Estimation of Multi-Mycotoxin Contamination in South African Compound Feeds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling and Sample Preparation
| Compound Feed | N a | Major ingredients |
|---|---|---|
| Poultry | 62 | |
| Breeder | 14 | Cereals (mainly maize), maize germ, soya oilcake, sunflower oilcake |
| Broiler | 28 | Cereals (mainly maize), maize germ, soya oilcake, sunflower oilcake |
| Layer | 20 | Cereals (mainly maize), maize germ, soya oilcake, sunflower oilcake |
| Cattle b | 25 | |
| Calf grower | 8 | Cereals (mainly maize), maize germ, cottonseed, soya oilcake, sunflower oilcake |
| Dairy | 11 | Cereals (mainly maize), maize germ, cottonseed, sunflower oilcake, Lucerne meal |
| Finisher | 6 | Cereals (mainly maize), maize germ, cottonseed, sunflower oilcake, Lucerne meal |
| Others | 5 | |
| Horse | 3 | Cereals (mainly maize), maize germ, full fat soya, Lucerne meal |
| Swine | 2 | Cereals (mainly maize), maize germ, soya oilcake, sunflower oilcake |
| Total | 92 |
2.2. Experimental
2.2.1. Reagents and Apparatus
2.2.2. Methods of Mycotoxin Analyses
2.2.2.1. Dexynivalenol, Fumonisin B1, Fumonisin B2, HT-2, T-2, Ochratoxin and Zearalenone
| ESI | SRM Transition (m/z→m/z) | Collision | ~tR | ||
|---|---|---|---|---|---|
| Mycotoxin | (+ or -) | Quantitative | Qualitative | Energy | (min) |
| DON | - | 341.0→265.0 | 341.0→247.0 | 18 | 6.1 |
| HT2 | + | 442.2→263.0 | 442.2→215.0 | 18 | 8.9 |
| T2 | + | 484.2→185.0 | 484.2→305.0 | 18 | 9.3 |
| ZON | - | 317.0→174.5 | 317.0→130.5 | 25 | 10.1 |
| FB1 | + | 722.5→334.4 | 722.5→352.2 | 40 | 9.2 |
| OTA | + | 404.0→239.0 | 404.0→358.4 | 20 | 9.9 |
| FB2 | + | 706.4→336.2 | 706.4→354.2 | 35 | 10.2 |
| Meloxicam (IS) | + | - | 352.0→115.0 | 20 | 8.6/9.1 |
2.2.2.2. Aflatoxins
2.2.2.3. Method Performance and Quantification of Mycotoxins
| Mycotoxin | Spiking mycotoxins levels(µg/kg) a | Apparent Recovery b (%) | LOD (µg/kg) | LOQ (µg/kg) | Expanded Measurement Uncertainty c (%) |
|---|---|---|---|---|---|
| DON | 300 | 106 ± 5.6 | 72 | 115 | 30 |
| ZON | 30.0 | 127 ± 26.0 | 3.5 | 5.2 | 40 |
| T2 | 40.0 | 149 ± 27.2 | 1.0 | 2.5 | 17 |
| HT2 | 40.0 | 167 ± 17.3 | 2.5 | 8.0 | 18 |
| FB1 | 100 | 104 ± 9.5 | 9.0 | 30 | 32 |
| FB2 | 100 | 113 ± 13.4 | 30 | 53 | 30 |
| OTA | 5.20 | 145 ± 14.5 | 2.7 | 4.6 | 33 |
| AFB1 | 2.70 | 94 ± 14.5 | 0.10 | 0.20 | 32 |
| AFB2 | 1.20 | 104 ± 11.5 | 0.10 | 0.20 | 32 |
| AFG1 | 2.10 | 100 ± 21.8 | 0.10 | 0.20 | 32 |
| AFG2 | 2.10 | 105 ± 10.9 | 0.10 | 0.20 | 32 |
3. Results and Discussion
| Mycotoxins contamination a | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AF | FB | OTA | DON | ZEA | ||||||||||||
| Feed | N b | N + ve c | Mean d | Max | N + ve c | Mean d | Max | N + ve c | Mean d | Max | N +vec | Mean d | Max | N + ve c | Mean d | Max |
| Chicken | 62 | 14 (8) | 0.7 ± 0.7 | 1.8 | 62 (54) | 903 ± 757 | 2999 | 0 (0) | - | - | 62 (35) | 620 ± 386 | 1980 | 62 (24) | 100 ± 125 | 610 |
| Cattle | 25 | 13 (13) | 14.7 ± 22.8 | 71.8 | 25 (23) | 975 ± 686 | 2497 | 4 (4) | 9.9 ± 4.9 | 17.1 | 24 (24) | 891 ± 512 | 2280 | 24 (19) | 72 ± 43 | 123 |
| Horse | 3 | 1 (1) | 0.4 ± 0 | 0.4 | 3 (3) | 693 ± 617 | 1338 | 0 (0) | - | - | 3 (3) | 742 ± 338 | 1133 | 3 (2) | 43 ± 5 | 46 |
| Swine | 2 | 0 (0) | - | - | 2 (1) | 313 ± 0 | 313 | 0 (0) | - | - | 2 (2) | 1,875 ± 713 | 2352 | 2 (2) | 148 ± 30 | 170 |
| Total | 92 | 28 (22) | 9.0 ± 18.7 | 71.8 | 92 (81) | 916 ± 727 | 2999 | 4 (4) | 9.9 ± 4.9 | 17.1 | 91 (64) | 696 ± 490 | 2352 | 91 (47) | 88 ± 95 | 610 |
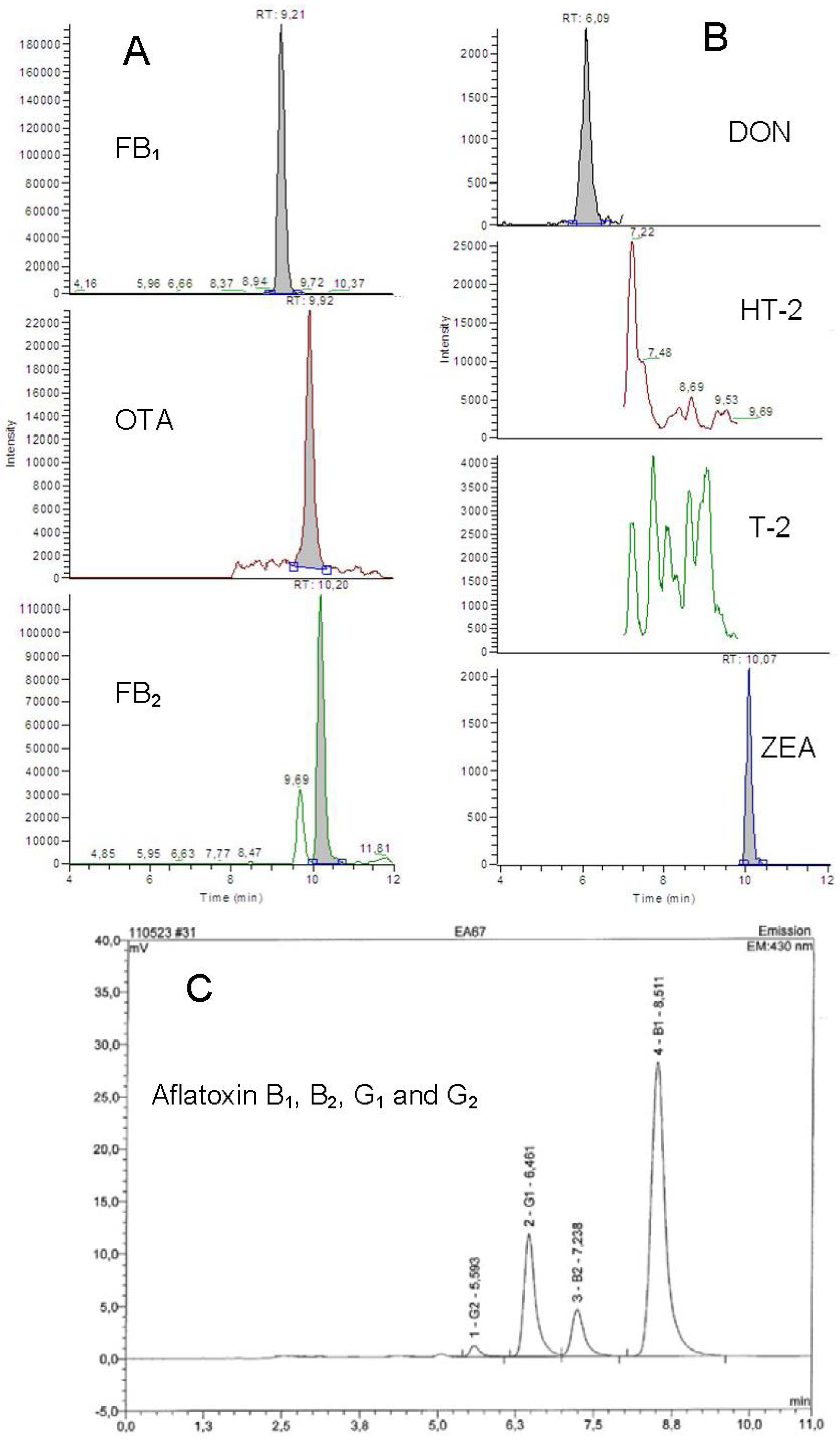
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Cusumano, V.; Rossano, F.; Merendino, R.A.; Arena, A.; Costa, G.B.; Mancuso, G.; Baroni, A.; Losi, E. Immunobiological activities of mould products: functional impairment of human monocytes exposed to aflatoxin B1. Res. Microbiol. 1996, 147, 385–391. [Google Scholar] [CrossRef]
- Sharma, R.P. Immunotoxicity of mycotoxins. J. Dairy Sci. 1993, 76, 892–897. [Google Scholar] [CrossRef]
- Marguardt, R.R. Effects of molds and their toxins on livestock performance: A western Canadian perspective. Anim. Feed Sci. Technol. 1996, 58, 77–89. [Google Scholar] [CrossRef]
- Fink-Gremmels, J.; Malekinejad, H. Clinical effects and biochemical mechanisms associated with exposure to the mycoestrogen zearalenone. Anim. Feed Sci. Technol. 2007, 137, 326–341. [Google Scholar] [CrossRef]
- Duarte, S.C.; Lino, C.M.; Pena, A. Ochratoxin A in feed of food-producing animals: An undesirable mycotoxin with health and performance effects. Vet. Microbiol. 2011, 154, 1–13. [Google Scholar] [CrossRef]
- World Health Organization. AFRO Food Safety Newsletter. World Health Organization Food safety (FOS). Mycotoxins in African Foods: Implications to Food Safety and Health. Issue No. 2. July 2006. Available online: http://www.google.com.hk/url?sa=t&rct=j&q= Mycotoxins+in+African+foods:+Implications+to+food+safety+and+health&source=web&cd=1&cad=rja&ved=0CCoQFjAA&url=http%3A%2F%2Fwww.afro.who.int%2Findex.php%3Foption%3Dcom_docman%26task%3Ddoc_download%26gid%3D1724&ei=EfB8UJqlKKqk4gSG8oGYCg&usg=AFQjCNGv5Dli0nQVuHsZFN6QHh7fKG5DNw (accessed on 4 October 2011).
- Bastianello, S.S.; Nesbit, J.W.; Williams, M.C.; Lange, A.L. Pathological findings in a natural outbreak of aflatoxicosis in dogs. Onderstepoort J. Vet. Res. 1987, 54, 635–640. [Google Scholar]
- Arnot, L.F.; Duncan, N.M.; Coetzer, H.; Botha, C.J. An outbreak of canine aflatoxicosis in Gauteng Province, South Africa. J. S. Afr. Vet. Assoc. 2012, 83, 1–4. [Google Scholar]
- Mbambo, S. National Department of Agriculture, Forestry and Fisheries, Pretoria, Gauteng, South Africa. Personal communication, 2011. [Google Scholar]
- Reiter, E.V.; Dutton, M.F.; Mwanza, M.; Agus, A.; Prawano, D.; Häggblom, P.; Razzazi-Fazeli, E.; Zentek, J.; Andersson, G.; Njobeh, P.B. Uncertainty from sampling in measurement of aflatoxins in animal feedstuffs: Application of the Eurachem/CITAC guidelines. Analyst 2011, 136, 4059–4069. [Google Scholar] [CrossRef]
- Tevell Åberg, A.; Solyakov, A.; Bondesson, U. Development and in-house validation of an LC-MS/MS method for the simultaneous quantification of the mycotoxins deoxynivalenol, zearalenone, T-2 and HT-2 toxin, ochratoxin A, and fumonisin B1 and B2 in vegetable animal feed. Food Addit. Contam. Part A 2012. submitted for publication. [Google Scholar]
- Barmark, A.L.; Larsson, K. Immunoaffinity column cleanup/liquid chromatographic determination of aflatoxins: An interlaboratory study. J. AOAC Int. 1994, 77, 46–53. [Google Scholar]
- Government Gazette, Fertilizers, Farm Feeds, Agricultural Remedies and Stock Remedies Act (Act No. 36 of 1947). No. R70; Pretoria, South Africa, 2010; pp. 4–18.
- Gelderblom, W.C.A.; Jaskiewics, K.; Marasas, W.F.O.; Thiel, P.G.; Horak, M.J.; Vleggaar, R.; Kriek, N.P.J. Fumonisin-novel mycotoxins with cancer promoting activity produced by Fusarium moniliforme. Appl. Environ. Microbiol. 1988, 54, 1806–1811. [Google Scholar]
- Dutton, M.F.; Kinsey, A. Occurrence of mycotoxins in cereals and animal feedstuffs in Natal, South Africa 1994. Mycopathologia 1996, 131, 31–36. [Google Scholar] [CrossRef]
- Mngadi, P.T.; Govinden, R.; Odhav, B. Co-occurring mycotoxins in animal feeds. Afr. J. Biotechnol. 2008, 7, 2239–2243. [Google Scholar]
- Griessler, K. A survey of mycotoxins in feed samples from South Africa. Pluimvee Poultry Bulletin 2009, 419. [Google Scholar]
- Stoev, S.D.; Denev, S.; Dutton, M.F.; Njobeh, P.B.; Mosonik, S.J.; Steenkamp, P.A.; Petkov, J. Complex etiology and pathology of mycotoxic nephropathy in South African pigs. Mycotoxin Res. 2010, 26, 31–46. [Google Scholar] [CrossRef]
- Rodrigues, I.; Handl, J.; Binder, E.M. Mycotoxin occurrence in commodities, feeds and feed ingredients sourced in the Middle East and Africa. Food Addit. Contam. Part B 2011, 4, 168–179. [Google Scholar] [CrossRef]
- Gonzalez, H.H.; Martinez, E.J.; Pacin, A.M.; Pacin, S.L.; Resnik, S.L.; Sydenham, E.W. Natural co-occurrence of fumonisins, deoxynivalenol, zearalenone and aflatoxins in field trial corn in Argentina. Food Addit. Contam. 1999, 16, 565–569. [Google Scholar] [CrossRef]
- Dragon, Y.P.; Bidlack, W.R.; Cohen, S.M.; Goldsworthy, T.L.; Hard, G.C.; Howard, P.C.; Riley, R.T.; Voss, K.A. Implications of apoptosis for toxicity, carcinogenicity, and risk assessment: Fumonisin B1 as an example. Toxicol. Sci. 2001, 61, 6–17. [Google Scholar] [CrossRef]
- Stoev, S.D.; Hald, B.; Mantle, P. Porcine nephropathy in Bulgaria: A progressive syndrome of complex of uncertain (mycotoxin) etiology. Vet. Rec. 1998, 142, 190–194. [Google Scholar] [CrossRef]
- Stoev, S.D. Complex etiology, prophylaxis and hygiene control in mycotoxic nephropathies in farm animals and humans. Int. J. Mol. Sci. 2008, 9, 578–605. [Google Scholar] [CrossRef]
- Stoev, S.D.; Dutton, M.F.; Njobeh, P.B.; Mosonik, S.J.; Steenkamp, P.A. Mycotoxic nephropathy in Bulgarian pigs and chickins: Complex ethiology and pathology. Food Addit. Contam. Part A 2010, 27, 72–88. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer, Toxins Derived from Fusarium graminearum, F. culmorum and F. crookwellense: Zearalenone, Deoxynivalenol, Nivalenol and Fusarenone X. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Some Naturally Occurring Substances; Food Items and Constituents, Hetercyclic Aromatic Amines and Mycotoxins; IARC: Lyon, France, 1993; 56, pp. 445–466.
- Benford, D.; Boyle, B.; Dekant, W.; Fuchs, R.; Gaylor, D.W.; Hard, G.; McGregor, D.B.; Pitt, J.I.; Plestina, R.; Shephard, G.; et al. Ochratoxin A; The Joint FAO/WHO Expert Committee on Food Additives: Geneva, Switzerland, 2001; pp. 1–172. [Google Scholar]
- Tanaka, T.; Hasegawa, A.; Yamamato, S.; Lee, S.; Sigiura, Y.; Ueno, Y. Worldwide contamination of cereals by Fusarium mycotoxins nivalenol, deoxynivalenol and zearalenone. A survey of 19 countries. J. Agric. Food. Chem. 1988, 36, 979–983. [Google Scholar] [CrossRef]
- Wu, X.; Murphy, P.; Cunnick, J.; Hendrich, S. Synthesis and characterization of deoxynivalenol glucuronide: Its comparative immunotoxicity with deoxynivalenol. Food Chem. Toxicol. 2007, 45, 1846–1855. [Google Scholar] [CrossRef]
- Sibanda, L.; Marovatsanga, L.T.; Pestka, J.J. Review of mycotoxin work in sub-Saharan Africa. Food Control 1997, 8, 21–29. [Google Scholar] [CrossRef]
- South African Grain Laboratories. Mycotoxin results over ten years-Summary of season 1999/2000 to 2008/. 2009. Available online: http://www.sagl.co.za (accessed on 25 October, 2011).
- Mashinini, K.; Dutton, M.F. The incidence of fungi and mycotoxins in South African wheat and wheat-based products. J. Environ. Sci. Health. Part B 2006, 41, 285–296. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EC) No 1881/2006 of 19 December, as Amended, on Setting Maximum Levels of Certain Contaminants in Foodstuffs. Off. J. Eur. Union 2006, 364, 5–24.
- Breytenbach, L. Animal Feed Manufacturers’ Association, Centurion, Gauteng, South Africa. Personal communication, 2011. [Google Scholar]
- Stoev, S.D.; Gundasheva, D.; Zarkov, I.; Mircheva, T.; Zapryanova, D.; Denev, S.; Mitev, Y.; Daskalov, H.; Dutton, M.; Mwanza, M. Experimental mycotoxic nephropathy in pigs provoked by a mouldy diet containing ochratoxin A and fumonisin B1. Exp. Toxicol. Pathol. 2011, in press. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Njobeh, P.B.; Dutton, M.F.; Åberg, A.T.; Haggblom, P. Estimation of Multi-Mycotoxin Contamination in South African Compound Feeds. Toxins 2012, 4, 836-848. https://doi.org/10.3390/toxins4100836
Njobeh PB, Dutton MF, Åberg AT, Haggblom P. Estimation of Multi-Mycotoxin Contamination in South African Compound Feeds. Toxins. 2012; 4(10):836-848. https://doi.org/10.3390/toxins4100836
Chicago/Turabian StyleNjobeh, Patrick B., Mike F. Dutton, Annica Tevell Åberg, and Per Haggblom. 2012. "Estimation of Multi-Mycotoxin Contamination in South African Compound Feeds" Toxins 4, no. 10: 836-848. https://doi.org/10.3390/toxins4100836
APA StyleNjobeh, P. B., Dutton, M. F., Åberg, A. T., & Haggblom, P. (2012). Estimation of Multi-Mycotoxin Contamination in South African Compound Feeds. Toxins, 4(10), 836-848. https://doi.org/10.3390/toxins4100836





