Man-Made Synthetic Receptors for Capture and Analysis of Ochratoxin A
Abstract
:1. Introduction
| Issues | Immunoaffinity Chromatography | Molecularly Imprinted Polymers | Aptamers | Binding Peptides |
|---|---|---|---|---|
| binding structures | anti-OTA antibodies grafted onto chromatography-type solid supports | cross-linked synthetic polymers prepared in presence of OTA or OTA-mimic molecules | oligonucleotides selected through a sequential affinity purification/PCR amplification process | linear peptides selected through a sequential screening of a of a spatially addressable peptide combinatorial library |
| binding affinity | high | medium | medium | low |
| binding site density | low | high | low | low |
| binding kinetics | slow dissociation | slow dissociation | fast dissociation | fast dissociation |
| binding selectivity | high | high | high | high |
| reproducibility | limited | very high | very high | very high |
| non-specific binding | negligible | significant in water | negligible | negligible |
| resistance to extreme pH | no | yes | no | no |
| resistance to organic solvents | limited | yes | yes | yes |
| resistance to denaturing agents | no | yes | yes | yes |
| resistance to microorganisms | no | yes | no | no |
| needs of a solid support | yes | no | yes | yes |
| reuse | difficult, mainly monouse | yes | yes | yes |
| costs | low to medium | low | low | low |
| commercial availability as read-to-use | yes | yes | no | no |
| literature | large | growing | growing | limited |
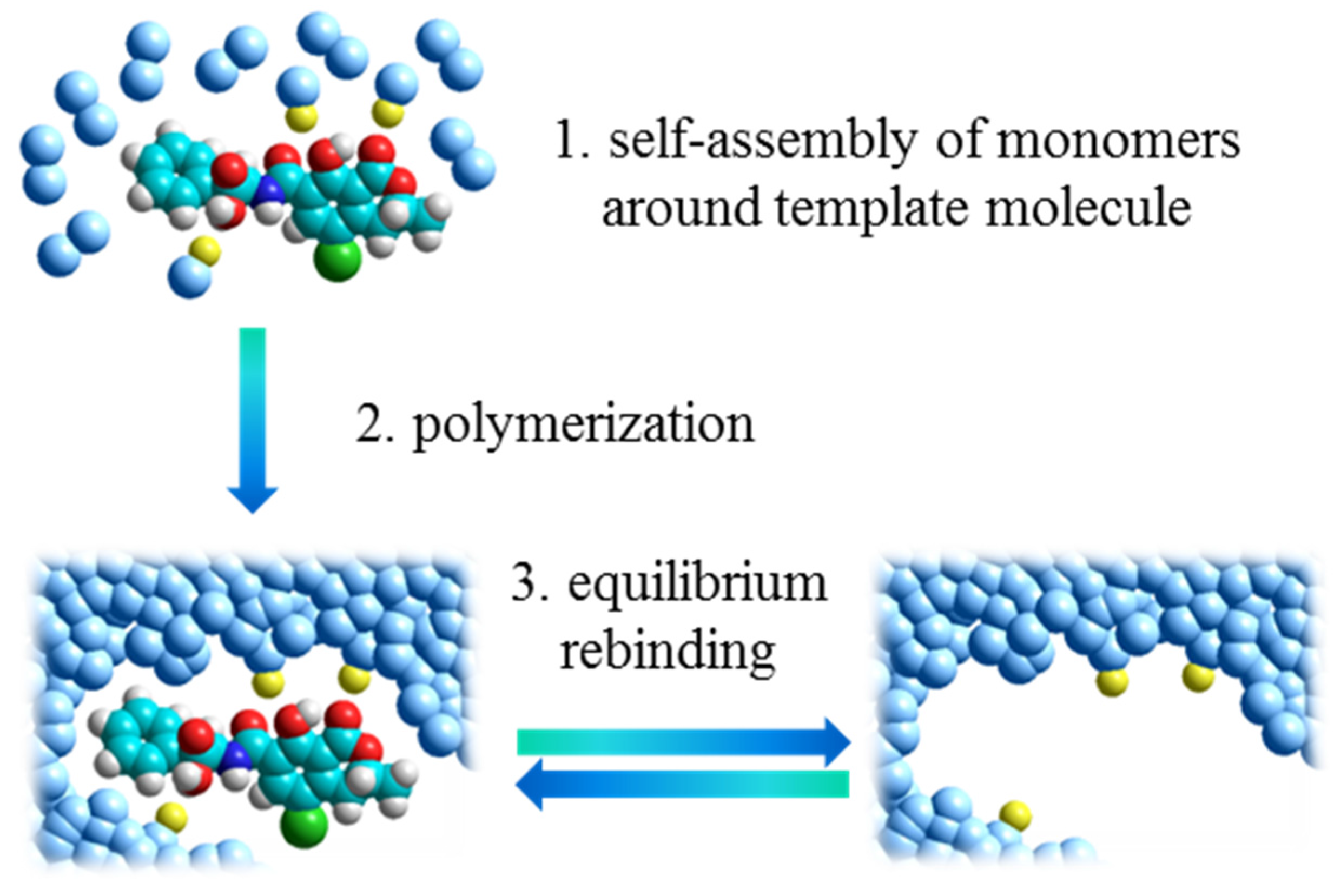
2. Molecularly Imprinted Polymers
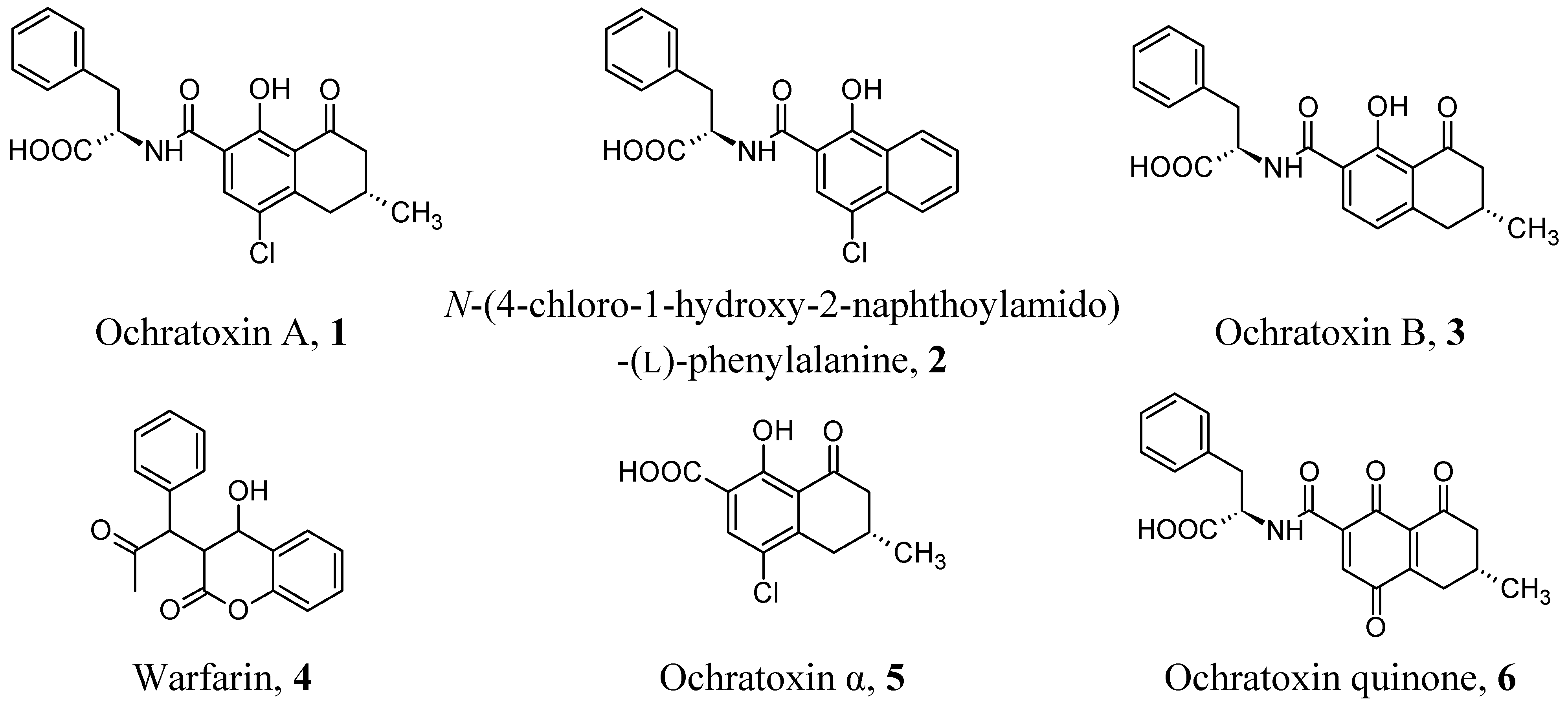
2.1. OTA Imprinting by Template Mimics
2.2. Direct OTA Imprinting
2.3. OTA Imprinting in Polypyrrole Layers
2.4. Commercial OTA-Imprinted Polymers
3. Aptamers
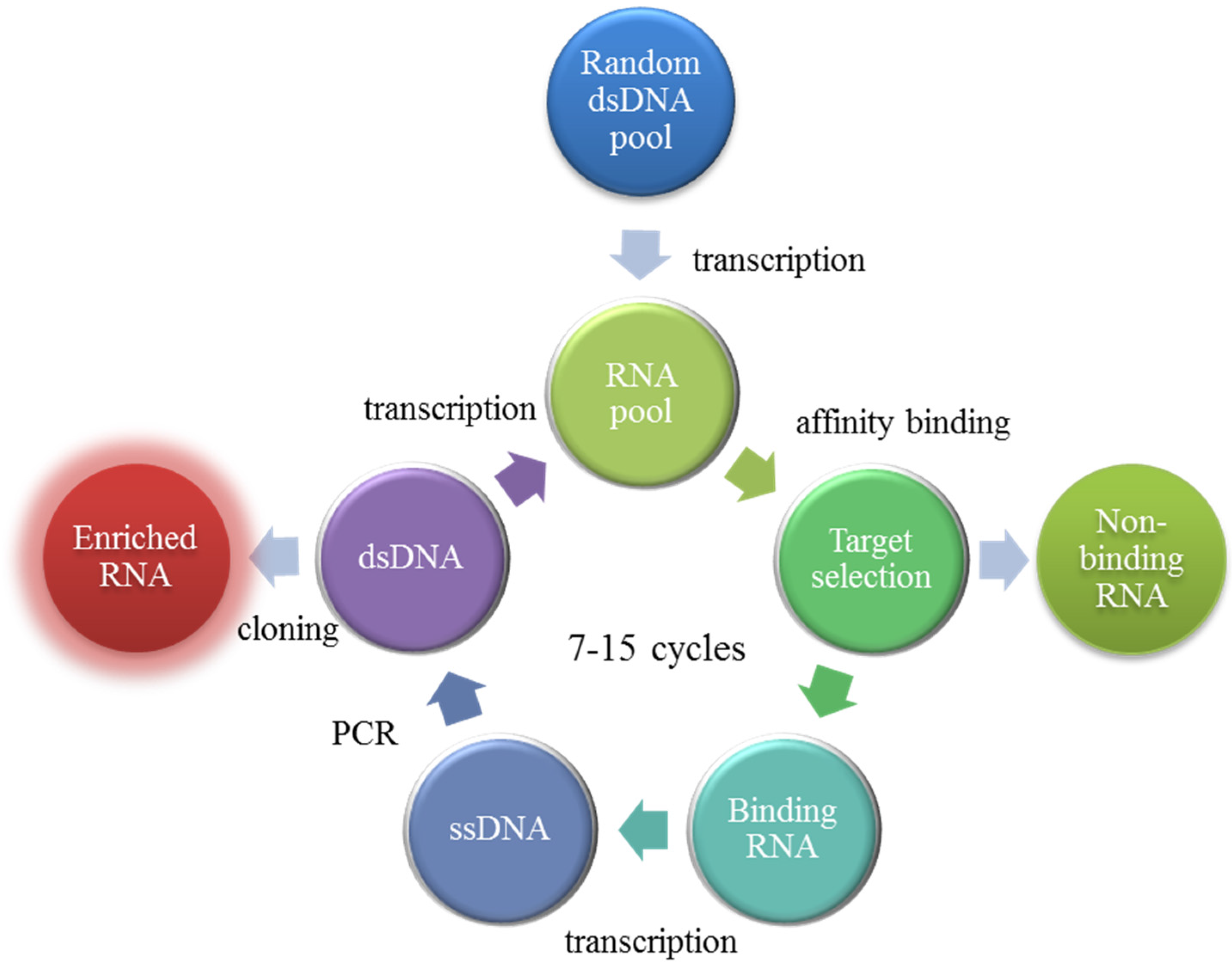
OTA-Binding Aptamers as Oligosorbents
| Aptamer | Sequence | Kd (µM) |
|---|---|---|
| 1.12 | GCATCTGATCGTAAAGG | 0.36 |
| 1.12.2 | GATCGTAAAGGGAGCATCGGACA | 0.2 |
| 1.12.5 | GATCGTAAAGGGAGCATCGGACAACG | 0.8 |
| 1.12.8 | GATCGTAAAGGGAGCATCGG | 0.2 |
| 1.12.9 | GATCGTAAAGGGAGCAT | 1.6 |
| 1.12.11 | GATCGTAAAGGGAGCATCG | 0.4 |
| 1.12.12 | GATCGTAAAGGGAGCATC | 0.5 |
| 1.13 | GAAACGCCCG | 6.7 |
| 1.14 | GCAGTCCTAGATCCTTGG | 0.99 |
| 1.4 | GCACGATGGGGAAACCCCCGTTG | 19.3 |
| 2.2 | ACTGTCCGTCTTAGGGCATTGG | 1.6 |
| 2.3 | TCAGTCCCGATCA ATTGG | 1.7 |
| 2.4 | CCAAATCGGACGCCTGTTTTAATGGGG | 19.5 |
| 2.6 | CGTACGGTAACGTCCTCTTAGGGT | 7.1 |
| 2.9 | CAGGTGGCAGATCCTGG | 0.96 |
| 2.10 | ACATGCGACTGACCGGTTATTGAGGG | 4.3 |
| 2.11 | CCTGACGATCTTGAGG | 2.5 |
| 2.12 | CCTTGTAGATCTTGTAGG | 0.97 |
| 2.13 | GCAGTACGATCGGATGTAGG | 1.9 |
4. Combinatorial Peptides
OTA-Binding Combinatorial Peptides

5. Conclusions
Author Contributions
Conflicts of Interest
References
- Duarte, S.C.; Lino, C.M.; Pena, A. Mycotoxin food and feed regulation and the specific case of Ochratoxin A: A review of the worldwide status. Food Additiv. Contam. A 2010, 27, 1440–1450. [Google Scholar] [CrossRef] [PubMed]
- El Khoury, A.; Atoui, A. Ochratoxin A: General overview and actual molecular status. Toxins 2010, 2, 461–493. [Google Scholar] [CrossRef] [PubMed]
- Reddy, L.; Bhoola, K. Ochratoxins-food contaminants: Impact on human health. Toxins 2010, 2, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Klaric, M.S.; Rasic, D.; Peraica, M. Deleterious effects of mycotoxin combinations involving Ochratoxin A. Toxins 2013, 5, 1965–1987. [Google Scholar] [CrossRef] [PubMed]
- Sorrenti, V.; Di Giacomo, C.; Acquaviva, R.; Barbagallo, I.; Bognanno, M.; Galvano, F. Toxicity of Ochratoxin A and its modulation by antioxidants: A review. Toxins 2013, 5, 1742–1766. [Google Scholar] [CrossRef] [PubMed]
- Bezeria da Rocha, M.E.; Oliveira Freire, F.C.; Feitosa Maia, F.B.; Florindo Guedes, M.I.; Rondina, D. Mycotoxins and their effects on human and animal health. Food Contr. 2014, 36, 159–165. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation 100/03. Off. J. Eur. Commun. 2003, L285, 33–37. [Google Scholar]
- European Commission. Commission Regulation 683/04. Off. J. Eur. Commun. 2004, L106, 3–5. [Google Scholar]
- European Commission. Commission Regulation 1881/06. Off. J. Eur. Commun. 2006, L364, 5–24. [Google Scholar]
- Meulenberg, E.P. Immunochemical methods for Ochratoxin A detection: A review. Toxins 2012, 4, 244–266. [Google Scholar] [CrossRef] [PubMed]
- Anfossi, L.; Baggiani, C.; Giovannoli, C.; D’Arco, G.; Giraudi, G. Lateral-flow immunoassays for mycotoxins and phycotoxins: A review. Anal. Bioanal. Chem. 2013, 405, 467–480. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.W.; Subrahmanyam, S.; Piletsky, S.A. Analytical methods for determination of mycotoxins: A review. Anal. Chim. Acta 2009, 632, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Berthiller, F.; Burdaspal, P.A.; Crews, C.; Iha, M.H.; Krska, R.; Lattanzio, V.M.T.; MacDonald, S.; Malone, R.J.; Maragos, C.; Solfrizzo, M.; et al. Developments in mycotoxin analysis: An update for 2012–2013. World Mycotox. J. 2014, 7, 3–33. [Google Scholar] [CrossRef]
- Berthiller, F.; Brera, C.; Crews, C.; Iha, M.H.; Krska, R.; Lattanzio, V.M.T.; MacDonald, S.; Malone, R.J.; Maragos, C.; Solfrizzo, M.; et al. Developments in mycotoxin analysis: An update for 2013–2014. World Mycotox. J. 2015, 8, 5–35. [Google Scholar] [CrossRef]
- Pichon, V.; Delaunay-Bertoncini, M.; Hennion, M.C. Immunosorbents in sample preparation. In Sampling and Sample Preparation for Field and Laboratory—Comprehensive Analytical Chemistry Ser; Pawliszyn, J., Ed.; Elsevier Science: Amsterdam, The Netherlands, 2002; Volume 37, pp. 1081–1100. [Google Scholar]
- Senyuva, H.Z.; Gilbert, J. Immunoaffinity column clean-up techniques in food analysis: A review. J. Chromatogr. B 2010, 878, 115–132. [Google Scholar] [CrossRef] [PubMed]
- Sellergren, B. Molecularly Imprinted Polymers: Man-Made Mimics of Antibodies and Their Applications in Analytical Chemistry, 1st ed.; Elsevier Science: Amsterdam, The Netherlands, 2001. [Google Scholar]
- Yan, M.; Ramström, O. Molecularly Imprinted Materials, 1st ed.; Marcel Dekker: New York, NY, USA, 2004. [Google Scholar]
- Haupt, K. Molecular Imprinting, 1st ed.; Springer-Verlag: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Beltran, A.; Borrull, F.; Cormack, P.A.G.; Marcè, R.M. Molecularly-imprinted polymers: Useful sorbents for selective extractions. Trends Anal. Chem. 2010, 29, 1363–1375. [Google Scholar] [CrossRef]
- Martin-Esteban, A. Molecularly-imprinted polymers as a versatile, highly selective tool in sample preparation. Trends Anal. Chem. 2013, 45, 169–181. [Google Scholar] [CrossRef]
- Baggiani, C.; Anfossi, L.; Giovannoli, C. Molecular imprinted polymers as synthetic receptors for the analysis of myco- and phyco-toxins. Analyst 2008, 133, 719–730. [Google Scholar] [CrossRef] [PubMed]
- Baggiani, C.; Giraudi, G.; Vanni, A. A molecular imprinted polymer with recognition properties towards the carcinogenic mycotoxin Ochratoxin A. Bioseparation 2002, 10, 389–394. [Google Scholar] [CrossRef]
- Jodlbauer, J.; Maier, M.M.; Lindner, W. Towards Ochratoxin A selective molecularly imprinted polymers for solid-phase extraction. J. Chromatogr. A 2002, 945, 45–63. [Google Scholar] [CrossRef]
- Baggiani, C.; Biagioli, F.; Anfossi, L.; Giovannoli, C.; Passini, C.; Giraudi, G. Effect of the mimic structure on the molecular recognition properties of molecularly imprinted polymers for Ochratoxin A prepared by a fragmental approach. React. Funct. Polym. 2013, 73, 833–837. [Google Scholar] [CrossRef]
- Maier, N.M.; Buttinger, G.; Welhartizki, S.; Gavioli, E.; Lindner, W. Molecularly imprinted polymer-assisted sample clean-up of Ochratoxin A from red wine: Merits and limitations. J. Chromatogr. B 2004, 804, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Giovannoli, C.; Passini, C.; Di Nardo, F.; Anfossi, L.; Baggiani, C. Determination of Ochratoxin A in italian red wines by molecularly imprinted solid phase extraction and HPLC analysis. J. Agric. Food Chem. 2014, 62, 5220–5225. [Google Scholar] [CrossRef] [PubMed]
- Vidal, J.C.; Duato, P.; Bonel, L.; Castillo, J.R. Molecularly imprinted on-line solid-phase extraction coupled with fluorescence detection for the determination of Ochratoxin A in wheat samples. Anal. Lett. 2012, 45, 51–62. [Google Scholar] [CrossRef]
- Turner, N.W.; Piletska, E.V.; Karim, K.; Whitcombe, M.; Malecha, M.; Magan, N.; Baggiani, C.; Piletsky, S.A. Effect of the solvent on recognition properties of molecularly imprinted polymer specific for Ochratoxin A. Biosens. Bioelectron. 2004, 20, 1060–1067. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.N.; Lai, E.P.C.; Miller, J.D. Analysis of wheat extracts for Ochratoxin A by molecularly imprinted solid-phase extraction and pulsed elution. Anal. Bioanal. Chem. 2004, 378, 1903–1906. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.C.C.; Krushkova, S.; Lai, E.P.C.; Dabek-Zlotorzynska, E. Molecularly-imprinted polypyrrole-modified stainless steel frits for selective solid phase preconcentration of Ochratoxin A. Anal. Bioanal. Chem. 2005, 382, 1534–1540. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.C.C.; Lai, E.P.C. Molecularly imprinted polypyrrole modified carbon nanotubes on stainless steel frit for selective micro solid phase pre-concentration of Ochratoxin A. React. Funct. Polym. 2006, 66, 702–711. [Google Scholar] [CrossRef]
- Yu, J.C.C.; Lai, E.P.C. Determination of Ochratoxin A in red wines by multiple pulsed elutions from molecularly imprinted polypyrrole. Food Chem. 2007, 105, 301–310. [Google Scholar] [CrossRef]
- Wei, Y.; Qiu, L.H.; Yu, J.C.C.; Lai, E.P.C. Molecularly imprinted solid phase extraction in a syringe needle packed with polypyrrole-encapsulated carbon nanotubes for determination of Ochratoxin a in red wine. Food Sci. Technol. Int. 2007, 13, 375–380. [Google Scholar] [CrossRef]
- Ali, W.H.; Derrien, D.; Alix, F.; Perollier, C.; Lepine, O.; Bayoudh, S.; Chapuis-Hugon, F.; Pichon, V. Solid-phase extraction using molecularly imprinted polymers for selective extraction of a mycotoxin in cereals. J. Chromatogr. A 2010, 1217, 6668–6673. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.P.; Saad, B.; Khayoon, W.S.; Salleh, B. Molecularly imprinted polymer as sorbent in micro-solid phase extraction of Ochratoxin A in coffee, grape juice and urine. Talanta 2012, 88, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.L.; Zhou, S.J.; Kong, W.J.; Yang, M.H.; Wan, L.; Yang, S.H. Molecularly imprinted polymer-based solid phase clean-up for analysis of Ochratoxin A in ginger and LC-MS/MS confirmation. Food Control 2013, 33, 337–343. [Google Scholar] [CrossRef]
- Cao, J.L.; Kong, W.J.; Zhou, S.J.; Yin, L.H.; Wan, L.; Yang, M.H. Molecularly imprinted polymer-based solid phase clean-up for analysis of Ochratoxin A in beer, red wine, and grape juice. J. Sep. Sci. 2013, 36, 1291–1297. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.P.; Saad, B.; Salleh, B.; Mat, I. Micro-solid phase extraction of Ochratoxin A, and its determination in urine using capillary electrophoresis. Microchim. Acta 2013, 180, 1149–1156. [Google Scholar] [CrossRef]
- Xie, L.; Sheng, P.; Kong, W.; Zhao, X.; Ouyang, Z.; Yang, M. Solid-phase extraction using molecularly imprinted polymer for determination of Ochratoxin A in human urine. World Mycotox. J. 2015, 8, 37–43. [Google Scholar] [CrossRef]
- Prelle, A.; Spadaro, D.; Denca, A.; Garibaldi, A.; Gullino, M.L. Comparison of clean-up methods for Ochratoxin A on wine, beer, roasted coffee and chili commercialized in Italy. Toxins 2013, 5, 1827–1844. [Google Scholar] [CrossRef] [PubMed]
- Hermann, T.; Patel, D.J. Adaptive recognition by nucleic acid aptamers. Science 2000, 287, 820–825. [Google Scholar] [CrossRef] [PubMed]
- Ellington, A.D.; Szostak, J.W. In vitro selection of RNA molecules that bind specific ligands. Nature 1990, 346, 818–822. [Google Scholar] [CrossRef] [PubMed]
- Stoltenburg, R.; Reinemann, C.; Strehlitz, B. Selex—A (r)evolutionary method to generate high-affinity nucleic acid ligands. Biomol. Eng. 2007, 24, 381–403. [Google Scholar] [CrossRef] [PubMed]
- Mascini, M.; Palchetti, I.; Tombelli, S. Nucleic acid and peptide aptamers: Fundamentals and bioanalytical aspects. Angew. Chem. Int. Ed. 2012, 51, 1316–1332. [Google Scholar] [CrossRef] [PubMed]
- Tombelli, S.; Minunni, M.; Mascini, M. Aptamers-based assays for diagnostics, environmental and food analysis. Biomol. Eng. 2007, 24, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Wang, L.; Li, J.; Fan, C.; Zhao, J. Aptamer-based biosensors. Trends Anal. Chem. 2008, 27, 108–117. [Google Scholar] [CrossRef]
- Giovannoli, C.; Baggiani, C.; Anfossi, L.; Giraudi, G. Aptamers and molecularly imprinted polymers as artificial biomimetic receptors in affinity capillary electrophoresis and electrochromatography. Electrophoresis 2008, 29, 3349–3365. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Wu, M.; Le, X.C.; Li, X.F. Applications of aptamer affinity chromatography. Trends Anal. Chem. 2012, 41, 46–57. [Google Scholar] [CrossRef]
- Cruz-Aguado, J.A.; Penner, G. Determination of Ochratoxin A with a DNA Aptamer. J. Agric. Food Chem. 2008, 56, 10456–10461. [Google Scholar] [CrossRef] [PubMed]
- Chapuis-Hugon, F.; du Boisbaudry, A.; Madru, B.; Pichon, V. New extraction sorbent based on aptamers for the determination of Ochratoxin A in red wine. Anal. Bioanal. Chem. 2011, 400, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- Ali, W.H.; Pichon, V. Characterization of oligosorbents and application to the purification of Ochratoxin A from wheat extracts. Anal. Bioanal. Chem. 2014, 406, 1233–1240. [Google Scholar] [PubMed]
- De Girolamo, A.; McKeague, M.; Miller, J.D.; DeRosa, M.C.; Visconti, A. Determination of Ochratoxin A in wheat after clean-up through a DNA aptamer-based solid phase extraction column. Food Chem. 2011, 127, 1378–1384. [Google Scholar] [CrossRef] [PubMed]
- Rhouati, A.; Paniel, N.; Meraihi, Z.; Marty, J.L. Development of an oligosorbent for detection of Ochratoxin A. Food Control. 2011, 22, 1790–1796. [Google Scholar] [CrossRef]
- Yang, X.; Kong, W.; Hu, Y.; Yang, M.; Huang, L.; Zhao, M.; Ouyang, Z. Aptamer-affinity column clean-up coupled with ultra high performance liquid chromatography and fluorescence detection for the rapid determination of Ochratoxin A in ginger powder. J. Sep. Sci. 2014, 37, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Hu, J.; Zhu, B.; Lu, L.; Huang, X.; Pang, D. Aptamer-targeted magnetic nanospheres as a solid-phase extraction sorbent for determination of Ochratoxin A in food samples. J. Chromatogr. A 2011, 1218, 7341–7346. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Hu, Y.; Kong, W.; Chu, X.; Yang, M.; Zhao, M.; Ouyang, Z. Ultra-fast liquid chromatography with tandem mass spectrometry determination of Ochratoxin A in traditional Chinese medicines based on vortex-assisted solid-liquid microextraction and aptamer-affinity column clean-up. J. Sep. Sci. 2014, 37, 3052–3059. [Google Scholar] [CrossRef] [PubMed]
- Brothier, F.; Pichon, V. Miniaturized DNA aptamer-based monolithic sorbent for selective extraction of a target analyte coupled on-line to nanoLC. Anal. Bioanal. Chem. 2014, 406, 7875–7886. [Google Scholar] [CrossRef] [PubMed]
- Merrifield, R.B. Solid phase peptide synthesis. I. The synthesis of a tetrapeptide. J. Am. Chem. Soc. 1963, 85, 2149–2154. [Google Scholar] [CrossRef]
- Gray, B.P.; Brown, K.C. Combinatorial peptide libraries: Mining for cell-binding peptides. Chem. Rev. 2014, 114, 1020–1081. [Google Scholar] [CrossRef] [PubMed]
- Righetti, P.G.; Candiano, G.; Citterio, A.; Boschetti, E. Combinatorial peptide ligand libraries as a “trojan horse” in deep discovery proteomics. Anal. Chem. 2015, 87, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Li, T.Y. Peptide and peptidomimetic chiral selectors in liquid chromatography. J. Sep. Sci. 2006, 28, 1927–1931. [Google Scholar] [CrossRef]
- Tozzi, C.; Anfossi, L.; Giraudi, G.; Giovannoli, C.; Baggiani, C.; Vanni, A. Chromatographic characterisation of an estrogen-binding affinity column containing tetrapeptides selected by a combinatorial-binding approach. J. Chromatogr. A 2002, 966, 71–79. [Google Scholar] [CrossRef]
- Tozzi, C.; Anfossi, L.; Baggiani, C.; Giovannoli, C.; Giraudi, G. A combinatorial approach to obtain affinity media with binding properties towards the aflatoxins. Anal. Bioanal. Chem. 2003, 375, 994–999. [Google Scholar] [PubMed]
- Giraudi, G.; Anfossi, L.; Baggiani, C.; Giovannoli, C.; Tozzi, C. Solid-phase extraction of Ochratoxin A from wine based on a binding hexapeptide prepared by combinatorial synthesis. J. Chromatogr. A 2007, 1175, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Tozzi, C.; Anfossi, L.; Baggiani, C.; Giovannoli, C.; Giraudi, G. Synthetic peptides as artificial receptors towards proteins from genetically modified organisms. Biosens. Bioelectron. 2008, 24, 493–497. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baggiani, C.; Giovannoli, C.; Anfossi, L. Man-Made Synthetic Receptors for Capture and Analysis of Ochratoxin A. Toxins 2015, 7, 4083-4098. https://doi.org/10.3390/toxins7104083
Baggiani C, Giovannoli C, Anfossi L. Man-Made Synthetic Receptors for Capture and Analysis of Ochratoxin A. Toxins. 2015; 7(10):4083-4098. https://doi.org/10.3390/toxins7104083
Chicago/Turabian StyleBaggiani, Claudio, Cristina Giovannoli, and Laura Anfossi. 2015. "Man-Made Synthetic Receptors for Capture and Analysis of Ochratoxin A" Toxins 7, no. 10: 4083-4098. https://doi.org/10.3390/toxins7104083
APA StyleBaggiani, C., Giovannoli, C., & Anfossi, L. (2015). Man-Made Synthetic Receptors for Capture and Analysis of Ochratoxin A. Toxins, 7(10), 4083-4098. https://doi.org/10.3390/toxins7104083