Galectin-8A Inhibits Cry11Aa Binding to ALP1 and APN 2 Receptors and Toxicity Against Aedes aegypti Larvae
Abstract
1. Introduction
2. Results
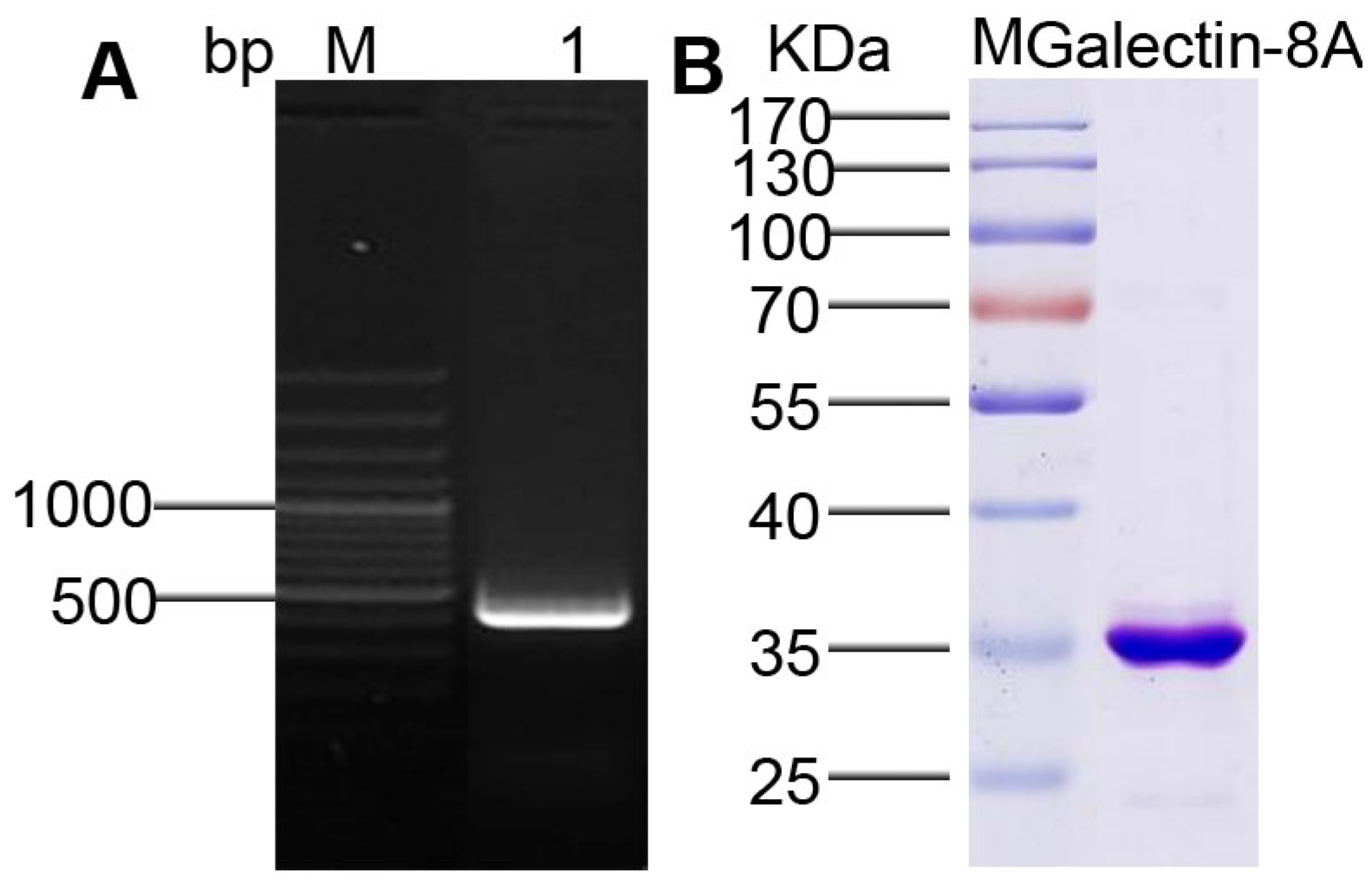
2.1. The Recombinant Galectin-8A Protein Purified from Ae. aegypti
2.2. Galectin-8A Protein Inhibits Larvicidal Activity of Bt Toxins
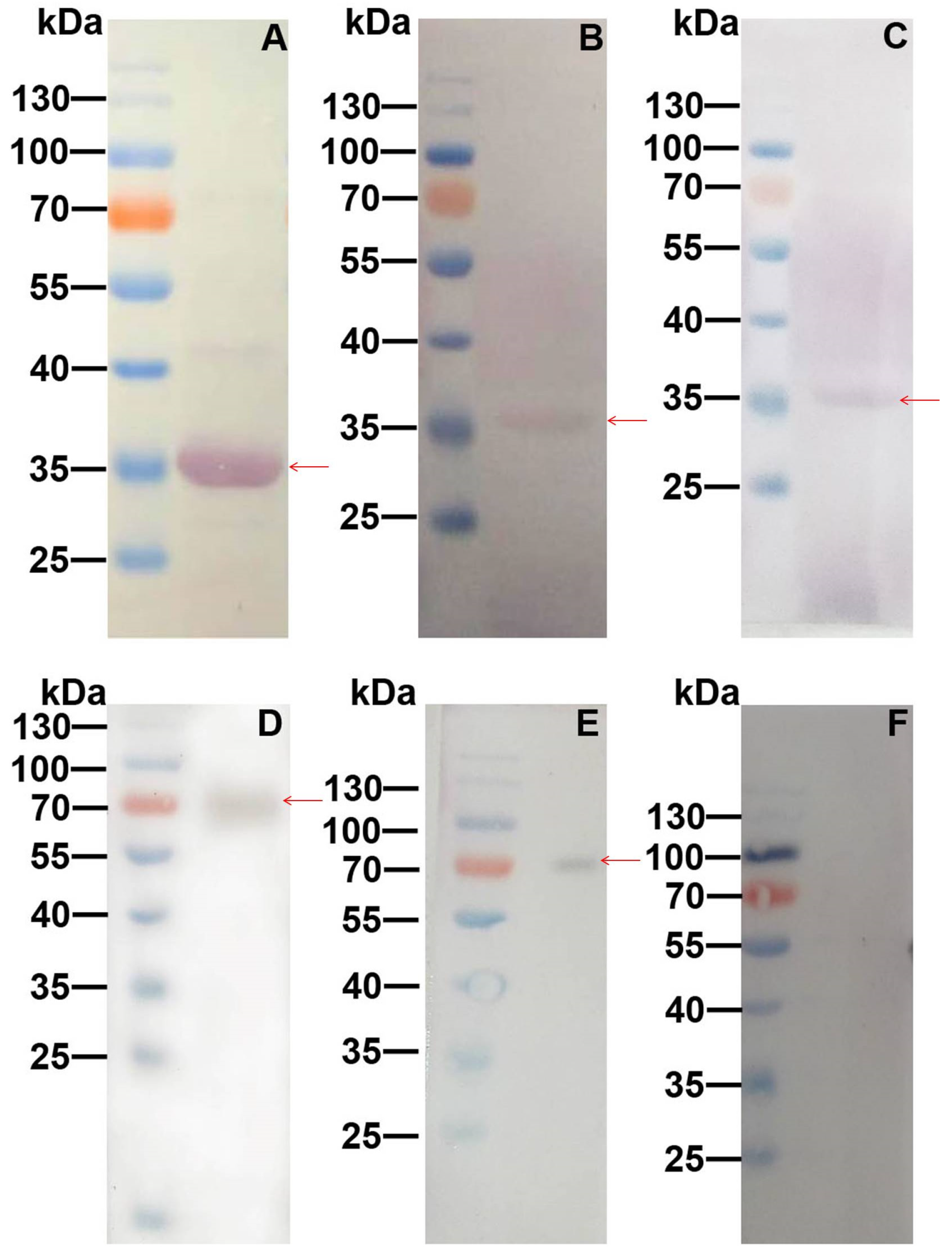
2.3. Galectin-8A and Cry11Aa Binds to BBMV
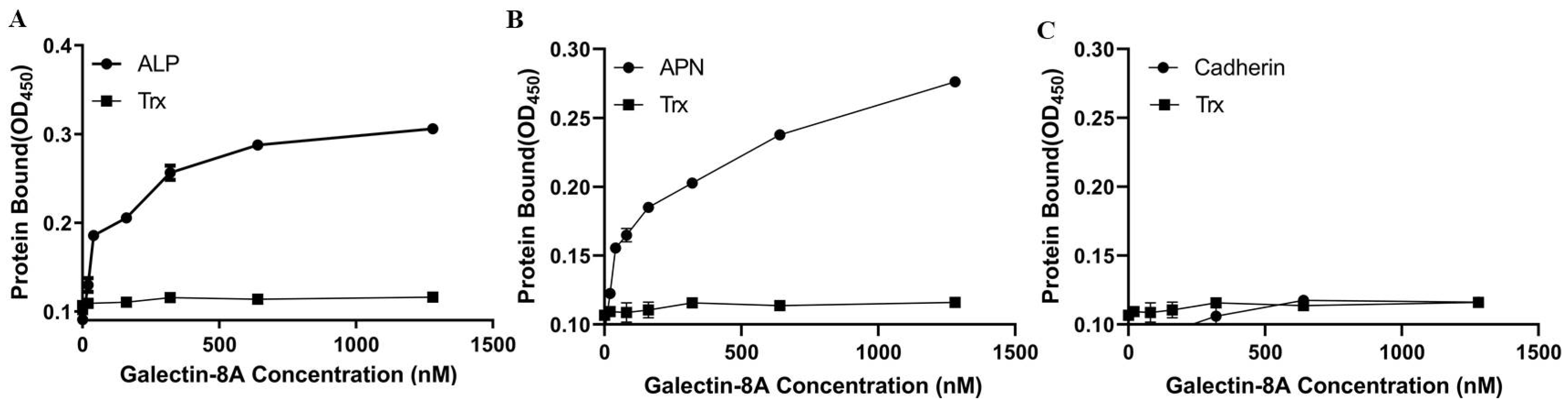
2.4. Galectin-8A Binds to Individual Toxin Receptors
2.5. Three-Dimensional Protein Structures of Galectin-8A and Molecular Docking
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Mosquitoes and Bacterial Strains
5.2. Expression and Purification of Recombinant Galectin-8A
5.3. Bioassays
5.4. Preparation of Ae. aegypti Brush Border Membrane Vesicles (BBMVs)
5.5. Western and Far-Western Blot Analysis
5.6. Octet Red System
5.7. Elisa
5.8. Modeling and Protein Docking Analyses of Galectin-8A, Cry11Aa, ALP, APN, and Cadherin
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Algamdi, A.G.; Mahyoub, J.A. Detection of insecticide detoxification enzymes activities in Aedes aegypti mosquito, the vector of dengue fever in Saudi Arabia. Main Group Chem. 2022, 21, 1053–1063. [Google Scholar] [CrossRef]
- Debojyati, D.; Semanti, G. Analyzing the molecular signature genes and pathways of dengue fever, Dengue hemorrhagic fever and Dengue shock syndrome caused by Dengue virus in India. Mol. Biotechnol. 2025, 14, 1–7. [Google Scholar] [CrossRef]
- Wang, Y.; Li, C.; Zhao, S.; Lin, G.; Jiang, X.; Yin, S.; He, M.; Wu, Q.; Guo, Z.; Wei, Y.; et al. Evaluation of dengue fever vulnerability in south and southeast asian countries: A multidimensional approach. J. Infect. Public Health 2025, 18, 102849. [Google Scholar] [CrossRef]
- Uddin, M.M.; Islam, A.; Islam, S.; Ekra, J.E.; Watanabe, K. Insecticide Susceptibility and Knockdown Resistance Mutations in Aedes aegypti and Culex species in Bangladesh. Acta Trop. 2025, 269, 107736. [Google Scholar] [CrossRef] [PubMed]
- Contessoto, V.G.; Dudchenko, O.; Aiden, E.L.; Wolynes, P.G.; Pierro, M. Interphase chromosomes of the Aedes aegypti mosquito are liquid crystalline and can sense mechanical cues. Biophys. J. 2022, 122, 20a. [Google Scholar]
- Abbasi, E.; Vahedi, M.; Bagheri, M.; Gholizadeh, S.; Alipour, H.; Moemenbellah-Fard, M.D. Monitoring of synthetic insecticides resistance and mechanisms among malaria vector mosquitoes in Iran: A systematic review. Heliyon 2022, 8, e08830. [Google Scholar] [CrossRef] [PubMed]
- Lin, O.; Janssens, L.; Stoks, R. Synthetic predator cues impair immune function and make the biological pesticide Bti more lethal for vector mosquitoes. Ecol. Appl. 2015, 26, 355–366. [Google Scholar]
- Zhao, L.; Alto, B.W.; Dagne, D. Transcriptional Profile for Detoxification Enzymes AeaGGT1 and AaeGGT2 from Aedes aegypti (Diptera: Culicidae) in Response to Larvicides. J. Med. Entomol. 2017, 4, 878–887. [Google Scholar] [CrossRef]
- Britch, S.C.; Linthicum, K.J.; Aldridge, R.L.; Golden, F.V.; Walker, T.W. Visualizing Efficacy of Pesticides Against Disease Vector Mosquitoes in the Field. J. Vis. Exp. Jove 2019, 145, 58440. [Google Scholar]
- Wang, P.; Zhang, C.; Guo, M.; Guo, S.; Zhu, Y.; Zheng, J.; Zhu, L.; Ruan, L.; Peng, D.; Sun, M. Complete genome sequence of Bacillus thuringiensis YBT-1518, a typical strain with high toxicity to nematodes. J. Biotechnol. 2014, 171, 1–2. [Google Scholar] [CrossRef]
- Thammasittirong, A.; Thammasittirong, S.N.R. Cry4Ba toxin of Bacillus thuringiensis subsp. israelensis uses both domains II and III to bind to its receptor-Aedes aegypti alkaline phosphatase. Heliyon 2023, 9, e19458. [Google Scholar] [CrossRef]
- Palma, L.; Muoz, D.; Berry, C.; Murillo, J.; Caballero, P. Bacillus thuringiensis toxins: An overview of their biocidal activity. Toxins 2014, 6, 3296–3325. [Google Scholar] [CrossRef] [PubMed]
- Vega-Cabrera, A.; Cancino-Rodezno, A.; Porta, H.; Pardo-Lopez, L. Aedes aegypti Mos20 Cells Internalizes Cry Toxins by Endocytosis, and Actin Has a Role in the Defense against Cry11Aa Toxin. Toxins 2014, 6, 464–487. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.B.; Chen, J.; Aimanova, K.G.; Gill, S.S. Aedes cadherin mediates the in vivo toxicity of the Cry11Aa toxin to Aedes aegypti. Peptides 2015, 68, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Pardo-Lopez, L.; Soberon, M.; Bravo, A. Bacillus thuringiensis insecticidal three-domain Cry toxins: Mode of action, insect resistance and consequences for crop protection. FEMS Microbiol. Rev. 2013, 37, 3–22. [Google Scholar] [CrossRef]
- Boonserm, P.; Davis, P.; Ellar, D.J.; Li, J. Crystal structure of the mosquito-larvicidal toxin Cry4Ba and its biological implications. J. Mol. Biol. 2005, 348, 363–382. [Google Scholar] [CrossRef]
- Boonserm, P.; Mo, M.; Angsuthanasombat, C.; Lescar, J. Structure of the Functional Form of the Mosquito Larvicidal Cry4Aa Toxin from Bacillus thuringiensis at a 2.8-Angstrom Resolution. J. Bacteriol. 2006, 188, 3391–3401. [Google Scholar] [CrossRef]
- Derbyshire, D.J.; Ellar, D.J.; Li, J. Crystallization of the Bacillus thuringiensis toxin Cry1Ac and its complex with the receptor ligand N-acetyl-D-galactosamine. Acta Crystallogr. 2010, 57, 1938–1944. [Google Scholar]
- Galitsky, N.; Cody, V.; Wojtczak, A.; Ghosh, D.; Luft, J.R.; Pangborn, W.; English, L. Structure of the insecticidal bacterial δ-endotoxin Cry3Bb1 of Bacillus thuringiensis. Acta Crystallogr. 2001, 57, 1101–1109. [Google Scholar]
- Li, J.; Derbyshire, D.J.; Promdonkoy, B.; Ellar, D.J. Structural implications for the transformation of the Bacillus thuringiensis delta-endotoxins from water-soluble to membrane-inserted forms. Biochem. Soc. Trans. 2001, 29, 571. [Google Scholar] [CrossRef]
- Rui, Z.; Gang, H.; Andacht, T.M.; Adang, M.J. A 106-kDa aminopeptidase is a putative receptor for Bacillus thuringiensis Cry11Ba toxin in the mosquito Anopheles gambiae. Biochemistry 2008, 47, 11263. [Google Scholar]
- Zhang, L.-L.; Hu, X.-H.; Wu, S.-Q.; Batool, K.; Chowdhury, M.; Lin, Y.; Zhang, J.; Gill, S.S.; Guan, X.; Yu, X.-Q. Aedes aegypti Galectin Competes with Cry11Aa for Binding to ALP1 To Modulate Cry Toxicity. J. Agric. Food Chem. 2018, 66, 13435–13443. [Google Scholar] [CrossRef]
- Fernandez, L.E.; Aimanova, K.G.; Gill, S.S.; Bravo, A.; Soberon, M. A GPI-anchored alkaline phosphatase is a functional midgut receptor of Cry11Aa toxin in Aedes aegypti larvae. Biochem. J. 2006, 394, 77–84. [Google Scholar] [CrossRef]
- Jurat-Fuentes, J.L.; Adang, M.J. Characterization of a Cry1Ac-receptor alkaline phosphatase in susceptible and resistant Heliothis virescens larvae. Febs J. 2010, 271, 3127–3135. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, G.; Hu, X.; Liu, J.; Li, M.; Batool, K.; Chen, M.; Wang, J.; Xu, J.; Huang, T.; et al. Cry11Aa Interacts with the ATP-Binding Protein from Culex quinquefasciatus To Improve the Toxicity. J. Agric. Food Chem. 2017, 65, 10884–10890. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Aimanova, K.G.; Fernandez, L.E.; Bravo, A.; Gill, S.S. Aedes aegypti cadherin serves as a putative receptor of the Cry11Aa toxin from Bacillus thuringiensis subsp. israelensis. Biochem. J. 2009, 424, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Chen, H.; Xu, J.; Zhao, G.; Huang, X.; Liu, J.; Batool, K.; Wu, C.; Wu, S.; Huang, E.; et al. Function of Aedes aegypti galectin-6 in modulation of Cry11Aa toxicity. Pestic. Biochem. Physiol. 2020, 162, 96–104. [Google Scholar] [CrossRef]
- Batool, K.; Alam, I.; Jin, L.; Xu, J.; Zhang, L. CTLGA9 interacts with ALP1 and APN receptors to modulate Cry11Aa toxicity in Aedes aegypti. J. Agric. Food Chem. 2019, 67, 8896–8904. [Google Scholar] [CrossRef]
- Zelensky, A.N.; Gready, J.E. The C-type lectin-like domain superfamily. Febs J. 2005, 272, 6179–6217. [Google Scholar] [CrossRef]
- Ling, E.; Yu, X.Q. Cellular encapsulation and melanization are enhanced by immulectins, pattern recognition receptors from the tobacco hornworm Manduca sexta. Dev. Comp. Immunol. 2006, 30, 289–299. [Google Scholar] [CrossRef]
- Yu, X.Q.; Kanost, M.R. Immulectin-2, a lipopolysaccharide-specific lectin from an insect, Manduca sexta, is induced in response to gram-negative bacteria. J. Biol. Chem. 2000, 275, 37373–37381. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, E.; Lin, J.; Gelbič, I.; Zhang, Q.; Guan, Y.; Huang, T.; Guan, X. A novel mosquitocidal Bacillus thuringiensis strain LLP29 isolated from the phylloplane of Magnolia denudata. Microbiol. Res. 2010, 165, 133–141. [Google Scholar] [CrossRef]
- Abdul-Rauf, M.; Ellar, D.J. Isolation and Characterization of Brush Border Membrane Vesicles from Whole Aedes aegypti Larvae. J. Invertebr. Pathol. 1999, 73, 45–51. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, X.; Huang, X.; Liu, J.; Zhao, G.; Wu, S.; Yu, X.; Xu, L.; Guan, X.; Zhang, L. Galectin-8A Inhibits Cry11Aa Binding to ALP1 and APN 2 Receptors and Toxicity Against Aedes aegypti Larvae. Toxins 2025, 17, 451. https://doi.org/10.3390/toxins17090451
Hu X, Huang X, Liu J, Zhao G, Wu S, Yu X, Xu L, Guan X, Zhang L. Galectin-8A Inhibits Cry11Aa Binding to ALP1 and APN 2 Receptors and Toxicity Against Aedes aegypti Larvae. Toxins. 2025; 17(9):451. https://doi.org/10.3390/toxins17090451
Chicago/Turabian StyleHu, Xiaohua, Xianhui Huang, Jiannan Liu, Guohui Zhao, Songqing Wu, Xiaoqiang Yu, Lei Xu, Xiong Guan, and Lingling Zhang. 2025. "Galectin-8A Inhibits Cry11Aa Binding to ALP1 and APN 2 Receptors and Toxicity Against Aedes aegypti Larvae" Toxins 17, no. 9: 451. https://doi.org/10.3390/toxins17090451
APA StyleHu, X., Huang, X., Liu, J., Zhao, G., Wu, S., Yu, X., Xu, L., Guan, X., & Zhang, L. (2025). Galectin-8A Inhibits Cry11Aa Binding to ALP1 and APN 2 Receptors and Toxicity Against Aedes aegypti Larvae. Toxins, 17(9), 451. https://doi.org/10.3390/toxins17090451





