Triggering of Suicidal Erythrocyte Death by Penta-O-galloyl-β-d-glucose
Abstract
:1. Introduction
2. Results and Discussion
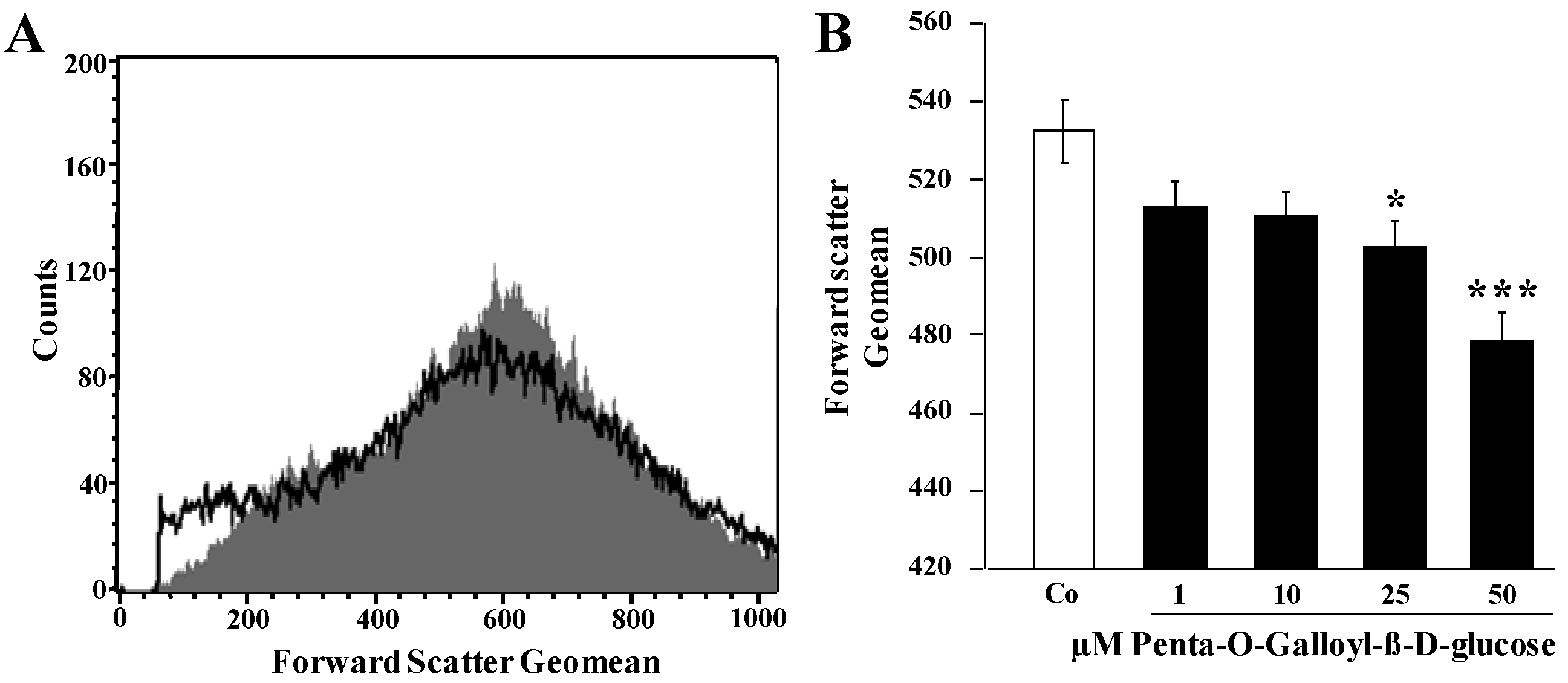
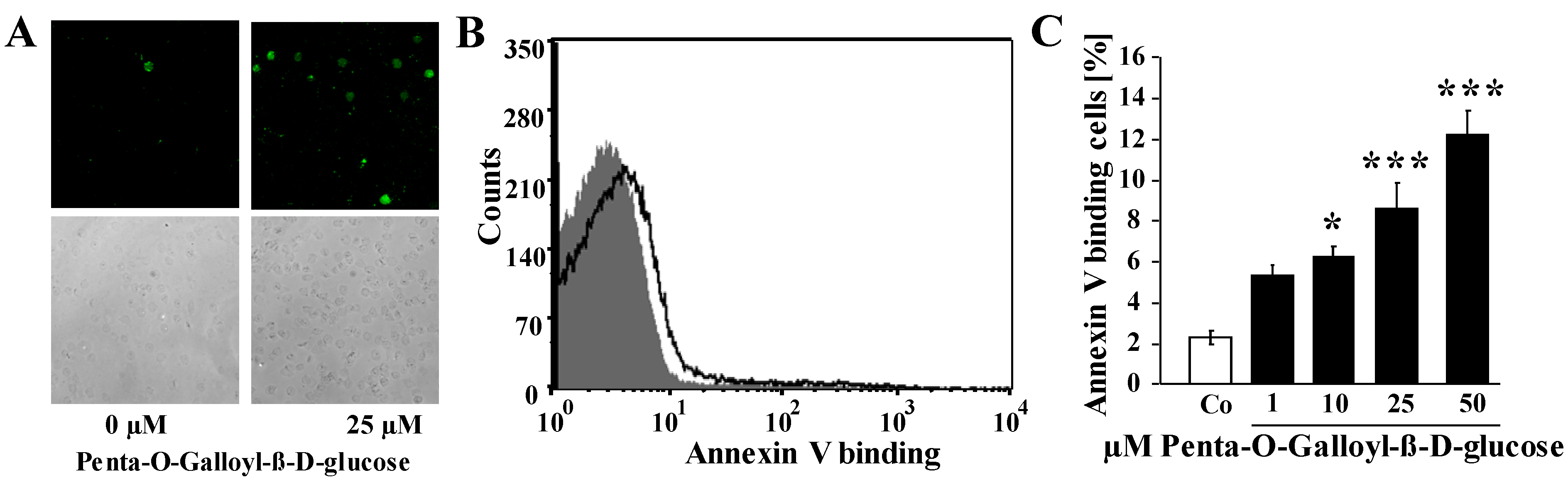
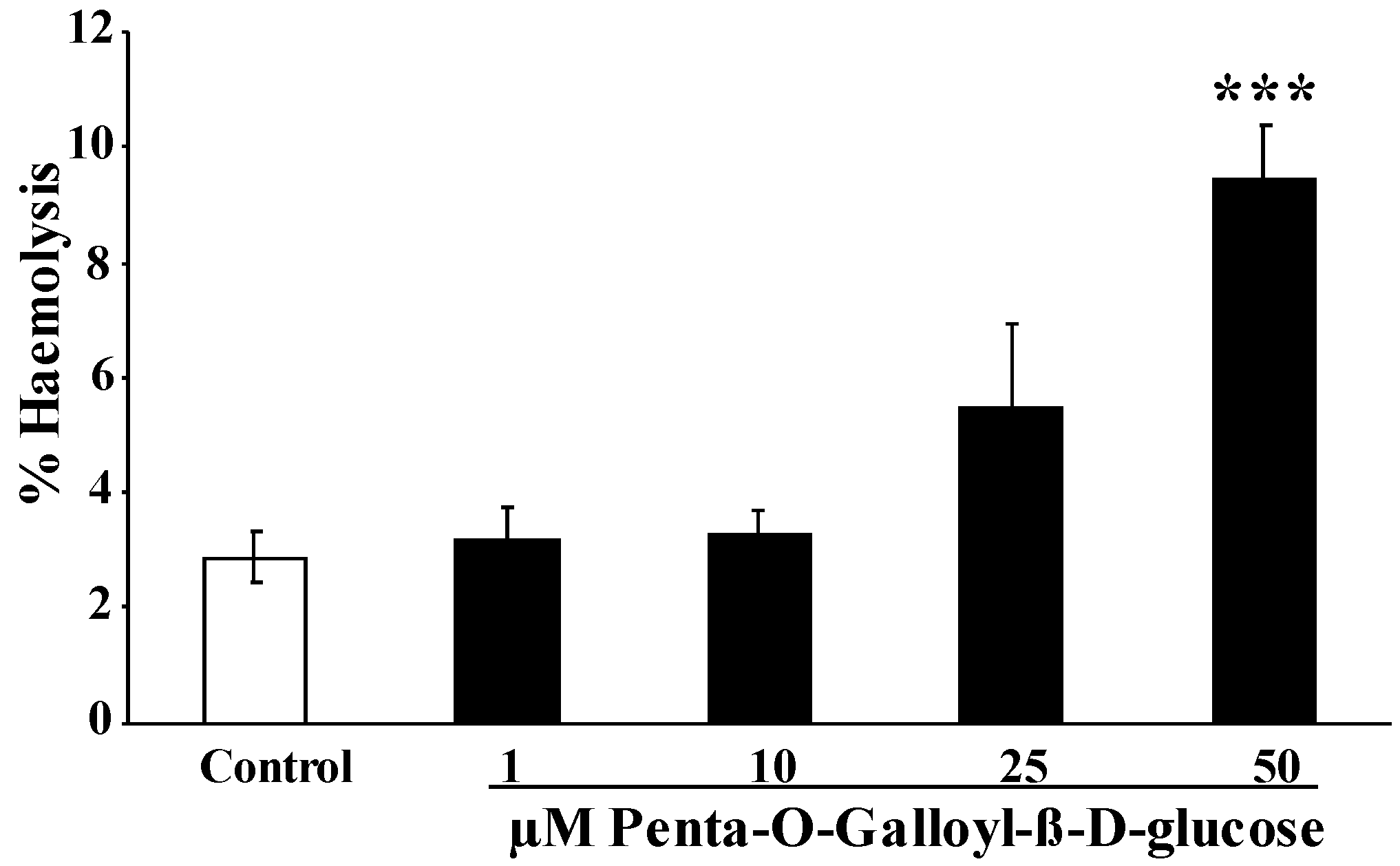
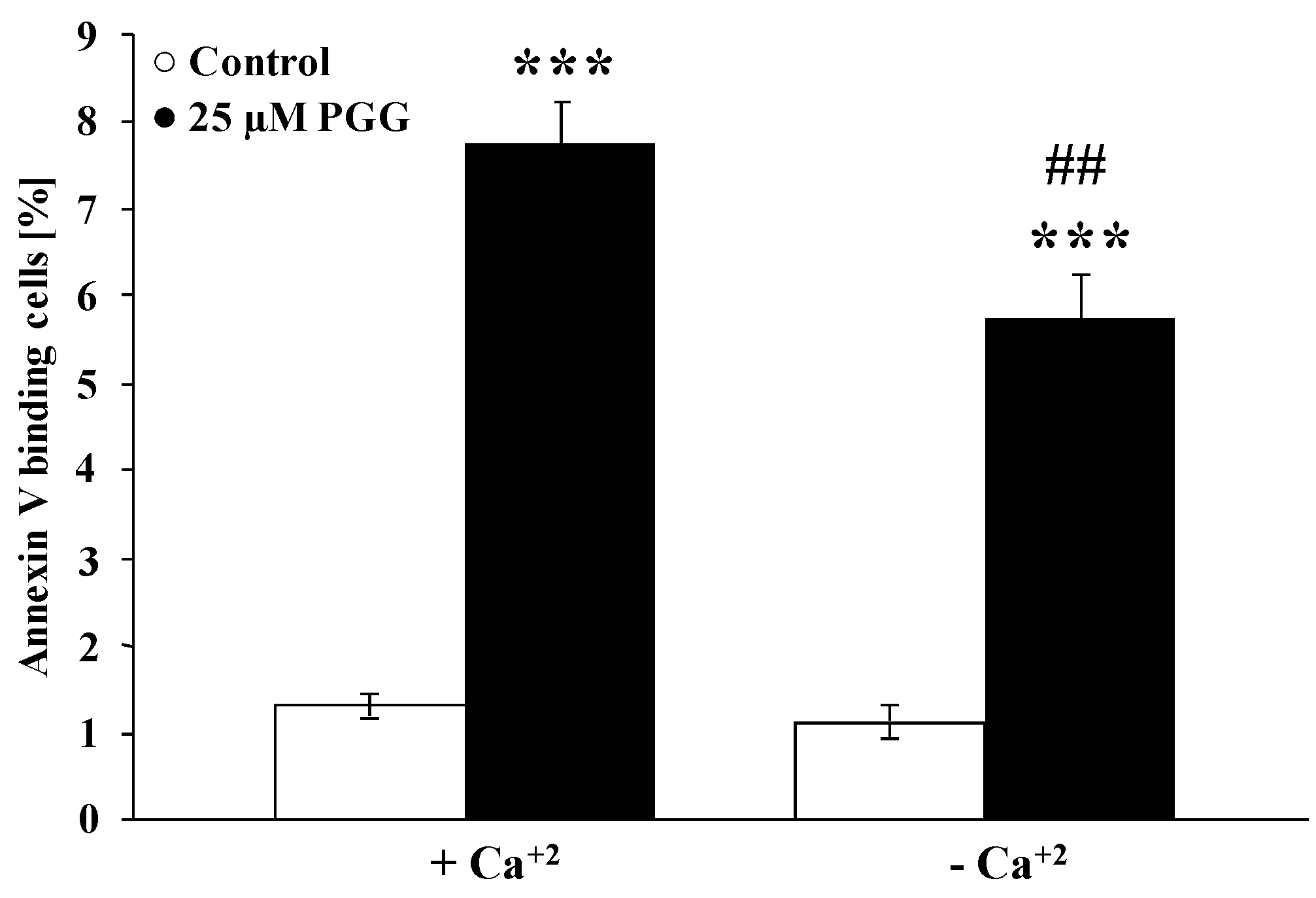

3. Experimental Section
3.1. Erythrocytes, Solutions, and Chemicals
3.2. Confocal Microscopy and Immunofluorescence
3.3. FACS Analysis of Annexin V Binding and Forward Scatter
3.4. Measurement of Intracellular Ca2+
3.5. Measurement of Hemolysis
3.6. Determination of Ceramide Formation
3.7. Confocal Microscopy and Immunofluorescence
3.8. Statistics
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Zhang, J.; Li, L.; Kim, S.H.; Hagerman, A.E.; Lu, J. Anti-cancer, anti-diabetic and other pharmacologic and biological activities of penta-galloyl-glucose. Pharm. Res. 2009, 26, 2066–2080. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, J.; Lee, H.J.; Kim, S.H.; Lu, J. Penta-O-galloyl-beta-d-glucose induces s- and g(1)-cell cycle arrests in prostate cancer cells targeting DNA replication and cyclin d1. Carcinogenesis 2009, 30, 818–823. [Google Scholar] [CrossRef]
- Chai, Y.; Lee, H.J.; Shaik, A.A.; Nkhata, K.; Xing, C.; Zhang, J.; Jeong, S.J.; Kim, S.H.; Lu, J. Penta-O-galloyl-beta-d-glucose induces g1 arrest and DNA replicative s-phase arrest independently of cyclin-dependent kinase inhibitor 1a, cyclin-dependent kinase inhibitor 1b and p53 in human breast cancer cells and is orally active against triple negative xenograft growth. Breast Cancer Res. 2010, 12, R67. [Google Scholar] [CrossRef]
- Oh, G.S.; Pae, H.O.; Oh, H.; Hong, S.G.; Kim, I.K.; Chai, K.Y.; Yun, Y.G.; Kwon, T.O.; Chung, H.T. In vitro anti-proliferative effect of 1,2,3,4,6-penta-O-galloyl-beta-d-glucose on human hepatocellular carcinoma cell line, sk-hep-1 cells. Cancer Lett. 2001, 174, 17–24. [Google Scholar] [CrossRef]
- Kiss, A.K.; Filipek, A.; Zyzynska-Granica, B.; Naruszewicz, M. Effects of penta-O-galloyl-beta-d-glucose on human neutrophil function: Significant down-regulation of l-selectin expression. Phytother. Res. 2013, 27, 986–992. [Google Scholar] [CrossRef]
- Lee, H.J.; Jeong, S.J.; Lee, H.J.; Lee, E.O.; Bae, H.; Lieske, J.C.; Kim, S.H. 1,2,3,4,6-penta-O-galloyl-beta-d-glucose reduces renal crystallization and oxidative stress in a hyperoxaluric rat model. Kidney Int. 2011, 79, 538–545. [Google Scholar] [CrossRef]
- Bing, S.J.; Kim, M.J.; Park, E.; Ahn, G.; Kim, D.S.; Ko, R.K.; Lee, N.H.; Shin, T.; Park, J.W.; Jee, Y. 1,2,3,4,6-Penta-O-galloyl-beta-d-glucose protects splenocytes against radiation-induced apoptosis in murine splenocytes. Biol. Pharm. Bull. 2010, 33, 1122–1127. [Google Scholar] [CrossRef]
- Huang, C.; Lee, S.Y.; Lin, C.L.; Tu, T.H.; Chen, L.H.; Chen, Y.J.; Huang, H.C. Co-treatment with quercetin and 1,2,3,4,6-penta-O-galloyl-beta-d-glucose causes cell cycle arrest and apoptosis in human breast cancer mda-mb-231 and au565 cells. J. Agric. Food Chem. 2013, 61, 6430–6445. [Google Scholar] [CrossRef]
- Kwon, T.R.; Jeong, S.J.; Lee, H.J.; Lee, H.J.; Sohn, E.J.; Jung, J.H.; Kim, J.H.; Jung, D.B.; Lu, J.; Kim, S.H. Reactive oxygen species-mediated activation of jnk and down-regulation of daxx are critically involved in penta-O-galloyl-beta-d-glucose-induced apoptosis in chronic myeloid leukemia k562 cells. Biochem. Biophys. Res. Commun. 2012, 424, 530–537. [Google Scholar] [CrossRef]
- Ryu, H.G.; Jeong, S.J.; Kwon, H.Y.; Lee, H.J.; Lee, E.O.; Lee, M.H.; Choi, S.H.; Ahn, K.S.; Kim, S.H. Penta-O-galloyl-beta-d-glucose attenuates cisplatin-induced nephrotoxicity via reactive oxygen species reduction in renal epithelial cells and enhances antitumor activity in caki-2 renal cancer cells. Toxicol. in Vitro 2012, 26, 206–214. [Google Scholar] [CrossRef]
- Lee, H.J.; Seo, N.J.; Jeong, S.J.; Park, Y.; Jung, D.B.; Koh, W.; Lee, H.J.; Lee, E.O.; Ahn, K.S.; Ahn, K.S.; et al. Oral administration of penta-O-galloyl-beta-d-glucose suppresses triple-negative breast cancer xenograft growth and metastasis in strong association with jak1-stat3 inhibition. Carcinogenesis 2011, 32, 804–811. [Google Scholar] [CrossRef]
- Huh, J.E.; Lee, E.O.; Kim, M.S.; Kang, K.S.; Kim, C.H.; Cha, B.C.; Surh, Y.J.; Kim, S.H. Penta-O-galloyl-beta-d-glucose suppresses tumor growth via inhibition of angiogenesis and stimulation of apoptosis: Roles of cyclooxygenase-2 and mitogen-activated protein kinase pathways. Carcinogenesis 2005, 26, 1436–1445. [Google Scholar] [CrossRef]
- Pan, M.H.; Lin, J.H.; Lin-Shiau, S.Y.; Lin, J.K. Induction of apoptosis by penta-O-galloyl-beta-d-glucose through activation of caspase-3 in human leukemia hl-60 cells. Eur. J. Pharmacol. 1999, 381, 171–183. [Google Scholar] [CrossRef]
- Park, E.J.; Zhao, Y.Z.; An, R.B.; Kim, Y.C.; Sohn, D.H. 1,2,3,4,6-Penta-O-galloyl-beta-d-glucose from galla rhois protects primary rat hepatocytes from necrosis and apoptosis. Planta Med. 2008, 74, 1380–1383. [Google Scholar] [CrossRef]
- Lang, E.; Qadri, S.M.; Lang, F. Killing me softly—Suicidal erythrocyte death. Int. J.Biochem. Cell.Biol. 2012, 44, 1236–1243. [Google Scholar] [CrossRef]
- Foller, M.; Sopjani, M.; Koka, S.; Gu, S.; Mahmud, H.; Wang, K.; Floride, E.; Schleicher, E.; Schulz, E.; Munzel, T.; et al. Regulation of erythrocyte survival by amp-activated protein kinase. FASEB J. 2009, 23, 1072–1080. [Google Scholar] [CrossRef]
- Bookchin, R.M.; Ortiz, O.E.; Lew, V.L. Activation of calcium-dependent potassium channels in deoxygenated sickled red cells. Prog. Clin. Biol. Res. 1987, 240, 193–200. [Google Scholar]
- Brugnara, C.; De Franceschi, L.; Alper, S.L. Inhibition of Ca(2+)-dependent K+ transport and cell dehydration in sickle erythrocytes by clotrimazole and other imidazole derivatives. J. Clin. Invest. 1993, 92, 520–526. [Google Scholar] [CrossRef]
- Lang, P.A.; Kaiser, S.; Myssina, S.; Wieder, T.; Lang, F.; Huber, S.M. Role of Ca2+-activated K+ channels in human erythrocyte apoptosis. Am. J. Physiol. Cell. Physiol. 2003, 285, C1553–C1560. [Google Scholar] [CrossRef]
- Berg, C.P.; Engels, I.H.; Rothbart, A.; Lauber, K.; Renz, A.; Schlosser, S.F.; Schulze-Osthoff, K.; Wesselborg, S. Human mature red blood cells express caspase-3 and caspase-8, but are devoid of mitochondrial regulators of apoptosis. Cell. Death Differ. 2001, 8, 1197–1206. [Google Scholar] [CrossRef]
- Lau, I.P.; Chen, H.; Wang, J.; Ong, H.C.; Leung, K.C.; Ho, H.P.; Kong, S.K. In vitro effect of ctab- and peg-coated gold nanorods on the induction of eryptosis/erythroptosis in human erythrocytes. Nanotoxicology 2011. [Google Scholar] [CrossRef]
- Maellaro, E.; Leoncini, S.; Moretti, D.; Del Bello, B.; Tanganelli, I.; De Felice, C.; Ciccoli, L. Erythrocyte caspase-3 activation and oxidative imbalance in erythrocytes and in plasma of type 2 diabetic patients. Acta Diabetol. 2011. [Google Scholar] [CrossRef]
- Foller, M.; Feil, S.; Ghoreschi, K.; Koka, S.; Gerling, A.; Thunemann, M.; Hofmann, F.; Schuler, B.; Vogel, J.; Pichler, B.; et al. Anemia and splenomegaly in cgki-deficient mice. Proc. Natl. Acad. Sci. USA 2008, 105, 6771–6776. [Google Scholar] [CrossRef]
- Bhavsar, S.K.; Gu, S.; Bobbala, D.; Lang, F. Janus kinase 3 is expressed in erythrocytes, phosphorylated upon energy depletion and involved in the regulation of suicidal erythrocyte death. Cell. Physiol. Biochem. 2011, 27, 547–556. [Google Scholar] [CrossRef]
- Kucherenko, Y.V.; Huber, S.M.; Nielsen, S.; Lang, F. Decreased redox-sensitive erythrocyte cation channel activity in aquaporin 9-deficient mice. J. Membr. Biol. 2012, 245, 797–805. [Google Scholar] [CrossRef]
- Zelenak, C.; Eberhard, M.; Jilani, K.; Qadri, S.M.; Macek, B.; Lang, F. Protein kinase ck1alpha regulates erythrocyte survival. Cell. Physiol. Biochem. 2012, 29, 171–180. [Google Scholar] [CrossRef]
- Gatidis, S.; Zelenak, C.; Fajol, A.; Lang, E.; Jilani, K.; Michael, D.; Qadri, S.M.; Lang, F. P38 mapk activation and function following osmotic shock of erythrocytes. Cell. Physiol. Biochem. 2011, 28, 1279–1286. [Google Scholar] [CrossRef]
- Lupescu, A.; Shaik, N.; Jilani, K.; Zelenak, C.; Lang, E.; Pasham, V.; Zbidah, M.; Plate, A.; Bitzer, M.; Foller, M.; et al. Enhanced erythrocyte membrane exposure of phosphatidylserine following sorafenib treatment: An in vivo and in vitro study. Cell. Physiol. Biochem. 2012, 30, 876–888. [Google Scholar] [CrossRef]
- Shaik, N.; Lupescu, A.; Lang, F. Sunitinib-sensitive suicidal erythrocyte death. Cell. Physiol. Biochem. 2012, 30, 512–522. [Google Scholar] [CrossRef]
- Morad, S.A.; Cabot, M.C. Ceramide-orchestrated signalling in cancer cells. Nat. Rev. Cancer 2013, 13, 51–65. [Google Scholar] [CrossRef]
- Borst, O.; Abed, M.; Alesutan, I.; Towhid, S.T.; Qadri, S.M.; Foller, M.; Gawaz, M.; Lang, F. Dynamic adhesion of eryptotic erythrocytes to endothelial cells via cxcl16/sr-psox. Am. J. Physiol. Cell. Physiol. 2012, 302, C644–C651. [Google Scholar] [CrossRef]
- Andrews, D.A.; Low, P.S. Role of red blood cells in thrombosis. Curr. Opin. Hematol. 1999, 6, 76–82. [Google Scholar] [CrossRef]
- Closse, C.; Dachary-Prigent, J.; Boisseau, M.R. Phosphatidylserine-related adhesion of human erythrocytes to vascular endothelium. Br. J. Haematol. 1999, 107, 300–302. [Google Scholar] [CrossRef]
- Gallagher, P.G.; Chang, S.H.; Rettig, M.P.; Neely, J.E.; Hillery, C.A.; Smith, B.D.; Low, P.S. Altered erythrocyte endothelial adherence and membrane phospholipid asymmetry in hereditary hydrocytosis. Blood 2003, 101, 4625–4627. [Google Scholar] [CrossRef]
- Pandolfi, A.; Di Pietro, N.; Sirolli, V.; Giardinelli, A.; Di Silvestre, S.; Amoroso, L.; Di Tomo, P.; Capani, F.; Consoli, A.; Bonomini, M. Mechanisms of uremic erythrocyte-induced adhesion of human monocytes to cultured endothelial cells. J. Cell. Physiol. 2007, 213, 699–709. [Google Scholar] [CrossRef]
- Wood, B.L.; Gibson, D.F.; Tait, J.F. Increased erythrocyte phosphatidylserine exposure in sickle cell disease: Flow-cytometric measurement and clinical associations. Blood 1996, 88, 1873–1880. [Google Scholar]
- Chung, S.M.; Bae, O.N.; Lim, K.M.; Noh, J.Y.; Lee, M.Y.; Jung, Y.S.; Chung, J.H. Lysophosphatidic acid induces thrombogenic activity through phosphatidylserine exposure and procoagulant microvesicle generation in human erythrocytes. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 414–421. [Google Scholar]
- Zwaal, R.F.; Comfurius, P.; Bevers, E.M. Surface exposure of phosphatidylserine in pathological cells. Cell. Mol. Life Sci. 2005, 62, 971–988. [Google Scholar] [CrossRef]
- Braun, M.; Foller, M.; Gulbins, E.; Lang, F. Eryptosis triggered by bismuth. Biometals 2009, 22, 453–460. [Google Scholar] [CrossRef]
- Felder, K.M.; Hoelzle, K.; Ritzmann, M.; Kilchling, T.; Schiele, D.; Heinritzi, K.; Groebel, K.; Hoelzle, L.E. Hemotrophic mycoplasmas induce programmed cell death in red blood cells. Cell. Physiol. Biochem. 2011, 27, 557–564. [Google Scholar] [CrossRef]
- Ghashghaeinia, M.; Toulany, M.; Saki, M.; Bobbala, D.; Fehrenbacher, B.; Rupec, R.; Rodemann, H.P.; Ghoreschi, K.; Rocken, M.; Schaller, M.; et al. The nfkb pathway inhibitors bay 11-7082 and parthenolide induce programmed cell death in anucleated erythrocytes. Cell. Physiol. Biochem. 2011, 27, 45–54. [Google Scholar] [CrossRef]
- Lang, E.; Jilani, K.; Zelenak, C.; Pasham, V.; Bobbala, D.; Qadri, S.M.; Lang, F. Stimulation of suicidal erythrocyte death by benzethonium. Cell. Physiol. Biochem. 2011, 28, 347–354. [Google Scholar] [CrossRef]
- Nguyen, D.B.; Wagner-Britz, L.; Maia, S.; Steffen, P.; Wagner, C.; Kaestner, L.; Bernhardt, I. Regulation of phosphatidylserine exposure in red blood cells. Cell. Physiol. Biochem. 2011, 28, 847–856. [Google Scholar] [CrossRef]
- Qadri, S.M.; Bauer, J.; Zelenak, C.; Mahmud, H.; Kucherenko, Y.; Lee, S.H.; Ferlinz, K.; Lang, F. Sphingosine but not sphingosine-1-phosphate stimulates suicidal erythrocyte death. Cell. Physiol. Biochem. 2011, 28, 339–346. [Google Scholar] [CrossRef]
- Qadri, S.M.; Kucherenko, Y.; Lang, F. Beauvericin induced erythrocyte cell membrane scrambling. Toxicology 2011, 283, 24–31. [Google Scholar] [CrossRef]
- Qadri, S.M.; Kucherenko, Y.; Zelenak, C.; Jilani, K.; Lang, E.; Lang, F. Dicoumarol activates ca-permeable cation channels triggering erythrocyte cell membrane scrambling. Cell. Physiol. Biochem. 2011, 28, 857–864. [Google Scholar] [CrossRef]
- Zbidah, M.; Lupescu, A.; Shaik, N.; Lang, F. Gossypol-induced suicidal erythrocyte death. Toxicology 2012, 302, 101–105. [Google Scholar] [CrossRef]
- Abed, M.; Towhid, S.T.; Shaik, N.; Lang, F. Stimulation of suicidal death of erythrocytes by rifampicin. Toxicology 2012, 302, 123–128. [Google Scholar] [CrossRef]
- Lupescu, A.; Jilani, K.; Zbidah, M.; Lang, F. Induction of apoptotic erythrocyte death by rotenone. Toxicology 2012, 300, 132–137. [Google Scholar] [CrossRef]
- Lang, E.; Modicano, P.; Arnold, M.; Bissinger, R.; Faggio, C.; Abed, M.; Lang, F. Effect of thioridazine on erythrocytes. Toxins 2013, 5, 1918–1931. [Google Scholar] [CrossRef]
- Calderon-Salinas, J.V.; Munoz-Reyes, E.G.; Guerrero-Romero, J.F.; Rodriguez-Moran, M.; Bracho-Riquelme, R.L.; Carrera-Gracia, M.A.; Quintanar-Escorza, M.A. Eryptosis and oxidative damage in type 2 diabetic mellitus patients with chronic kidney disease. Mol. Cell. Biochem. 2011, 357, 171–179. [Google Scholar] [CrossRef]
- Myssina, S.; Huber, S.M.; Birka, C.; Lang, P.A.; Lang, K.S.; Friedrich, B.; Risler, T.; Wieder, T.; Lang, F. Inhibition of erythrocyte cation channels by erythropoietin. J. Am. Soc. Nephrol. 2003, 14, 2750–2757. [Google Scholar] [CrossRef]
- Lang, P.A.; Beringer, O.; Nicolay, J.P.; Amon, O.; Kempe, D.S.; Hermle, T.; Attanasio, P.; Akel, A.; Schafer, R.; Friedrich, B.; et al. Suicidal death of erythrocytes in recurrent hemolytic uremic syndrome. J. Mol. Med. 2006, 84, 378–388. [Google Scholar] [CrossRef]
- Kempe, D.S.; Akel, A.; Lang, P.A.; Hermle, T.; Biswas, R.; Muresanu, J.; Friedrich, B.; Dreischer, P.; Wolz, C.; Schumacher, U.; et al. Suicidal erythrocyte death in sepsis. J. Mol. Med. 2007, 85, 273–281. [Google Scholar] [CrossRef]
- Lang, P.A.; Kasinathan, R.S.; Brand, V.B.; Duranton, C.; Lang, C.; Koka, S.; Shumilina, E.; Kempe, D.S.; Tanneur, V.; Akel, A.; et al. Accelerated clearance of plasmodium-infected erythrocytes in sickle cell trait and annexin-a7 deficiency. Cell. Physiol. Biochem. 2009, 24, 415–428. [Google Scholar] [CrossRef]
- Foller, M.; Bobbala, D.; Koka, S.; Huber, S.M.; Gulbins, E.; Lang, F. Suicide for survival—Death of infected erythrocytes as a host mechanism to survive malaria. Cell. Physiol. Biochem. 2009, 24, 133–140. [Google Scholar] [CrossRef]
- Lang, P.A.; Schenck, M.; Nicolay, J.P.; Becker, J.U.; Kempe, D.S.; Lupescu, A.; Koka, S.; Eisele, K.; Klarl, B.A.; Rubben, H.; et al. Liver cell death and anemia in wilson disease involve acid sphingomyelinase and ceramide. Nat. Med. 2007, 13, 164–170. [Google Scholar] [CrossRef]
- Kempe, D.S.; Lang, P.A.; Duranton, C.; Akel, A.; Lang, K.S.; Huber, S.M.; Wieder, T.; Lang, F. Enhanced programmed cell death of iron-deficient erythrocytes. FASEB J. 2006, 20, 368–370. [Google Scholar]
- Birka, C.; Lang, P.A.; Kempe, D.S.; Hoefling, L.; Tanneur, V.; Duranton, C.; Nammi, S.; Henke, G.; Myssina, S.; Krikov, M.; et al. Enhanced susceptibility to erythrocyte “apoptosis” following phosphate depletion. Pflugers. Arch. 2004, 448, 471–477. [Google Scholar]
- Zappulla, D. Environmental stress, erythrocyte dysfunctions, inflammation, and the metabolic syndrome: Adaptations to CO2 increases? J. Cardiometab. Syndr. 2008, 3, 30–34. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0).
Share and Cite
Alzoubi, K.; Honisch, S.; Abed, M.; Lang, F. Triggering of Suicidal Erythrocyte Death by Penta-O-galloyl-β-d-glucose. Toxins 2014, 6, 54-65. https://doi.org/10.3390/toxins6010054
Alzoubi K, Honisch S, Abed M, Lang F. Triggering of Suicidal Erythrocyte Death by Penta-O-galloyl-β-d-glucose. Toxins. 2014; 6(1):54-65. https://doi.org/10.3390/toxins6010054
Chicago/Turabian StyleAlzoubi, Kousi, Sabina Honisch, Majed Abed, and Florian Lang. 2014. "Triggering of Suicidal Erythrocyte Death by Penta-O-galloyl-β-d-glucose" Toxins 6, no. 1: 54-65. https://doi.org/10.3390/toxins6010054
APA StyleAlzoubi, K., Honisch, S., Abed, M., & Lang, F. (2014). Triggering of Suicidal Erythrocyte Death by Penta-O-galloyl-β-d-glucose. Toxins, 6(1), 54-65. https://doi.org/10.3390/toxins6010054




