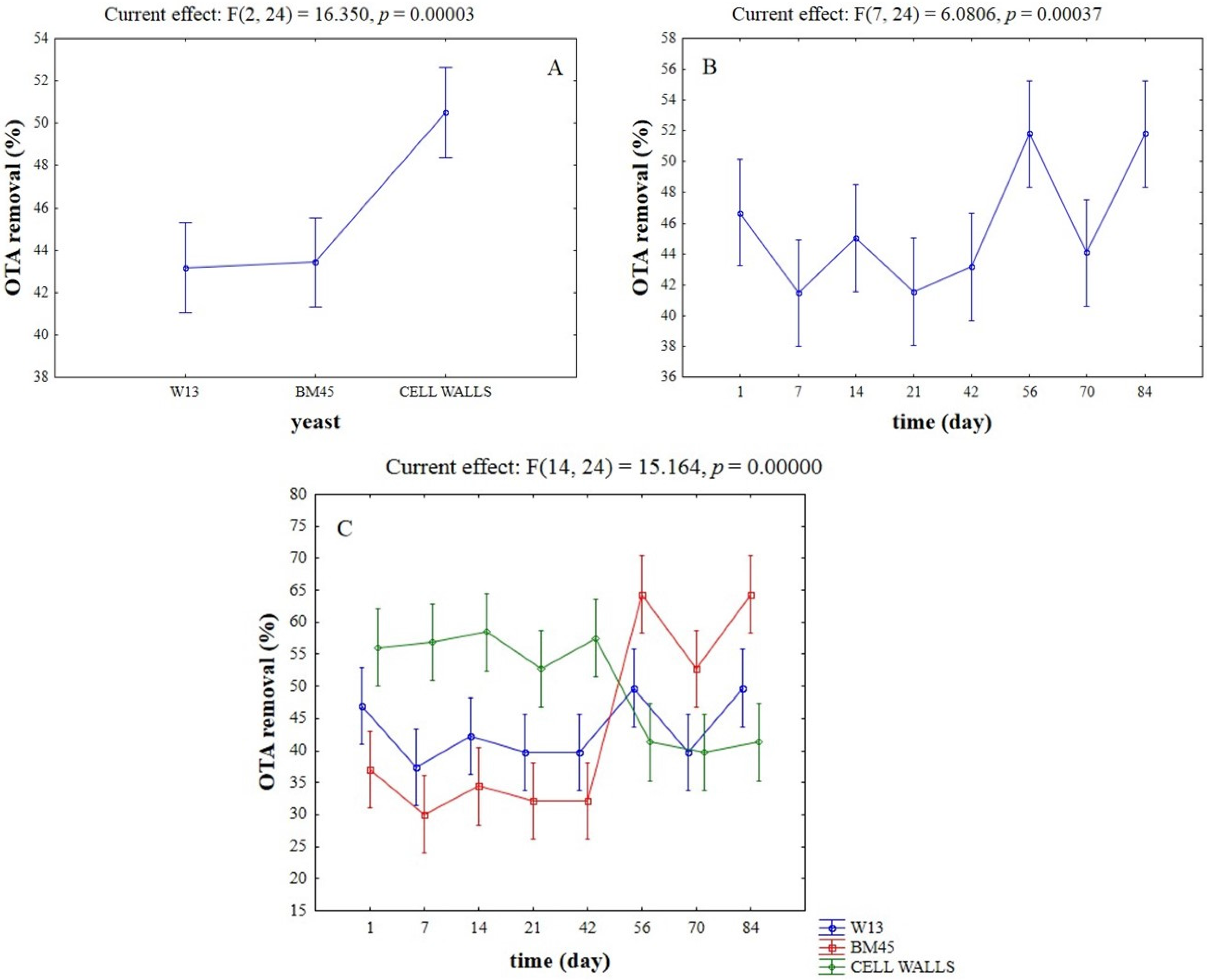
| Time (Day) | Control | W13 | BM45 | Cell Walls |
|---|---|---|---|---|
| 1 | 1.98 ± 0.00 | 1.35 ± 0.03 | 1.45 ± 0.06 | 1.27 ± 0.03 |
| 7 | 1.96 ± 0.03 | 1.43 ± 0.03 | 1.51 ± 0.03 | 1.25 ± 0.05 |
| 14 | 1.92 ± 0.03 | 1.35 ± 0.04 | 1.43 ± 0.03 | 1.21 ± 0.05 |
| 21 | 1.94 ± 0.00 | 1.39 ± 0.03 | 1.47 ± 0.03 | 1.27 ± 0.03 |
| 42 | 1.94 ± 0.00 | 1.39 ± 0.05 | 1.47 ± 0.03 | 1.23 ± 0.03 |
| 56 | 1.96 ± 0.03 | 1.31 ± 0.01 | 1.19 ± 0.06 | 1.39 ± 0.03 |
| 70 | 1.94 ± 0.00 | 1.39 ± 0.03 | 1.27 ± 0.04 | 1.39 ± 0.04 |
| 84 | 1.96 ± 0.03 | 1.31 ± 0.01 | 1.19 ± 0.03 | 1.39 ± 0.02 |
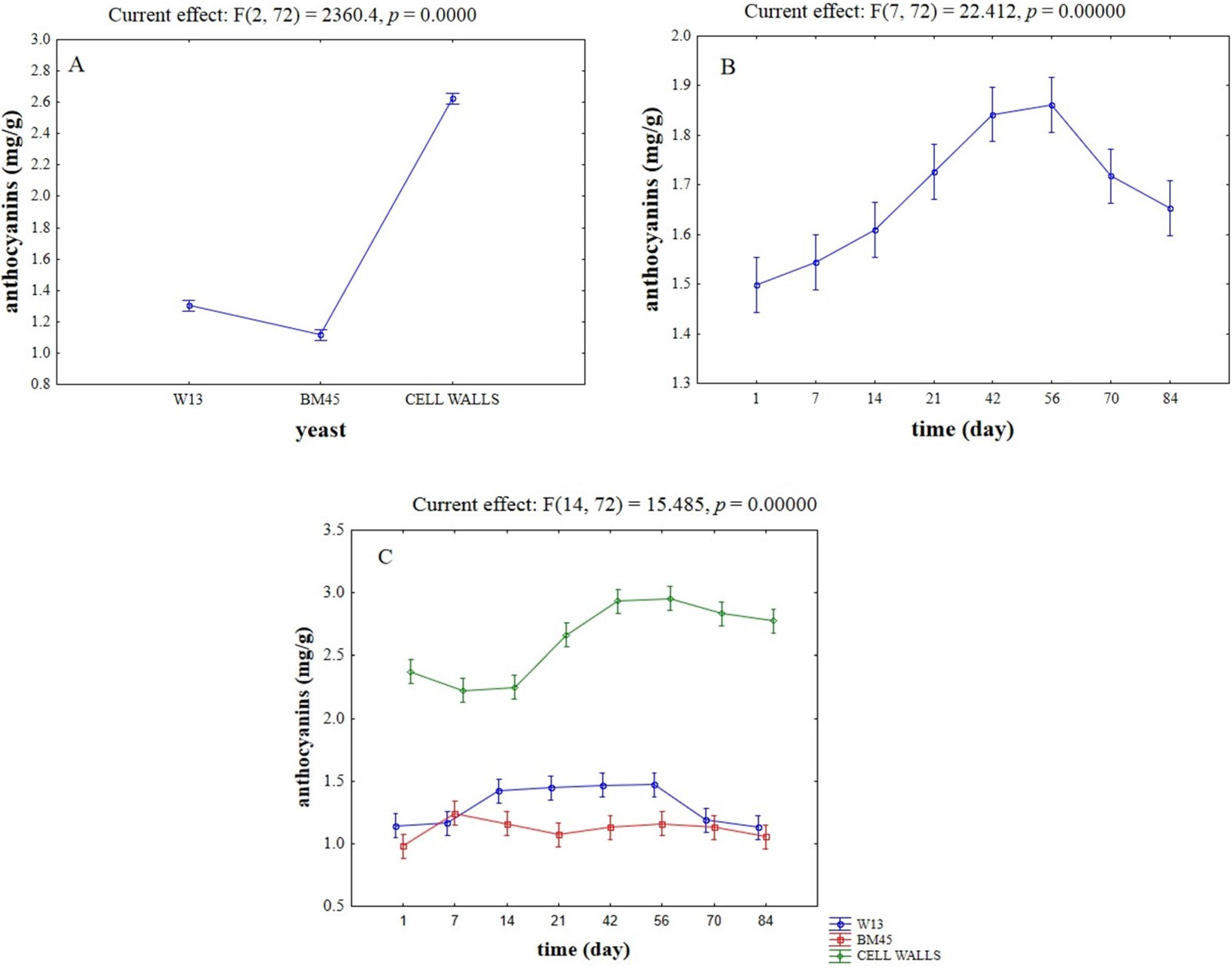
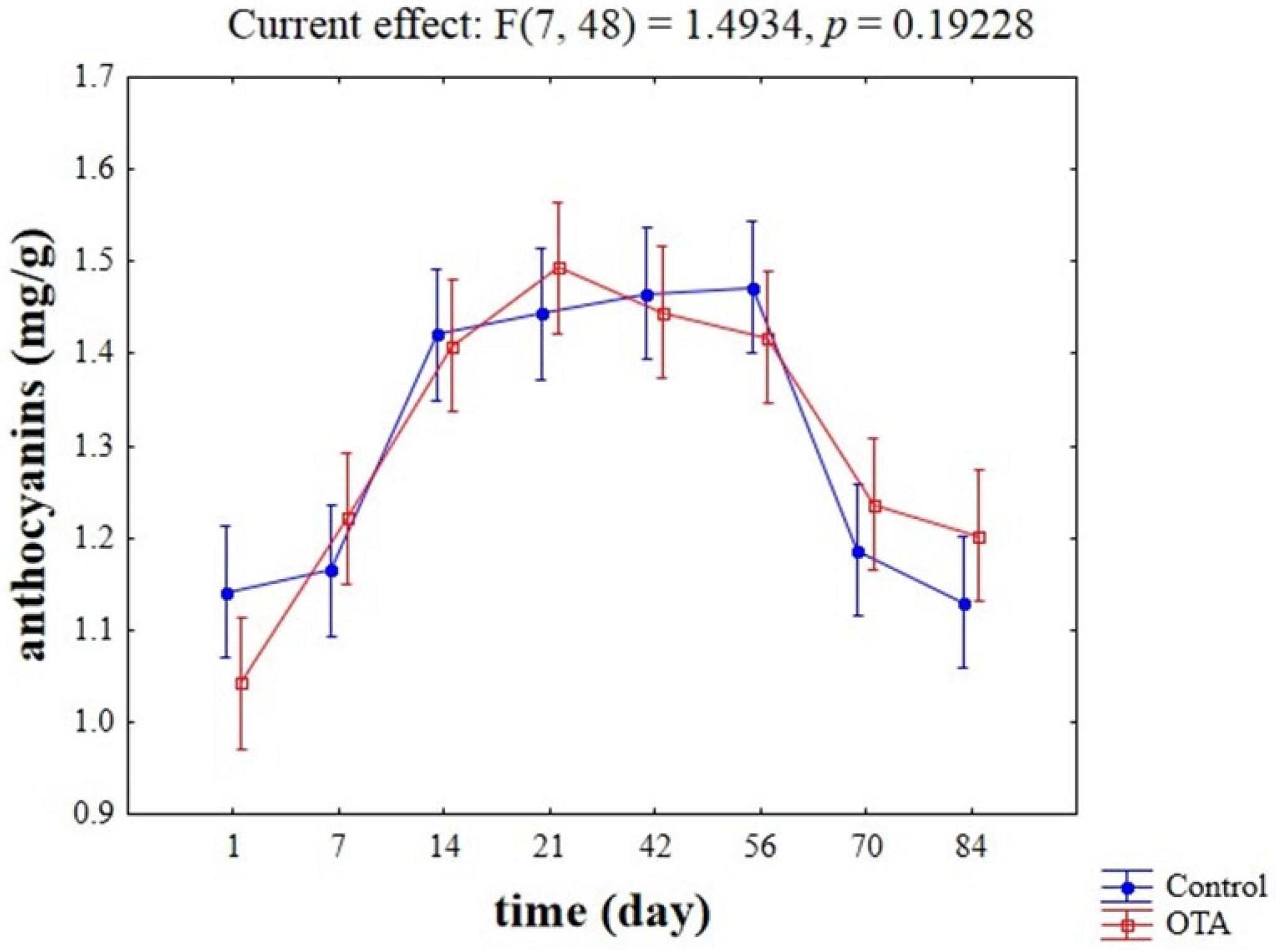
2.2. Evaluation of Potential Competitive Phenomena between OTA, Yeast Cells, and Anthocyanins
2.3. Effects of the Different Aging Techniques on Total Anthocyanins Content and Chromatic Characteristics of Wines
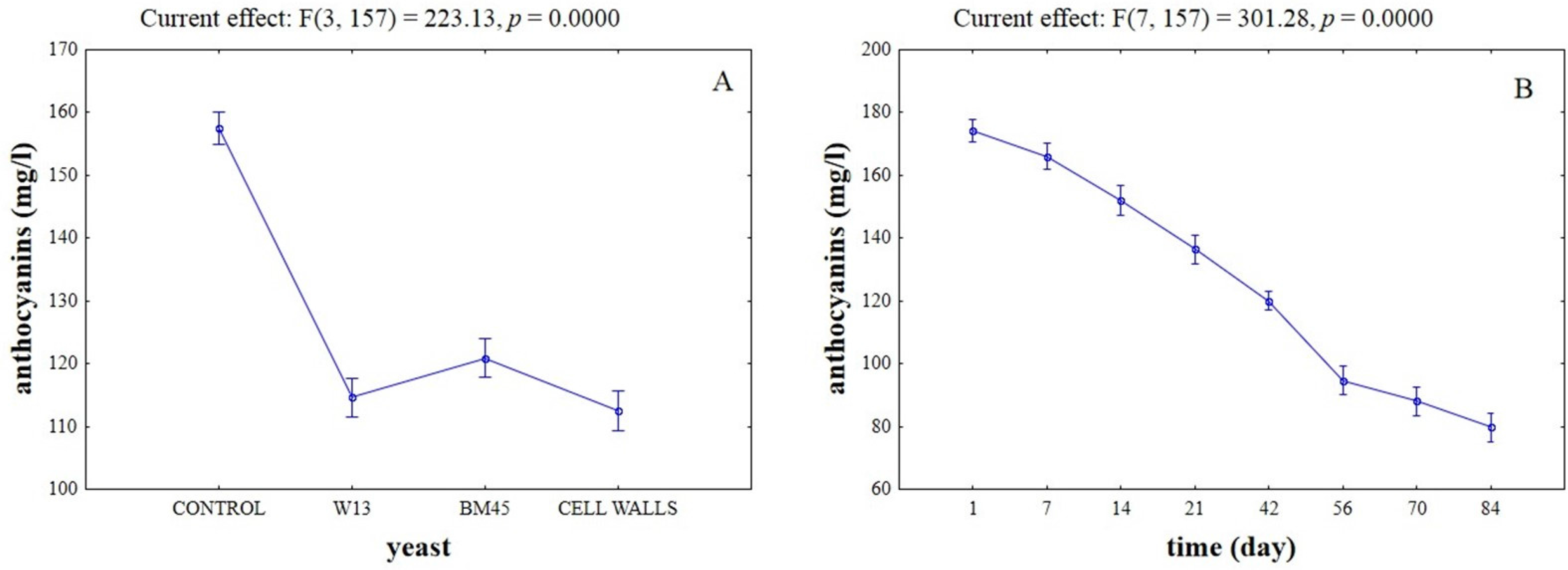
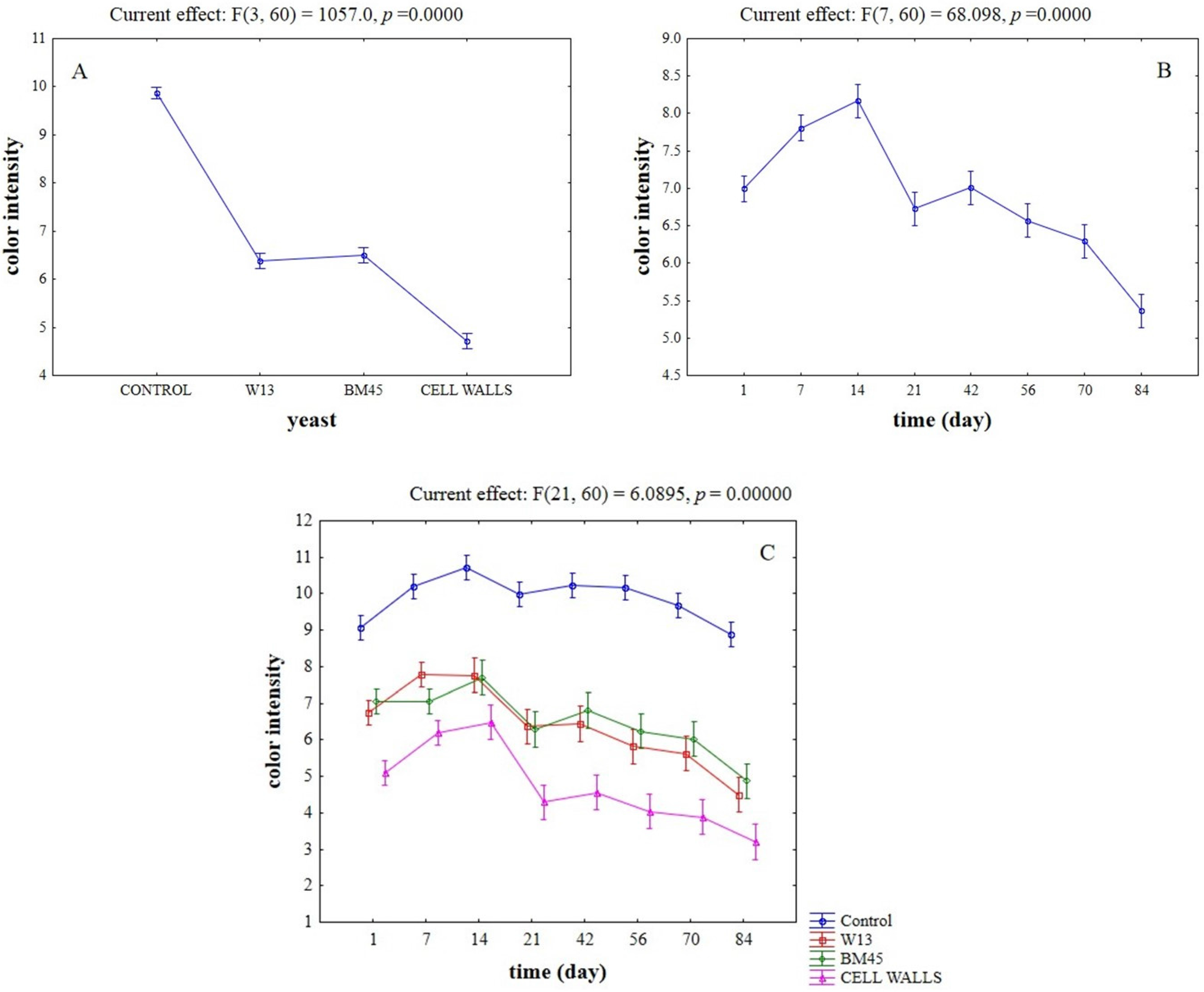
| Time (Day) | Control | W13 | ||
| no OTA | OTA | no OTA | OTA | |
| 1 | 191.82 ± 20.03 | 187.18 ± 25.22 | 172.41 ± 18.29 | 167.56 ± 15.54 |
| 7 | 186.90 ± 15.31 | 184.34 ± 3.39 | 154.52 ± 6.15 | 160.39 ± 11.12 |
| 14 | 174.37 ± 7.28 | 173.02 ± 8.09 | 137.24 ± 4.54 | 144.92 ± 8.12 |
| 21 | 171.54 ± 7.57 | 161.97 ± 8.26 | 124.31 ± 5.65 | 127.74 ± 4.85 |
| 42 | 152.77 ± 8.36 | 152.94 ± 6.52 | 103.18 ± 7.22 | 102.38 ± 3.71 |
| 46 | 136.70 ± 5.93 | 142.03 ± 3.97 | 83.07 ± 2.69 | 84.69 ± 5.83 |
| 70 | 128.82 ± 3.94 | 130.98 ± 2.25 | 72.26 ± 2.43 | 77.41 ± 3.74 |
| 84 | 117.10 ± 5.47 | 116.56 ± 1.17 | 70.14 ± 3.17 | 71.55 ± 4.37 |
| Time (Day) | BM45 | Cell Walls | ||
| no OTA | OTA | no OTA | OTA | |
| 1 | 166.75 ± 11.04 | 166.15 ± 23.48 | 166.05 ± 18.04 | 155.33 ± 11.85 |
| 7 | 160.57 ± 10.96 | 163.32 ± 7.84 | 161.90 ± 17.03 | 150.79 ± 6.25 |
| 14 | 148.36 ± 5.92 | 143.31 ± 6.81 | 148.16 ± 9.53 | 143.71 ± 5.04 |
| 21 | 123.90 ± 5.30 | 134.82 ± 7.93 | 125.92 ± 3.33 | 128.55 ± 9.08 |
| 42 | 111.93 ± 3.96 | 112.99 ± 6.24 | 112.18 ± 9.13 | 109.30 ± 10.91 |
| 46 | 91.97 ± 4.16 | 88.83 ± 5.59 | 67.00 ± 3.09 | 80.14 ± 1.88 |
| 70 | 84.49 ± 2.45 | 86.00 ± 5.42 | 66.80 ± 5.45 | 73.47 ± 5.45 |
| 84 | 79.13 ± 2.22 | 79.74 ± 3.24 | 52.65 ± 2.96 | 55.79 ± 2.31 |
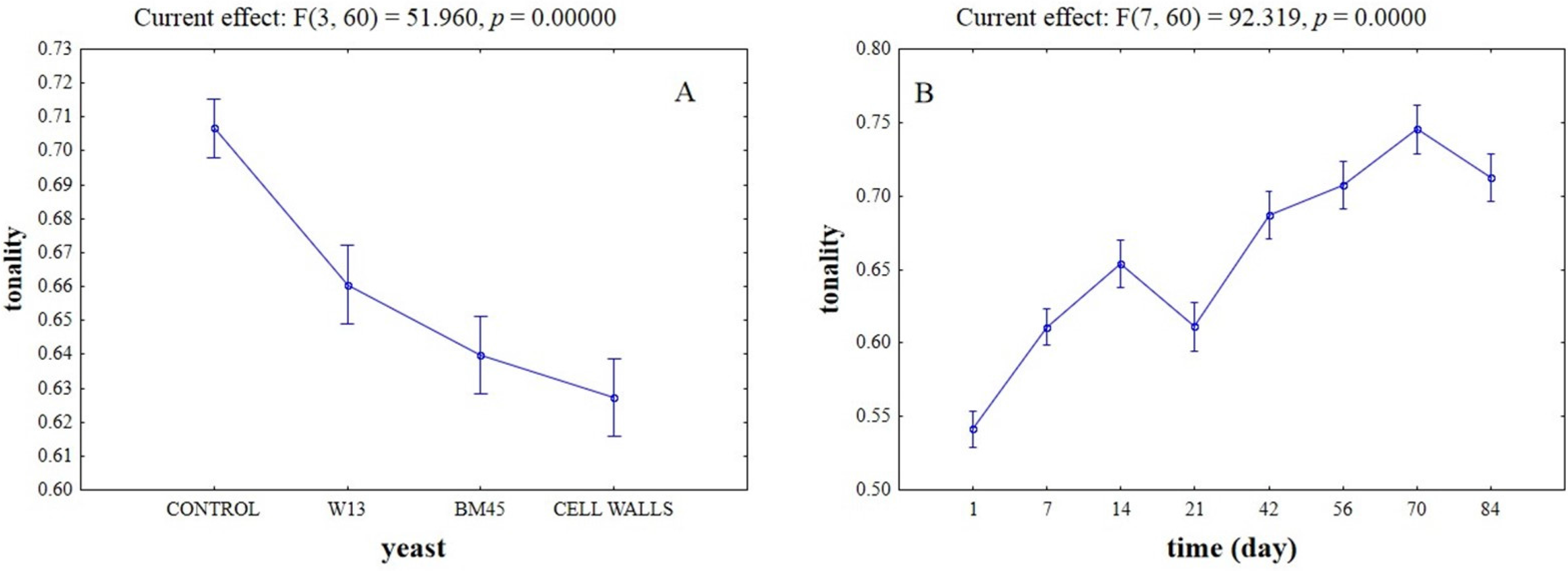

3. Discussion
4. Experimental Section
4.1. Yeast Strains
4.2. Preparation of Inactivated Yeasts
4.3. Yeast Cell Walls
4.4. Simulation of Wine Aging
4.5. Analytical Determinations
4.5.1. Extraction of Anthocyanins Adsorbed by the Yeast Cells
4.5.2. Determination of Total Anthocyanins
4.5.3. Determination of Chromatic Parameters
4.5.4. Quantification of OTA Concentration
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Comuzzo, P.; Tat, L.; Fenzi, D.; Brotto, L.; Battistutta, F.; Zironi, R. Interactions between yeast autolysates and volatile compounds in wine and model solution. Food Chem. 2011, 127, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Mekoue Nguela, J.; Sieczkowski, N.; Roi, S.; Vernhet, A. Sorption of grape proanthocyanidins and wine polyphenols by yeasts, inactivated yeasts, and yeast cell walls. J. Agric. Food Chem. 2015, 63, 660–670. [Google Scholar] [CrossRef] [PubMed]
- Vasserot, Y.; Caillet, S.; Maujean, A. Study of anthocyanin adsorption by yeast lees. Effect of some physicochemical parameters. Am. J. Enol. Vitic. 1997, 48, 433–437. [Google Scholar]
- Morata, A.; Gómez-Cordovés, M.C.; Suberviola, J.; Bartolomé, B.; Colomo, B.; Suárez, J.A. Adsorption of anthocyanins by yeast cell walls during the fermentation of red wines. J. Agric. Food Chem. 2003, 51, 4084–4088. [Google Scholar] [CrossRef] [PubMed]
- Mazauric, J.P.; Salmon, J.M. Interactions between yeast lees and wine polyphenols during simulation of wine aging: I. Analysis of remnant polyphenolic compounds in the resulting wines. J. Agric. Food Chem. 2005, 53, 5647–5653. [Google Scholar] [CrossRef] [PubMed]
- Mazauric, J.P.; Salmon, J.M. Interactions between yeast lees and wine polyphenols during simulation of wine aging: II. Analysis of desorbed polyphenol compounds from yeast lees. J. Agric. Food Chem. 2006, 54, 3876–3881. [Google Scholar] [CrossRef] [PubMed]
- Razmkhab, S.; Lopez-Toledano, A.; Ortega, J.M.; Mayen, M.; Merida, J.; Medina, M. Adsorption of phenolic compounds and browning products in white wines by yeasts and their cell walls. J. Agric. Food Chem. 2002, 50, 7432–7437. [Google Scholar] [CrossRef] [PubMed]
- Chalier, P.; Angot, B.; Delteil, D.; Doco, T.; Gunata, Z. Interactions between aroma compounds and whole mannoprotein isolated from Saccharomyces cerevisiae strains. Food Chem. 2007, 100, 22–30. [Google Scholar] [CrossRef]
- Palacios, S.; Vasserot, Y.; Maujean, A. Evidence for sulfur volatile products adsorption by yeast lees. Am. J. Enol. Vitic. 1997, 48, 525–526. [Google Scholar]
- Alexandre, H.; Lubbers, S.; Charpentier, C. Interactions between toxic fatty acids for yeasts and colloids, cellulose and yeast ghost using the equilibrium dialysis method in a modelwine system. Food Biotechnol. 1997, 11, 89–99. [Google Scholar] [CrossRef]
- Chassagne, D.; Guilloux-Benatier, M.; Alexandre, H.; Voilley, A. Sorption of wine volatile phenols by yeast lees. Food Chem. 2005, 91, 39–44. [Google Scholar] [CrossRef]
- Pradelles, R.; Ortiz-Julien, A.; Alexandre, H.; Chassagne, D. Effects of yeast wall composition on 4-ethylphenol sorption in model wine. J. Agric. Food Chem. 2008, 56, 11854–11861. [Google Scholar] [CrossRef] [PubMed]
- Palomero, F.; Ntanos, K.; Morata, A.; Benito, S.; Suárez-Lepe, J.A. Reduction of wine 4-ethylphenol concentration using lyophilized yeast as a bioadsorbent: Influence on anthocyanin content and chromatic variables. Eur. Food Res. Technol. 2011, 232, 971–977. [Google Scholar] [CrossRef]
- Pradelles, R.; Chassagne, D.; Vichi, S.; Gougeon, R.; Alexandre, H. (−)Geosmin sorption by enological yeasts in model wine and FTIR spectroscopy characterization of the sorbent. Food Chem. 2010, 120, 531–538. [Google Scholar] [CrossRef]
- Navarro, S.; Barba, A.; Oliva, J.; Navarro, G.; Pardo, F. Evolution of residual levels of six pesticides during elaboration of red wines. Effect of wine-making procedures in their disappearance. J. Agric. Food Chem. 1999, 47, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Petruzzi, L.; Sinigaglia, M.; Corbo, M.R.; Campaniello, D.; Speranza, B.; Bevilacqua, A. Decontamination of ochratoxin A by yeasts: Possible approaches and factors leading to toxin removal in wine. Appl. Microbiol. Biotechnol. 2014, 98, 6555–6567. [Google Scholar] [CrossRef] [PubMed]
- Giovannoli, C.; Passini, C.; Di Nardo, F.; Anfossi, L.; Baggiani, C. Determination of ochratoxin A in Italian red wines by molecularly imprinted solid phase extraction and HPLC analysis. J. Agric. Food Chem. 2014, 62, 5220–5225. [Google Scholar] [CrossRef] [PubMed]
- Solfrizzo, M.; Avantaggiato, G.; Panzarini, G.; Visconti, A. Removal of ochratoxin A from contaminated red wines by repassage over grape pomaces. J. Agric. Food Chem. 2010, 58, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Caridi, A.; Galvano, F.; Tafuri, A.; Ritieni, A. Ochratoxin A removal during winemaking. Enzyme Microb. Tech. 2006, 40, 122–126. [Google Scholar] [CrossRef]
- Cecchini, F.; Morassut, M.; García-Moruno, E.; Di Stefano, R. Influence of yeast strain on ochratoxin A content during fermentation of white and red must. Food Microbiol. 2006, 23, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Meca, G.; Blaiotta, G.; Ritieni, A. Reduction of ochratoxin A during the fermentation of Italian red wine Moscato. Food Control 2010, 21, 579–583. [Google Scholar] [CrossRef]
- Esti, M.; Benucci, I.; Liburdi, K.; Acciaro, G. Monitoring of ochratoxin A fate during alcoholic fermentation of wine must. Food Control 2012, 27, 53–56. [Google Scholar] [CrossRef]
- Bevilacqua, A.; Petruzzi, L.; Corbo, M.R.; Baiano, A.; Garofalo, C.; Sinigaglia, M. Ochratoxin A released back into the medium by Saccharomyces cerevisiae as a function of the strain, washing medium and fermentative conditions. J. Sci. Food Agric. 2014, 94, 3291–3295. [Google Scholar] [CrossRef] [PubMed]
- Petruzzi, L.; Bevilacqua, A.; Baiano, A.; Beneduce, L.; Corbo, M.R.; Sinigaglia, M. Study of Saccharomyces cerevisiae W13 as a functional starter for the removal of ochratoxin A. Food Control 2014, 35, 373–377. [Google Scholar] [CrossRef]
- Petruzzi, L.; Bevilacqua, A.; Baiano, A.; Beneduce, L.; Corbo, M.R.; Sinigaglia, M. In vitro removal of ochratoxin A by two strains of Saccharomyces cerevisiae and their performances under fermentative and stressing conditions. J. Appl. Microbiol. 2014, 116, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Petruzzi, L.; Bevilacqua, A.; Corbo, M.R.; Garofalo, C.; Baiano, A.; Sinigaglia, M. Selection of autochthonous Saccharomyces cerevisiae strains as wine starters using a polyphasic approach and ochratoxin A removal. J. Food Prot. 2014, 7, 1168–1177. [Google Scholar] [CrossRef] [PubMed]
- Petruzzi, L.; Corbo, M.R.; Baiano, A.; Beneduce, L.; Sinigaglia, M.; Bevilacqua, A. In vivo stability of the complex ochratoxin A—Saccharomyces cerevisiae starter strains. Food Control 2014, 50, 516–520. [Google Scholar] [CrossRef]
- Petruzzi, L.; Corbo, M.R.; Sinigaglia, M.; Bevilacqua, A. Yeast cells as adsorbing tools to remove ochratoxin A in a model wine. Int. J. Food Sci. Tech. 2014, 49, 936–940. [Google Scholar] [CrossRef]
- Núñez, Y.P.; Pueyo, E.; Carrascosa, A.V.; Martínez-Rodríguez, A.J. Effects of aging and heat treatment on whole yeast cells and yeast cell walls and on adsorption of ochratoxin A in a wine model system. J. Food Prot. 2008, 71, 1496–1499. [Google Scholar] [PubMed]
- Caridi, A. New perspectives in safety and quality enhancement of wine through selection of yeasts based on the parietal adsorption activity. Int. J. Food Microbiol. 2007, 120, 167–172. [Google Scholar] [CrossRef] [PubMed]
- García-Moruno, E.; Sanlorenzo, C.; Boccaccino, B.; Di Stefano, R. Treatment with yeast to reduce the concentration of ochratoxin A in red wine. Am. J. Enol. Vitic. 2005, 56, 73–76. [Google Scholar]
- Quintela, S.; Villarán, M.C.; de Armentia, I.L.; Elejalde, E. Ochratoxin A removal in wine: A review. Food Control 2013, 30, 439–445. [Google Scholar] [CrossRef]
- Nieto-Rojo, R.; Ancín-Azpilicueta, C.; Garrido, J.J. Sorption of 4-ethylguaiacol and 4-ethylphenol on yeast cell walls, using a synthetic wine. Food Chem. 2014, 152, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Del Barrio-Galán, R.; Pérez-Magariño, S.; Ortega-Heras, M. Effect of the aging on lees and other alternative techniques on the low molecular weight phenols of Tempranillo red wine aged in oak barrels. Anal. Chim. Acta 2012, 732, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska, M.; Nowak, A.; Czyzowska, A. Removal of ochratoxin A by wine Saccharomyces cerevisiae strains. Eur. Food Res. Technol. 2013, 236, 441–447. [Google Scholar] [CrossRef]
- Var, I.; Erginkaya, Z.; Kabak, B. Reduction of ochratoxin A levels in white wine by yeast treatments. J. Inst. Brew. 2009, 115, 30–34. [Google Scholar] [CrossRef]
- Bornet, A.; Teissedre, P.L. Chitosan, chitin-glucan and chitin effects on minerals (iron, lead, cadmium) and organic (ochratoxin A) contaminants in wines. Eur. Food Res. Technol. 2008, 226, 681–689. [Google Scholar] [CrossRef]
- Marquez, T.; Millan, C.; Salmon, J.-M. Plasma membrane sterols are involved in yeast’s ability to adsorb polyphenolic compounds resulting from wine model solution browning. J. Agric. Food Chem. 2009, 57, 8026–8032. [Google Scholar] [CrossRef] [PubMed]
- Escot, S.; Feuillat, M.; Dulau, L.; Charpentier, C. Release of polysaccharides by yeasts and the influence of released polysaccharides on colour stability and wine astringency. Aust. J. Grape Wine Res. 2001, 7, 153–159. [Google Scholar] [CrossRef]
- Guadalupe, Z.; Martínez, L.; Ayestarán, B. Yeast mannoproteins in red winemaking: Effect on polysaccharide, polyphenolic, and color composition. Am. J. Enol. Vitic. 2010, 61, 191–200. [Google Scholar]
- Guilloux-Benatier, M.; Chassagne, D. Comparison of components released by fermented or active dried yeasts after aging on lees in a model wine. J. Agric. Food Chem. 2003, 51, 746–751. [Google Scholar] [CrossRef] [PubMed]
- Pinna, M.V.; Budroni, M.; Farris, G.A.; Pusino, A. Fenhexamid adsorption behavior on soil amended with wine lees. J. Agric. Food Chem. 2008, 56, 10824–10828. [Google Scholar] [CrossRef] [PubMed]
- Paster, N. Means to prevent contamination with patulin in apple-derived produce and with ochratoxin A in wines. In Mycotoxins in Fruits and Vegetables; Barkai-Golan, R., Paster, N., Eds.; Elsevier: San Diego, CA, USA, 2008; pp. 351–386. [Google Scholar]
- Mussatto, S.I.; Santos, J.C.; Ricardo Filho, W.C.; Silva, S.S. A study on the recovery of xylitol by batch adsorption and crystallization from fermented sugarcane bagasse hydrolysate. J. Chem. Technol. Biotechnol. 2006, 81, 1840–1845. [Google Scholar] [CrossRef]
- Mauricio, J.C.; Millán, M.C.; Moreno, J.; Ortega, J.M. Changes in the urea concentration during controlled wine aging by two “flor” veil-forming yeasts. Biotechnol. Lett. 1995, 17, 401–406. [Google Scholar] [CrossRef]
- Patynowski, R.J.; Jiranek, V.; Markides, A.J. Yeast viability during fermentation and sur lie ageing of a defined medium and subsequent growth of Oenococcus oeni. Aust. J. Grape Wine Res. 2002, 8, 62–69. [Google Scholar] [CrossRef]
- Di Stefano, R.; Cravero, M.C.; Genilizzi, N. Metodi per lo studio dei polifenoli nei vini. L’Enotecnico 1989, 5, 83–89. (In Italian) [Google Scholar]
- Di Stefano, R.; Cravero, M.C. Metodi per lo studio dei polifenoli delle uve. Riv. Vitic. Enol. 1991, 2, 37–43. (in Italian). [Google Scholar]
- Glories, Y. La couleur des vins rouges. Mesure, origine et interprétation. Partie I. Connaiss. Vigne Vin 1984, 18, 195–217. (In Italian) [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petruzzi, L.; Baiano, A.; De Gianni, A.; Sinigaglia, M.; Corbo, M.R.; Bevilacqua, A. Differential Adsorption of Ochratoxin A and Anthocyanins by Inactivated Yeasts and Yeast Cell Walls during Simulation of Wine Aging. Toxins 2015, 7, 4350-4365. https://doi.org/10.3390/toxins7104350
Petruzzi L, Baiano A, De Gianni A, Sinigaglia M, Corbo MR, Bevilacqua A. Differential Adsorption of Ochratoxin A and Anthocyanins by Inactivated Yeasts and Yeast Cell Walls during Simulation of Wine Aging. Toxins. 2015; 7(10):4350-4365. https://doi.org/10.3390/toxins7104350
Chicago/Turabian StylePetruzzi, Leonardo, Antonietta Baiano, Antonio De Gianni, Milena Sinigaglia, Maria Rosaria Corbo, and Antonio Bevilacqua. 2015. "Differential Adsorption of Ochratoxin A and Anthocyanins by Inactivated Yeasts and Yeast Cell Walls during Simulation of Wine Aging" Toxins 7, no. 10: 4350-4365. https://doi.org/10.3390/toxins7104350
APA StylePetruzzi, L., Baiano, A., De Gianni, A., Sinigaglia, M., Corbo, M. R., & Bevilacqua, A. (2015). Differential Adsorption of Ochratoxin A and Anthocyanins by Inactivated Yeasts and Yeast Cell Walls during Simulation of Wine Aging. Toxins, 7(10), 4350-4365. https://doi.org/10.3390/toxins7104350







