Exploring the Potential of Genista ulicina Phytochemicals as Natural Biocontrol Agents: A Comparative In Vitro and In Silico Analysis
Abstract
1. Introduction
2. Results and Discussion
2.1. Identification of the Studied Plant
2.2. Extraction and Chemical Characterization
2.3. Antifungal Activity
2.4. Phytotoxic Activity
2.4.1. Phytotoxic Activity on Oxalis corniculata
2.4.2. Phytotoxic Activity on Euphorbia peplus
2.5. Molecular Docking Analyses
3. Conclusions
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Plant Material
4.3. Morphological Identification
4.4. Molecular Identification
4.5. Plant Extracts Preparation
4.6. GC-MS Analysis
4.7. Antifungal Assay
4.8. Leaf Puncture Assay
4.9. Statistical Analysis
4.10. Molecular Docking Analysis
4.10.1. Ligand Preparation
4.10.2. Protein Preparation
4.10.3. Validation by Re-Docking
4.10.4. Molecular Docking
4.10.5. Visualization and Interaction Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DCM | Dichloromethane |
References
- Rani, K.; Dhania, G. Bioremediation and biodegradation of pesticide from contaminated soil and water—A noval approach. Int. J. Curr. Microbiol. App. Sci. 2014, 3, 23–33. [Google Scholar]
- Rajmohan, K.-S.; Chandrasekaran, R.; Varjani, S. A review on occurrence of pesticides in environment and current technologies for their remediation and management. Indian J. Microbiol. 2020, 60, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Nega, A. Review on concepts in biological control of plant pathogens. J. Biol. Agric. Healthc. 2014, 4, 33–54. [Google Scholar]
- Zatout, R.; Benarbia, R.; Yalla, I. Phytochemical profiles and antimicrobial activities of Phellinus mushroom: Implications for agricultural health and crop protection. Agric. Res. J. 2024, 61, 340–345. [Google Scholar] [CrossRef]
- Hlaili, H.; Zorrilla, J.-G.; Salvatore, M.-M.; Abassi, M.; Russo, M.-T.; Martínez-González, M.-I.; Masi, M. Metabolite Screening From Pinus pinea Needles reveals (+)-isocupressic acid as a key phytotoxin for weed management. Phytochem. Anal. 2025. [Google Scholar] [CrossRef]
- Lengai, G.-M.; Muthomi, J.-W.; Mbega, E.-R. Phytochemical activity and role of botanical pesticides in pest management for sustainable agricultural crop production. Sci. Afr. 2020, 7, e00239. [Google Scholar] [CrossRef]
- Khursheed, A.; Rather, M.-A.; Jain, V.; Rasool, S.; Nazir, R.; Malik, N.-A.; Majid, S.-A. Plant based natural products as potential ecofriendly and safer biopesticides: A comprehensive overview of their advantages over conventional pesticides, limitations and regulatory aspects. Microb. Pathog. 2022, 173, 105854. [Google Scholar] [CrossRef]
- Vlaiculescu, A.; Varrone, C. Sustainable and eco-friendly alternatives to reduce the use of pesticides. In Pesticides in the Natural Environment; Elsevier: Amsterdam, The Netherlands, 2022; pp. 329–364. [Google Scholar]
- Verma, N.-S.; Kuldeep, D.-K.; Chouhan, M.; Prajapati, R.; Singh, S.-K. A review on eco-friendly pesticides and their rising importance in sustainable plant protection practices. Int. J. Plant Soil Sci. 2023, 35, 200–214. [Google Scholar] [CrossRef]
- He, B.; Hu, Y.; Wang, W.; Yan, W.; Ye, Y. The progress towards novel herbicide modes of action and targeted herbicide development. Agronomy 2022, 12, 2792. [Google Scholar] [CrossRef]
- Poslinski, H.; Hatley, M.; Tramell, J.; Song, B.-H. Harnessing phytochemicals in sustainable and green agriculture. J. Agric. Food Res. 2025, 19, 101633. [Google Scholar] [CrossRef]
- Del Prete, S.; Pagano, M. Enzyme inhibitors as multifaceted tools in medicine and agriculture. Molecules 2024, 29, 4314. [Google Scholar] [CrossRef] [PubMed]
- Aourahoun, K.-A.-K.; Fazouane, F.; Benayache, S.; Bettache, Z.-H.; Benayad, T.; Denni, N. Antioxidant and anti-inflammatory activity of phenolic extracts of Genista ferox (Fabaceae). Pak. J. Pharm. Sci. 2019, 32, 2643–2649. [Google Scholar] [PubMed]
- Laranjeira, I.-M.; Dias, A.-C.-P.; Pinto-Ribeiro, F.-L. Genista tridentata phytochemical characterization and biological activities: A systematic review. Biology 2023, 12, 1387. [Google Scholar] [CrossRef]
- Gibbs, P.-E. Taxonomic notes on some Canry Island in North African species of Cytisus and Genista. Lagascalia 1974, 4, 33–42. [Google Scholar]
- Bacchetta, G.; Brullo, S.; Velari, T.-C.; Chiapella, L.-F.; Kosovel, V. Taxonomic notes on the Genista ephedroides group (Fabaceae) from the Mediterranean area. Novon J. Bot. Nomencl. 2011, 21, 4–19. [Google Scholar] [CrossRef]
- Zheng, Z.; Scott, S.; Lukas, W.; Webb, M. A greedy algorithm for aligning DNA sequences. J. Comput. Biol. 2000, 7, 203–214. [Google Scholar] [CrossRef]
- Dananjaya, S.-H.-S.; Udayangani, R.-M.-C.; Shin, S.-Y.; Edussuriya, M.; Nikapitiya, C.; Lee, J.; De Zoysa, M. In vitro and in vivo antifungal efficacy of plant based lawsone against Fusarium oxysporum species complex. Microbiol. Res. 2017, 201, 21–29. [Google Scholar] [CrossRef]
- Rongai, D.; Pulcini, P.; Pesce, B.; Milano, F. Antifungal activity of some botanical extracts on Fusarium oxysporum. Open Life Sci. 2015, 10, 409–416. [Google Scholar] [CrossRef]
- Xu, J.; Zhao, X.; Han, X.; Du, Y. Antifungal activity of oligochitosan against Phytophthora capsici and other plant pathogenic fungi in vitro. Pestic. Biochem. Physiol. 2007, 87, 220–228. [Google Scholar] [CrossRef]
- Tang, C.-S.; Young, C.-C. Collection and identification of allelopathic compounds from the undisturbed root system of Bigalta limpograss (Hemarthria altissima). Plant Physiol. 1982, 69, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.-P.; Wang, Q.; Christie, P.; Li, X.L. Allelopathic potential of watermelon tissues and root exudates. Sci. Hortic. 2007, 112, 315–320. [Google Scholar] [CrossRef]
- Araniti, F.; Lupini, A.; Sorgonà, A.; Conforti, F.; Marrelli, M.; Statti, G.-A.; Abenavoli, M.-R. Allelopathic potential of Artemisia arborescens: Isolation, identification and quantification of phytotoxic compounds through fractionation-guided bioassays. Nat. Prod. Res. 2013, 27, 880–887. [Google Scholar] [CrossRef]
- Khan, A.-S.; Sakina; Nasrullah, A.; Ullah, S.; Ullah, Z.; Khan, Z.; Di, I.-U. An overview on phytotoxic perspective of ionic liquids and deep eutectic solvents: The role of chemical structure in the phytotoxicity. Chem. Biomol. Eng. Rev. 2023, 10, 174–194. [Google Scholar] [CrossRef]
- Dezfulian, M.-H.; Foreman, C.; Jalili, E.; Pal, M.; Dhaliwal, R.-K.; Roberto, D.-K.-A.; Imre, K.-M.; Kohalmi, S.-E.; Crosby, W.-L. Acetolactate synthase regulatory subunits play divergent and overlapping roles in branched-chain amino acid synthesis and Arabidopsis development. BMC Plant Biol. 2017, 17, 71. [Google Scholar] [CrossRef]
- Thomson, M.-K. Acetohydroxyacid synthase inhibitors (AHAS/ALS). In Modern Crop Protection Compounds; Krämer, W., Schirmer, U., Eds.; Wiley-VCH: Weinheim, Germany, 2007; pp. 27–45. [Google Scholar]
- Kraehmer, H.; van Almsick, A.; Beffa, R.; Dietrich, H.; Eckes, P.; Hacker, E.; Hain, R.; Strek, H.J.; Stuebler, H.; Willms, L. Herbicides as Weed Control Agents: State of the Art: II. Recent Achievements. Plant Physiol. 2014, 166, 1132–1148. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Qin, S.; Zhang, T.; Zhang, H.; Zhu, J.; Li, X.; Li, Y.; Zhao, F. Basing target enzyme study the enantioselective bioactivity action mechanism of flusulfinam, a novel HPPD inhibitor herbicide. Pestic. Biochem. Physiol. 2025, 209, 106346. [Google Scholar] [CrossRef]
- Koch, M.; Breithaupt, C.; Kiefersauer, R.; Freigang, J.; Huber, R.; Messerschmidt, A. Crystal structure of protoporphyrinogen IX oxidase: A key enzyme in haem and chlorophyll biosynthesis. EMBO J. 2004, 23, 1720–1728. [Google Scholar] [CrossRef]
- Fan, Q.; Shen, Y.; Yang, Y.; Zhang, Q. A review of remediation strategies for diphenyl ether herbicide contamination. Toxics 2024, 12, 397. [Google Scholar] [CrossRef]
- Takács, E.; Lázár, D.; Siakwa, A.; Klátyik, S.; Mörtl, M.; Kocsányi, L.; Barócsi, A.; Lenk, S.; Lengyel, E.; Székács, A. Ecotoxicological evaluation of safener and antimicrobial additives in isoxaflutole-based herbicide formulations. Toxics 2024, 12, 238. [Google Scholar] [CrossRef]
- Geoffroy, L.; Teisseire, H.; Couderchet, M.; Vernet, G. Effect of oxyfluorfen and diuron alone and in mixture on antioxidative enzymes of Scenedesmus obliquus. Pestic. Biochem. Physiol. 2002, 72, 178–185. [Google Scholar] [CrossRef]
- Halfaoui, F.; Touahria, S. Consequences of the degradation of plant formations in the region of Tamezguida, wilaya of Medea. Agrobiologia 2018, 8, 1165–1179. [Google Scholar]
- Mangold, J.; Parkinson, H. Plant identification basics. Mont. State Univ. Ext. Mt. Guide 2013, 9, 1–8. [Google Scholar]
- Murray, M.-G.; Thompson, W.-F. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980, 8, 4321–4326. [Google Scholar] [CrossRef]
- Gardes, M.; Bruns, T.-D. ITS primers withenhanced specificity for basidiomycetesapplication to the identification ofmycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef] [PubMed]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Zatout, R.; Christopher, B.; Cherfia, R.; Chaoua, S.; Chaouche, N.-K. A new record of Agaricus litoralis, a rare edible macro-fungus from Eastern Algeria. Mycopath 2023, 20, 38. [Google Scholar]
- Zatout, R.; Kacem Chaouche, N. Antibacterial activity screening of an edible mushroom Agaricus litoralis. Int. J. Bot. Stud. 2023, 8, 49–52. [Google Scholar]
- Salvatore, M.M.; Pappalardo, C.; Suarez, E.G.P.; Salvatore, F.; Andolfi, A.; Gesuele, R.; Galdiero, E.; Libralato, G.; Guida, M.; Siciliano, A. Ecotoxicological and metabolomic investigation of chronic exposure of Daphnia magna (Straus, 1820) to yttrium environmental concentrations. Aquat. Toxicol. 2024, 276, 107117. [Google Scholar] [CrossRef]
- Zatout, R.; Cimmino, A.; Cherfia, R.; Chaouche, N.-K. Isolation of tyrosol the main phytotoxic metabolite produced by the edible fungus Agaricus litoralis. Egypt. J. Chem. 2021, 64, 5741–5745. [Google Scholar] [CrossRef]
- Masi, M.; Sautua, F.; Zatout, R.; Castaldi, S.; Arrico, L.; Isticato, R.; Evidente, A. Phaseocyclopentenones A and B, phytotoxic penta-and tetrasubstituted cyclopentenones produced by Macrophomina phaseolina, the causal agent of charcoal rot of soybean in Argentina. J. Nat. Prod. 2021, 84, 459–465. [Google Scholar] [CrossRef]



| Compound | RI | Aerial Part | Roots | ||
|---|---|---|---|---|---|
| n-Hexane Extract | Dichloromethane Extract | n-Hexane Extract | Dichloromethane Extract | ||
| Benzyl alcohol, TMS | 1168 | 0.91 | |||
| Phenylethyl alcohol, TMS | 1234 | 0.41 | 2.18 | ||
| Octanoic acid, TMS | 1265 | 0.31 | 0.11 | ||
| Glycerol, 3TMS | 1283 | 0.68 | 0.55 | ||
| Carvacrol, TMS | 1338 | 0.11 | |||
| Nonanoic acid, TMS | 1362 | 0.16 | |||
| Eugenol, TMS | 1479 | 0.58 | 0.22 | ||
| Hydroxy-5-methoxy-3-methyl-5-oxopentanoic acid, 2TMS | 1508 | 5.17 | |||
| Methyl laurate | 1526 | 1.41 | |||
| Vanillin, TMS | 1549 | 1.07 | |||
| 2,4-Di-tert-butylphenoxytrimethylsilane | 1555 | 1.07 | |||
| Elemicin | 1569 | 2.94 | |||
| 3-Hydroxy-3-methylglutaric acid, 3TMS | 1613 | 3.07 | |||
| Vanillyl alcohol, 2TMS | 1648 | 0.43 | |||
| Lauric acid, TMS | 1657 | 8.10 | 0.32 | ||
| 8-Carbomethoxyoctanoic acid, TMS | 1680 | 2.10 | 6.86 | ||
| 2,6-Dimethoxyhydroquinone, 2TMS | 1689 | 3.03 | |||
| Syringaldehyde, TMS | 1720 | 2.99 | 46.77 | ||
| Methyl 3,4-dihydroxybenzoate, 2TMS | 1734 | 1.95 | |||
| Vanillic acid, 2TMS | 1775 | 3.99 | 2.02 | ||
| Azelaic acid, 2TMS | 1800 | 29.96 | 6.11 | ||
| Myristic acid, TMS | 1849 | 8.27 | |||
| Coniferyl aldehyde, TMS | 1863 | 0.87 | |||
| Syringic acid, 2TMS | 1910 | 9.32 | |||
| Isoprunetin, 2TMS | 1926 | 9.79 | |||
| Methyl palmitate | 1931 | 30.34 | 2.34 | ||
| Ferulic acid, methyl ester, TMS | 1969 | 6.08 | 5.22 | ||
| Methyl caffeate, 2TMS | 2028 | 30.29 | |||
| Sinapaldehyde, TMS | 2036 | 17.66 | |||
| Sinapyl alcohol, 2TMS | 2095 | 3.16 | |||
| Palmitic acid, TMS | 2051 | 39.25 | 17.39 | ||
| Linolelaidic acid, methyl ester | 2108 | 15.24 | |||
| Oleic acid, TMS | 2239 | 59.53 | |||
| Stearic acid, TMS | 2242 | 3.15 | |||
| 1-Monolinolein, 2TMS | 2769 | 0.55 | |||
| Daidzein, 2TMS | 2976 | 3.49 | |||
| Genistein, 3TMS | 3000 | 2.03 | |||
| Plant Part | % Inhibition | |||
|---|---|---|---|---|
| F. oxysporum | A. alternata | B. cinerea | ||
| Aerial | n-Hexane | 100 ± 0.0 a | 87 ± 1.6 b | 100 ± 0.0 a |
| DCM | 100 ± 0.0 a | 67 ± 2.1 c | 100 ± 0.0 a | |
| Root | n-Hexane | 96.4 ± 1.5 b | 98.2 ± 1.0 a | 100 ± 0.0 a |
| DCM | 94.7 ± 1.8 b | 97.1 ± 1.2 a | 100 ± 0.0 a | |
| Plant Part | Necrotic Area (mm2) | ||||
|---|---|---|---|---|---|
| 0.5 mg/mL | 1 mg/mL | 2 mg/mL | Control | ||
| Aerial | n-Hexane | 12.33 ± 0.62 bcd | 25.33 ± 0.70 bc | 48.50 ± 0.72 a | 0.50 ± 0.10 e |
| DCM | 10.90 ± 0.20 cd | 22.20 ± 0.36 cd | 42.60 ± 0.57 ab | 0.47 ± 0.06 e | |
| Root | n-Hexane | 14.27 ± 0.35 bc | 30.40 ± 0.62 b | 55.30 ± 0.75 a | 0.50 ± 0.10 e |
| DCM | 13.10 ± 0.30 bc | 27.73 ± 0.40 b | 51.30 ± 0.40 a | 0.47 ± 0.06 e | |
| Plant Part | Necrotic Area (mm2) | ||||
|---|---|---|---|---|---|
| 0.5 mg/mL | 1 mg/mL | 2 mg/mL | Control | ||
| Aerial | n-Hexane | 5.90 ± 0.20 cd | 12.43 ± 0.25 bc | 24.33 ± 0.56 ab | 0.20 ± 0.00 e |
| DCM | 4.10 ± 0.55 d | 6.40 ± 0.26 cd | 10.77 ± 0.26 c | 0.27 ± 0.06 e | |
| Root | n-Hexane | 7.13 ± 0.25 c | 14.97 ± 0.81 b | 26.57 ± 0.57 a | 0.27 ± 0.06 e |
| DCM | 6.37 ± 0.30 cd | 13.57 ± 0.15 bc | 25.40 ± 0.41 a | 0.20 ± 0.00 e | |
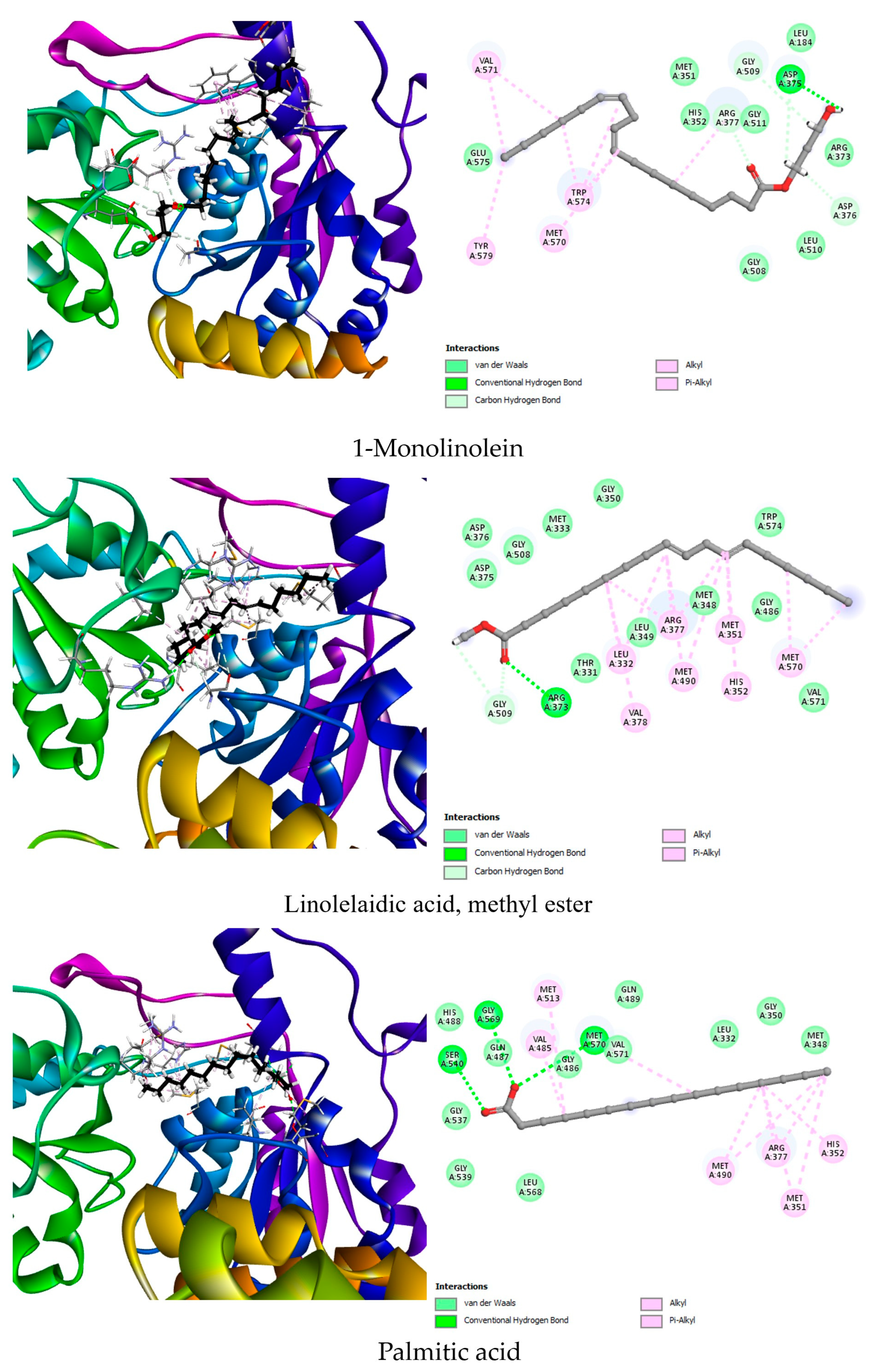
| Binding Energy (Kcal/mol) | Hydrogen Interactions (Distance Å) | Hydrophobic Interactions | |||
|---|---|---|---|---|---|
| AHAS (1YHZ) | Co-crystallized ligand | 1CS | −8.5 | Gly508 (2.56), Gly508 (2.84), Asp375 (2.54), Arg373 (2.52), Gly509 (3.23), Arg377 (2.60) | Trp574, Met570 |
| Best docked compounds | Linolelaidic acid, methyl ester | −7.6 | Gly509 (2.65), Arg373 (2.23) | Leu332 (2) Val378, Arg377 (2) Met490 (2), Met351, His352, Met570 (2) | |
| 1-Monolinolein | −6.8 | Gly509 (2.41), Arg377 (2.78), Asp375 (2.95), Asp375 (2.26), Asp376 (3.08) | Val571 (2), Tyr579, Met570, Trp574 (3), Arg377 | ||
| Palmitic acid | −6.7 | Ser540 (2.51), Gly569 (2.67), Met570 (2.49) | Met513, Val485, Met490 (2), Arg377 (2), Met351 (2) His352 | ||
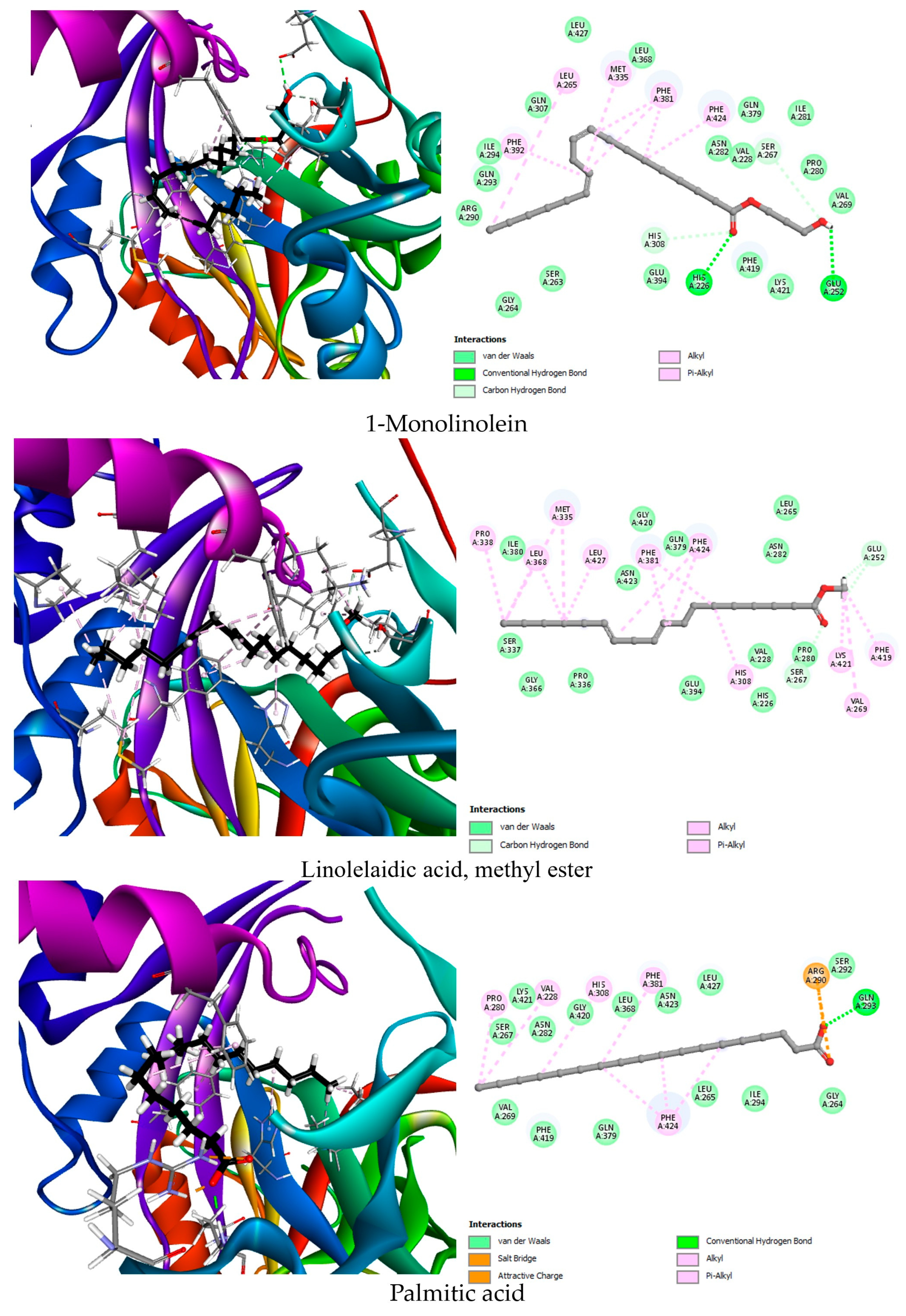
| HPPD (6J63) | Co-crystallized ligand | NDT | −6.9 | His226 (2.63), His308 (3.26), His308 (3.35), Lys421 (2.92) | Phe424 (2) |
| Best docked compounds | 1-Monolinolein | −7.7 | His226 (2.59), His308 (2.94), Glu252 (2.72), Ser267 (3.26) | Phe424, Phe381 (3), Met335, Leu265, Phe392 | |
| Linolelaidic acid, methyl ester | −7.5 | Glu252 (2.95), Ser267 (3.22) | Pro338, Leu368 (2), Met335 (2), Leu427, Phe381 (2), Phe424 (2), His308, Lys421, Val269, Phe419 | ||
| Stearic acid | −7.3 | Gln293 (2.63) | Pro280, Val228, His308, Phe381, Phe424 (3) | ||
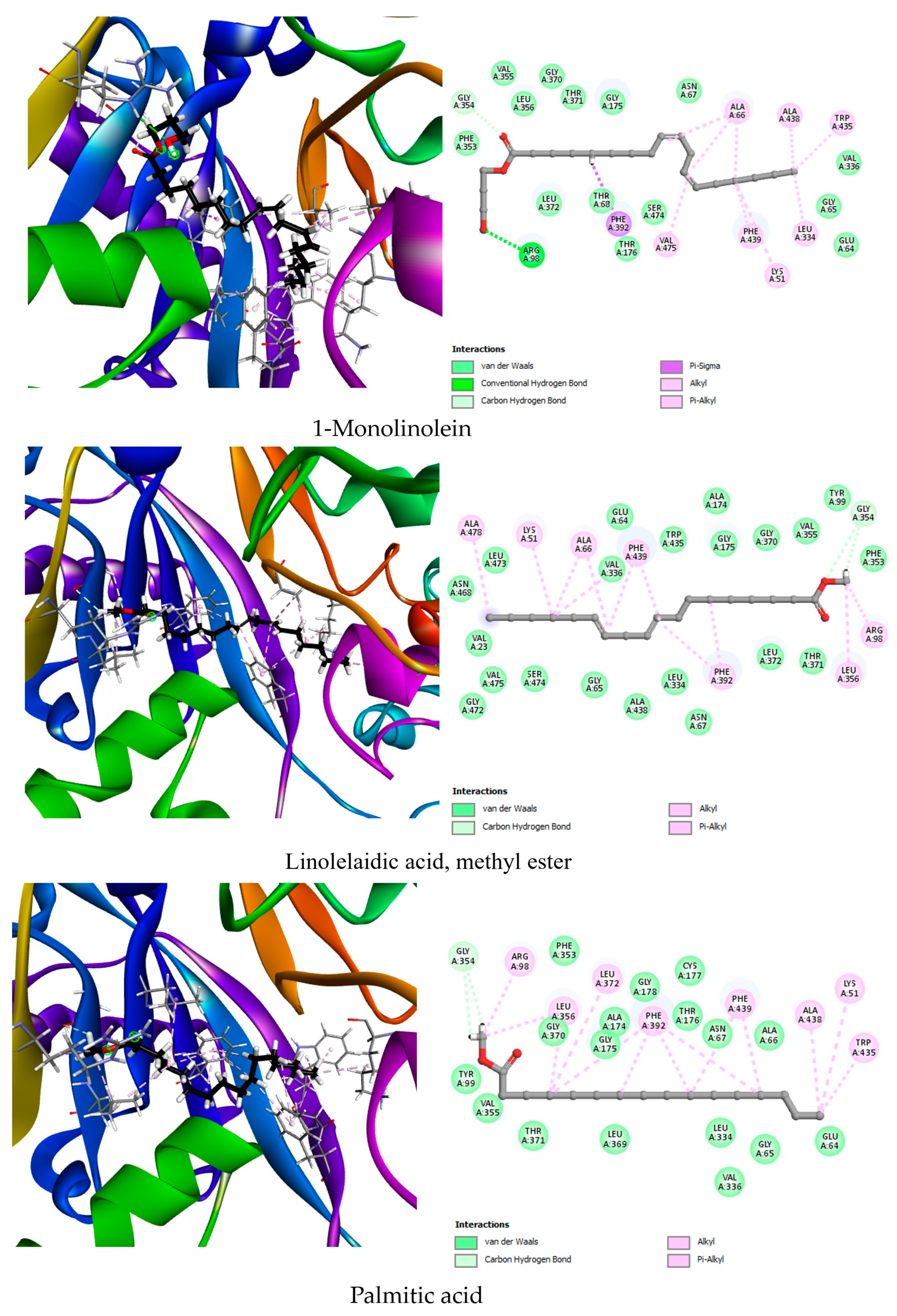
| PPO (1SEZ) | Co-crystallized ligand | OMN | −8.1 | Arg98 (2.33), Gly178 (2.65) | Leu372, Leu356, Phe392 (2), Leu334 |
| Best docked compounds | 1-Monolinolein | −8.7 | Gly354 (2.92), Arg98 (2.64) | Trp435, Ala438, Leu334, Ala66 (3), Phe439, Lys51, Val475, Phe392 | |
| Linolelaidic acid, methyl ester | −8.4 | Gly354 (2.91), Gly354 (3.06) | Ala478, Lys51, Ala66 (2), Phe439 (3), Phe392 (2), Arg98, Leu356 | ||
| Palmitic acid, methyl ester | −8.1 | Gly354 (3.13), Gly354 (3.24) | Arg98, Leu356 (2), Leu372, he392 (4), Phe439, Ala438, Lys51, Trp435 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zatout, R.; Benslama, O.; Makhlouf, F.Z.; Cimmino, A.; Salvatore, M.M.; Andolfi, A.; Kolla, R.M.; Masi, M. Exploring the Potential of Genista ulicina Phytochemicals as Natural Biocontrol Agents: A Comparative In Vitro and In Silico Analysis. Toxins 2025, 17, 452. https://doi.org/10.3390/toxins17090452
Zatout R, Benslama O, Makhlouf FZ, Cimmino A, Salvatore MM, Andolfi A, Kolla RM, Masi M. Exploring the Potential of Genista ulicina Phytochemicals as Natural Biocontrol Agents: A Comparative In Vitro and In Silico Analysis. Toxins. 2025; 17(9):452. https://doi.org/10.3390/toxins17090452
Chicago/Turabian StyleZatout, Roukia, Ouided Benslama, Fatima Zohra Makhlouf, Alessio Cimmino, Maria Michela Salvatore, Anna Andolfi, Radhia Manel Kolla, and Marco Masi. 2025. "Exploring the Potential of Genista ulicina Phytochemicals as Natural Biocontrol Agents: A Comparative In Vitro and In Silico Analysis" Toxins 17, no. 9: 452. https://doi.org/10.3390/toxins17090452
APA StyleZatout, R., Benslama, O., Makhlouf, F. Z., Cimmino, A., Salvatore, M. M., Andolfi, A., Kolla, R. M., & Masi, M. (2025). Exploring the Potential of Genista ulicina Phytochemicals as Natural Biocontrol Agents: A Comparative In Vitro and In Silico Analysis. Toxins, 17(9), 452. https://doi.org/10.3390/toxins17090452









