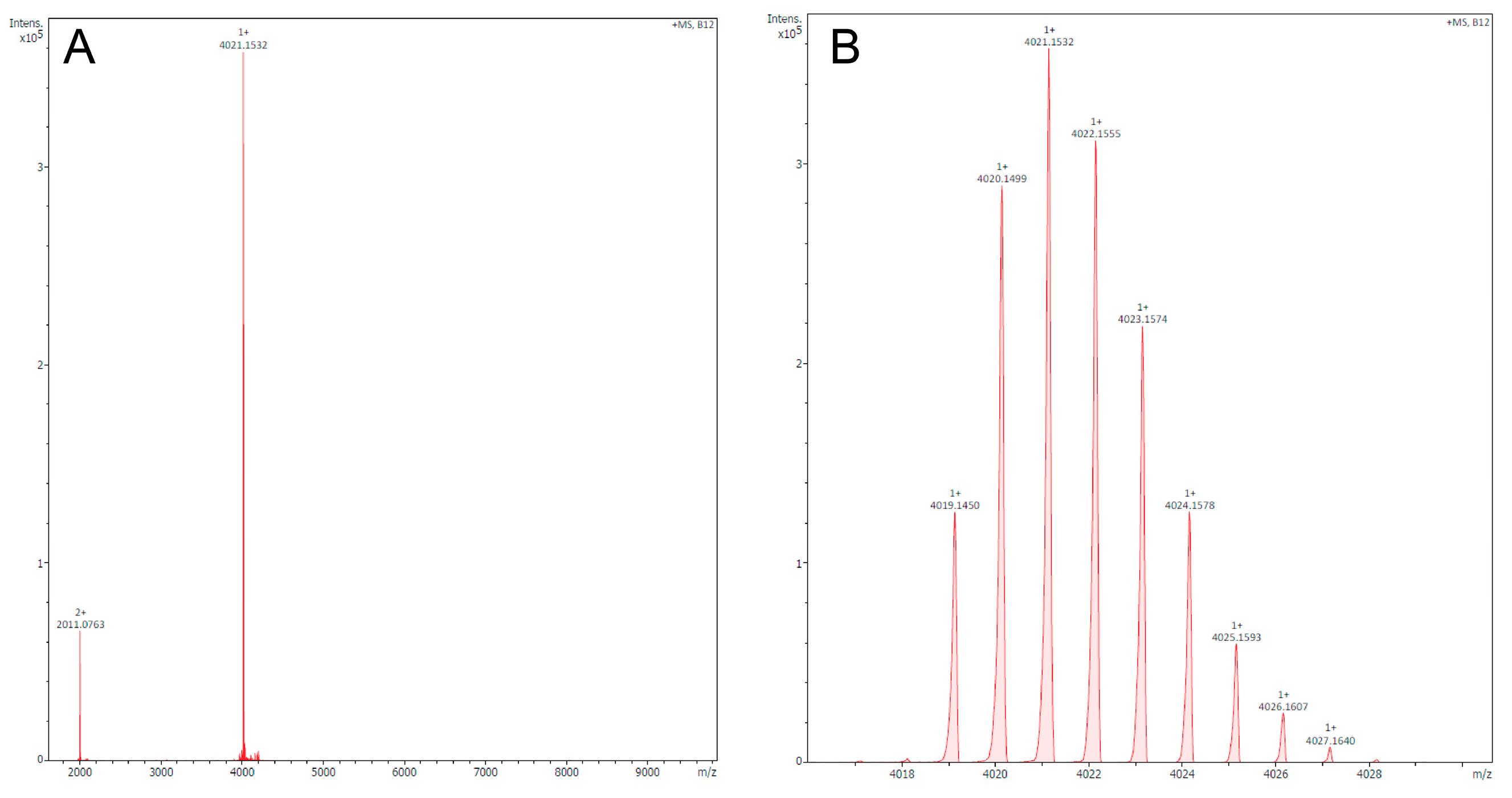
The Helix Ring Peptide U11 from the Venom of the Ant, Tetramorium bicarinatum, Acts as a Putative Pore-Forming Toxin, Not a New Kv1.3 Channel Blocker. Comment on Boy et al. A New Kv1.3 Channel Blocker from the Venom of the Ant Tetramorium bicarinatum. Toxins 2025, 17, 379
Conclusions
Conflicts of Interest
References
- Boy, G.; Jouvensal, L.; Téné, N.; Carayon, J.-L.; Bonnafé, E.; Paquet, F.; Treilhou, M.; Loth, K.; Billet, A. A New Kv1.3 Channel Blocker from the Venom of the Ant Tetramorium bicarinatum. Toxins 2025, 17, 379. [Google Scholar] [CrossRef] [PubMed]
- Barassé, V.; Jouvensal, L.; Boy, G.; Billet, A.; Ascoet, S.; Lefranc, B.; Leprince, J.; Dejean, A.; Lacotte, V.; Rahioui, I.; et al. Discovery of an Insect Neuroactive Helix Ring Peptide from Ant Venom. Toxins 2023, 15, 600. [Google Scholar] [CrossRef] [PubMed]
- Peigneur, S.; Tibery, D.; Tytgat, J. The Helix Ring Peptide U11 from the Venom of the Ant, Tetramorium bicarinatum, Acts as a Putative Pore-Forming Toxin. Membranes 2024, 14, 114. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Jiang, D.; Lan, X.; Liu, K.; Fan, C. Voltage-gated potassium channel 1.3: A promising molecular target in multiple disease therapy. Biomed. Pharmacother. 2024, 175, 116651. [Google Scholar] [CrossRef] [PubMed]
- Dauplais, M.; Lecoq, A.; Song, J.; Cotton, J.; Jamin, N.; Gilquin, B.; Roumestand, C.; Vita, C.; de Medeiros, C.L.C.; Rowan, E.G.; et al. On the convergent evolution of animal toxins. J. Biol. Chem. 1997, 272, 4302–4309. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, K.N.; Sivaraja, V.; Huys, I.; Sasaki, T.; Cheng, B.; Kumar, T.K.S.; Sato, K.; Tytgat, J.; Yu, C.; San, C.C.; et al. κ-Hefutoxin1, a Novel Toxin from the Scorpion Heterometrus fulvipes with unique structure and Function. J. Biol. Chem. 2002, 277, 30040–30047. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Peigneur, S.; Tibery, D.; Tytgat, J. The Helix Ring Peptide U11 from the Venom of the Ant, Tetramorium bicarinatum, Acts as a Putative Pore-Forming Toxin, Not a New Kv1.3 Channel Blocker. Comment on Boy et al. A New Kv1.3 Channel Blocker from the Venom of the Ant Tetramorium bicarinatum. Toxins 2025, 17, 379. Toxins 2026, 18, 44. https://doi.org/10.3390/toxins18010044
Peigneur S, Tibery D, Tytgat J. The Helix Ring Peptide U11 from the Venom of the Ant, Tetramorium bicarinatum, Acts as a Putative Pore-Forming Toxin, Not a New Kv1.3 Channel Blocker. Comment on Boy et al. A New Kv1.3 Channel Blocker from the Venom of the Ant Tetramorium bicarinatum. Toxins 2025, 17, 379. Toxins. 2026; 18(1):44. https://doi.org/10.3390/toxins18010044
Chicago/Turabian StylePeigneur, Steve, Diogo Tibery, and Jan Tytgat. 2026. "The Helix Ring Peptide U11 from the Venom of the Ant, Tetramorium bicarinatum, Acts as a Putative Pore-Forming Toxin, Not a New Kv1.3 Channel Blocker. Comment on Boy et al. A New Kv1.3 Channel Blocker from the Venom of the Ant Tetramorium bicarinatum. Toxins 2025, 17, 379" Toxins 18, no. 1: 44. https://doi.org/10.3390/toxins18010044
APA StylePeigneur, S., Tibery, D., & Tytgat, J. (2026). The Helix Ring Peptide U11 from the Venom of the Ant, Tetramorium bicarinatum, Acts as a Putative Pore-Forming Toxin, Not a New Kv1.3 Channel Blocker. Comment on Boy et al. A New Kv1.3 Channel Blocker from the Venom of the Ant Tetramorium bicarinatum. Toxins 2025, 17, 379. Toxins, 18(1), 44. https://doi.org/10.3390/toxins18010044





