Injection of Affibodies by a Self-Organizing Bacterial Syringe to Interfere with Intracellular Signaling
Abstract
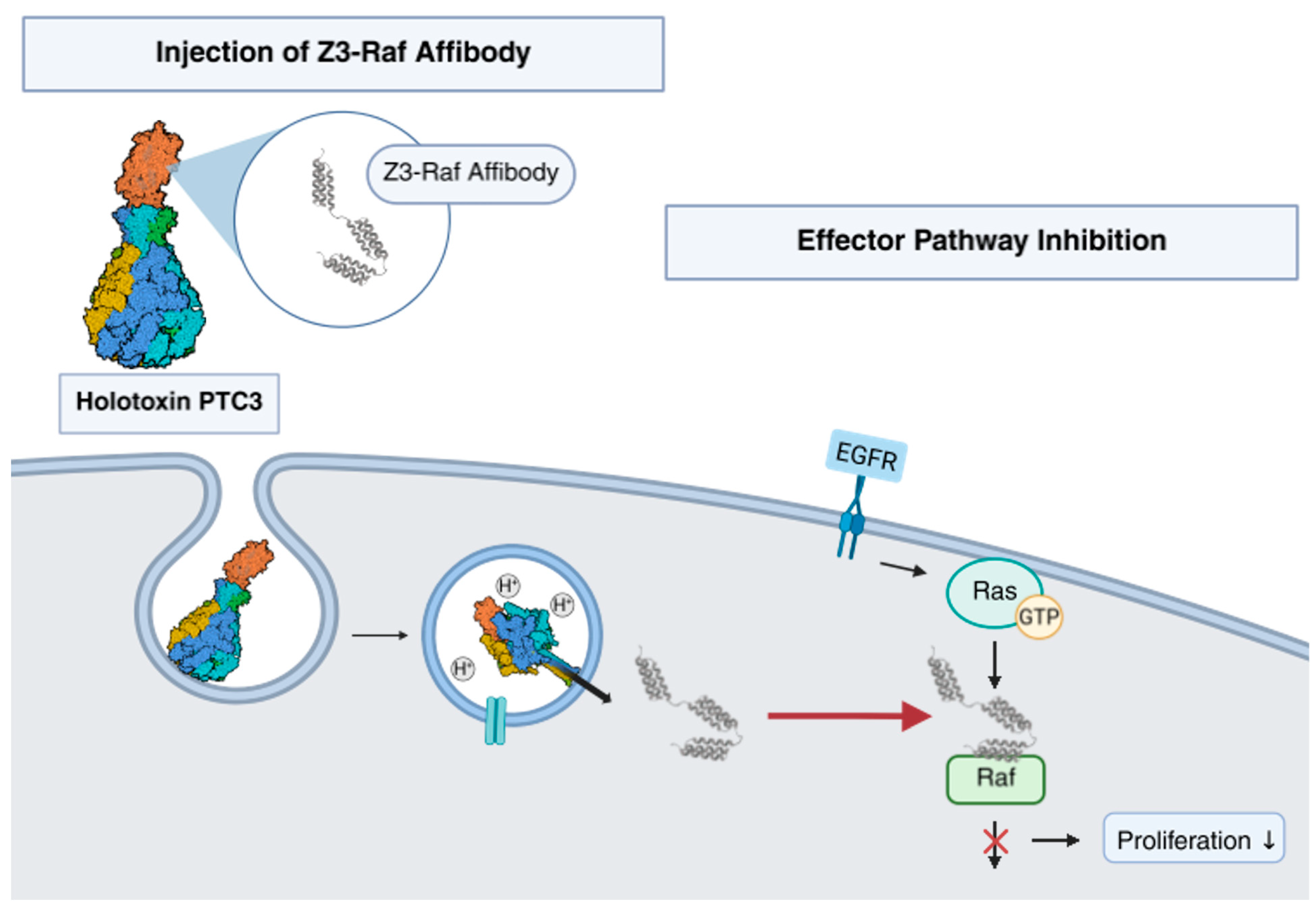
1. Introduction
2. Results
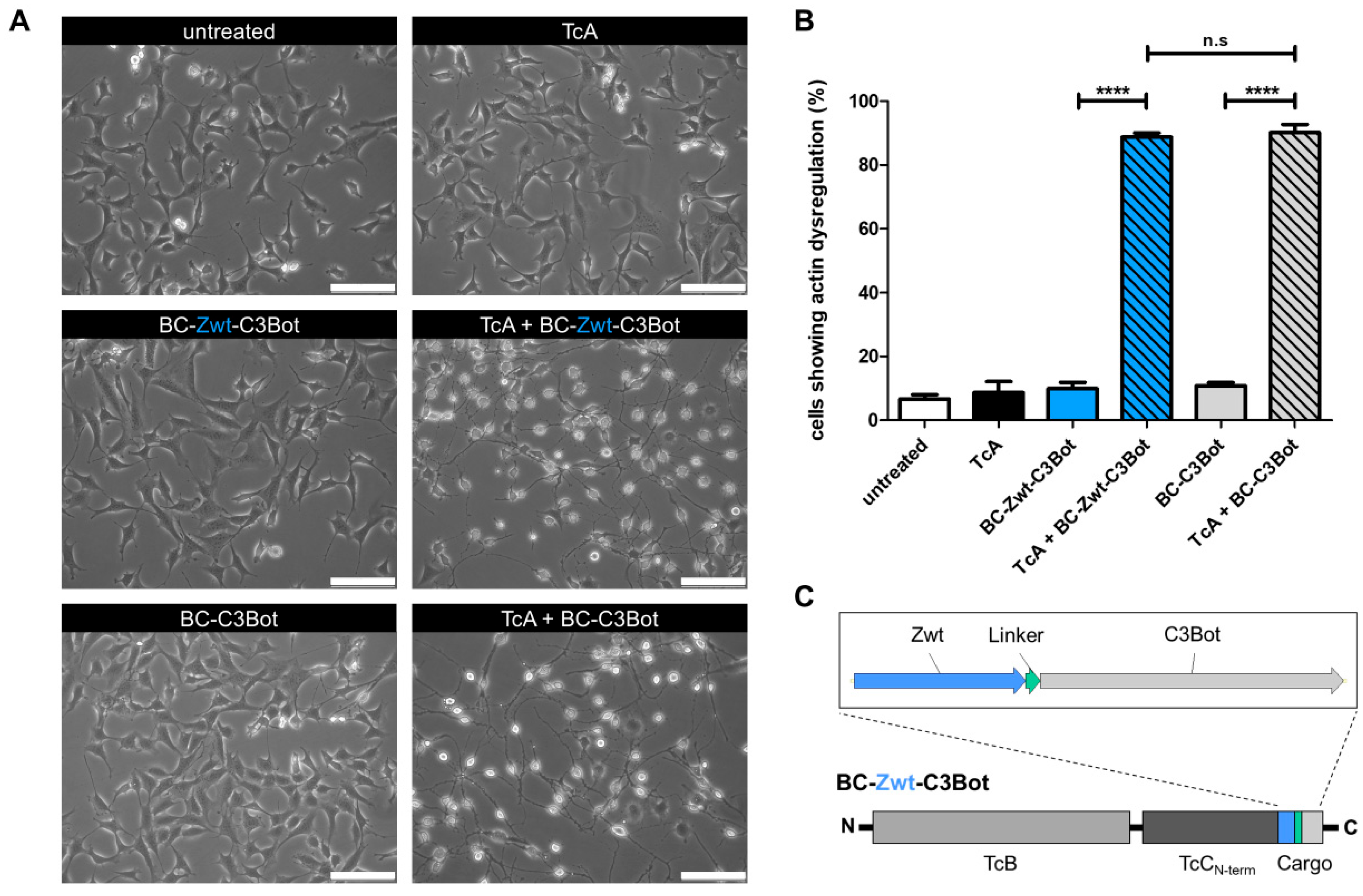
2.1. Injection of an Affibody–Toxin Fusion into Mammalian Cells
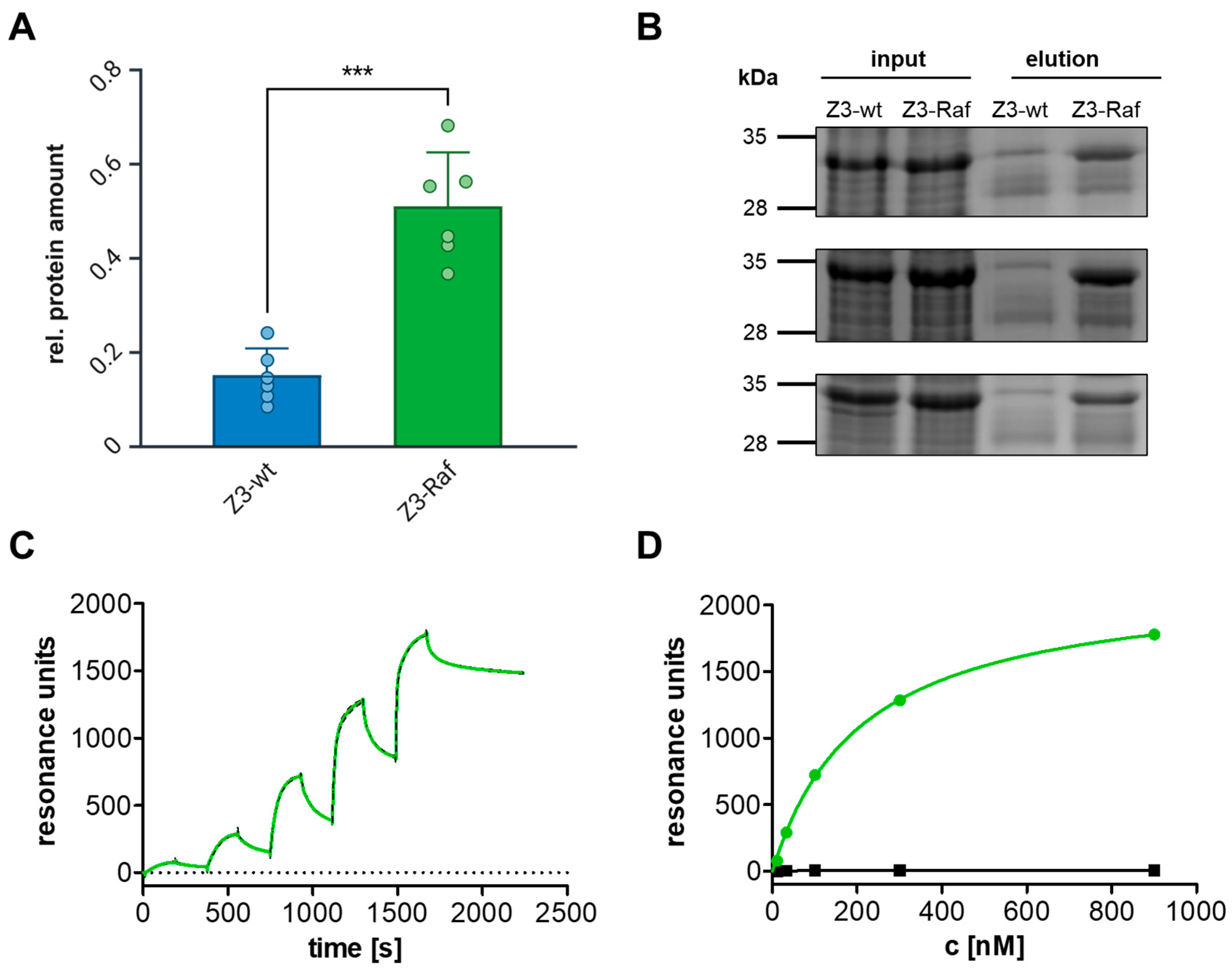
2.2. Z3-Affibody Construction and Binding Studies
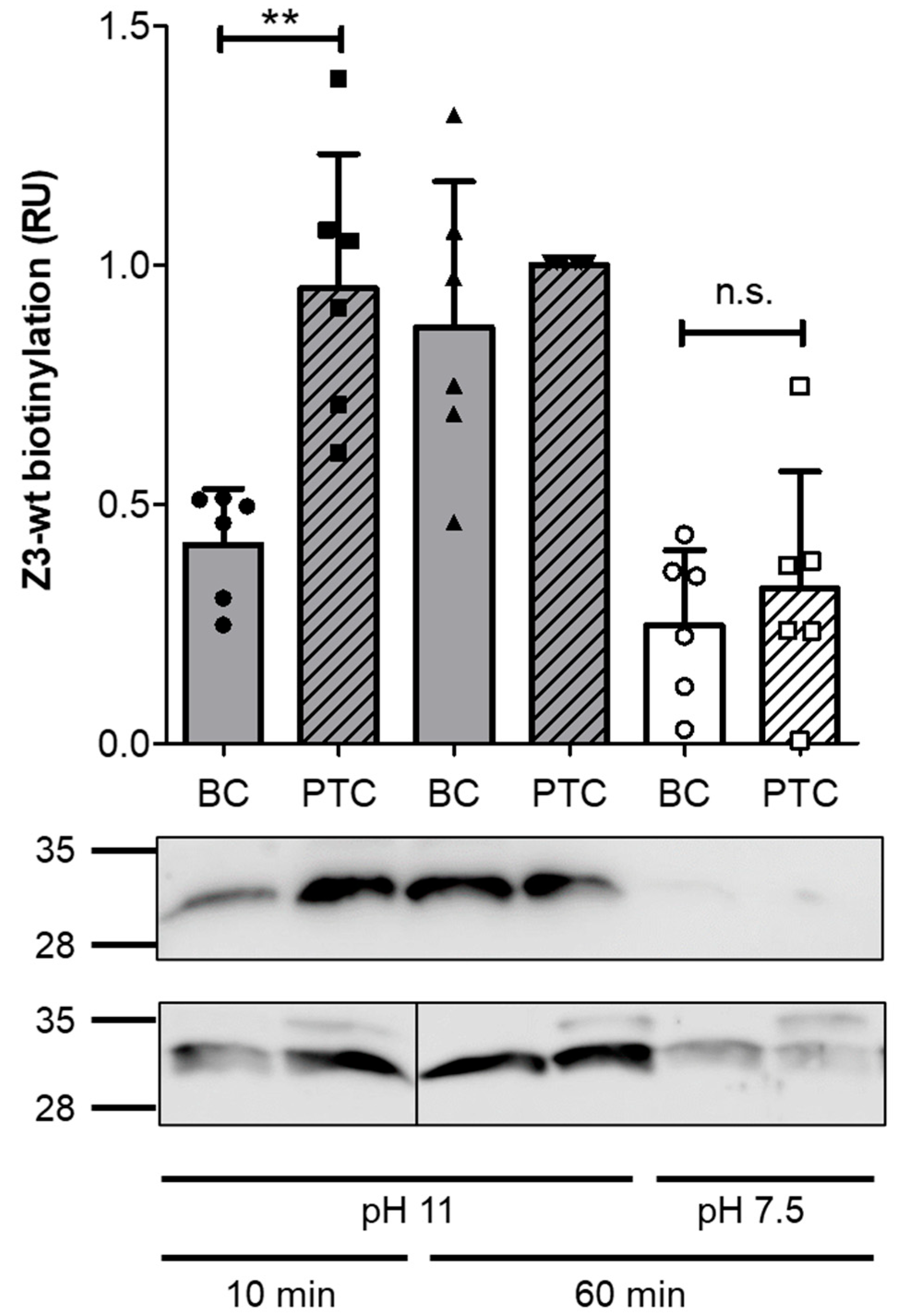
2.3. In Vitro Injection of Trimeric Affibodies
2.4. Effects of Z3-Affibody Expression on AP1 Activity
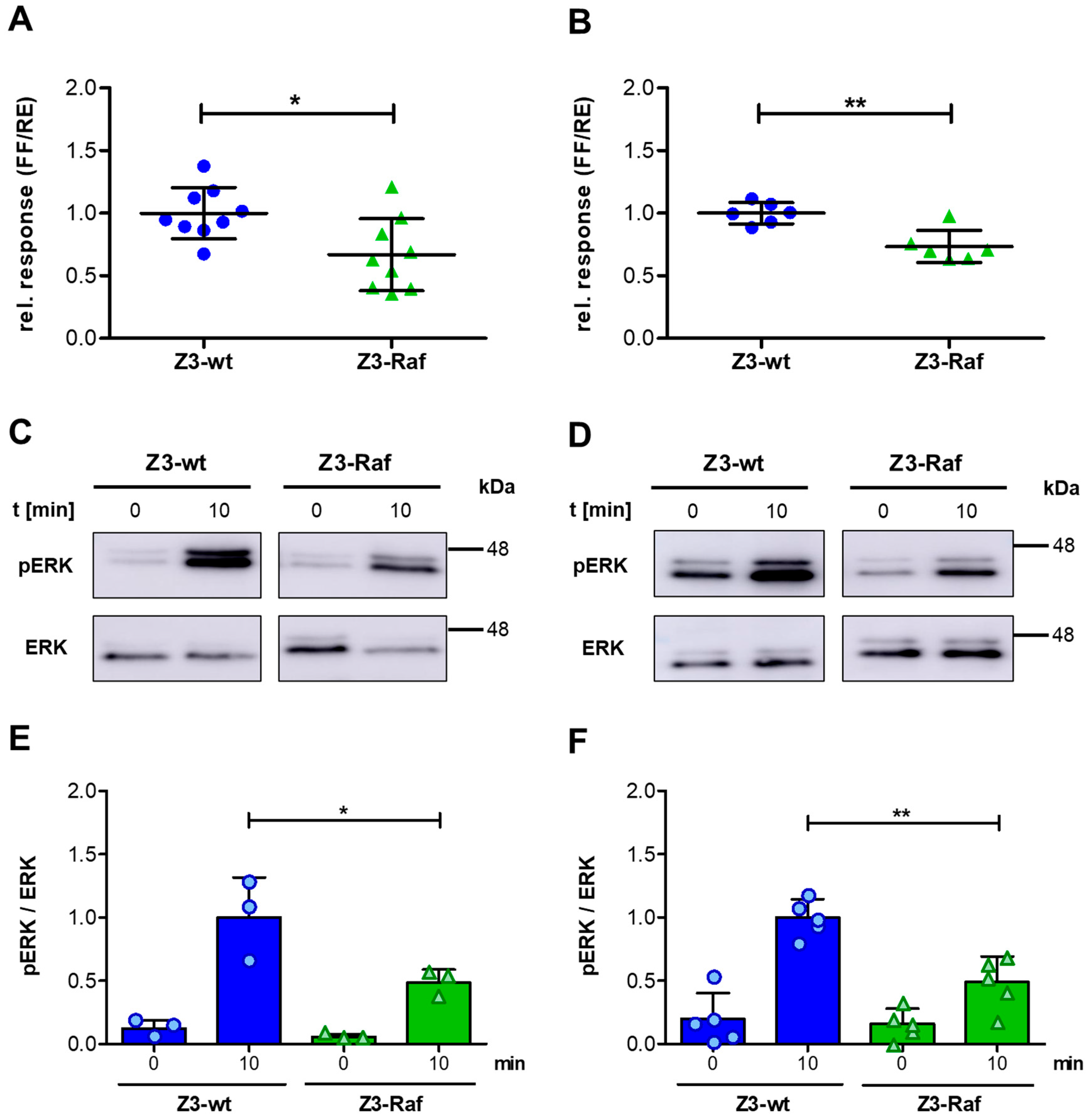
2.5. Effects of Z3-Affibody Injection on ERK Phosphorylation
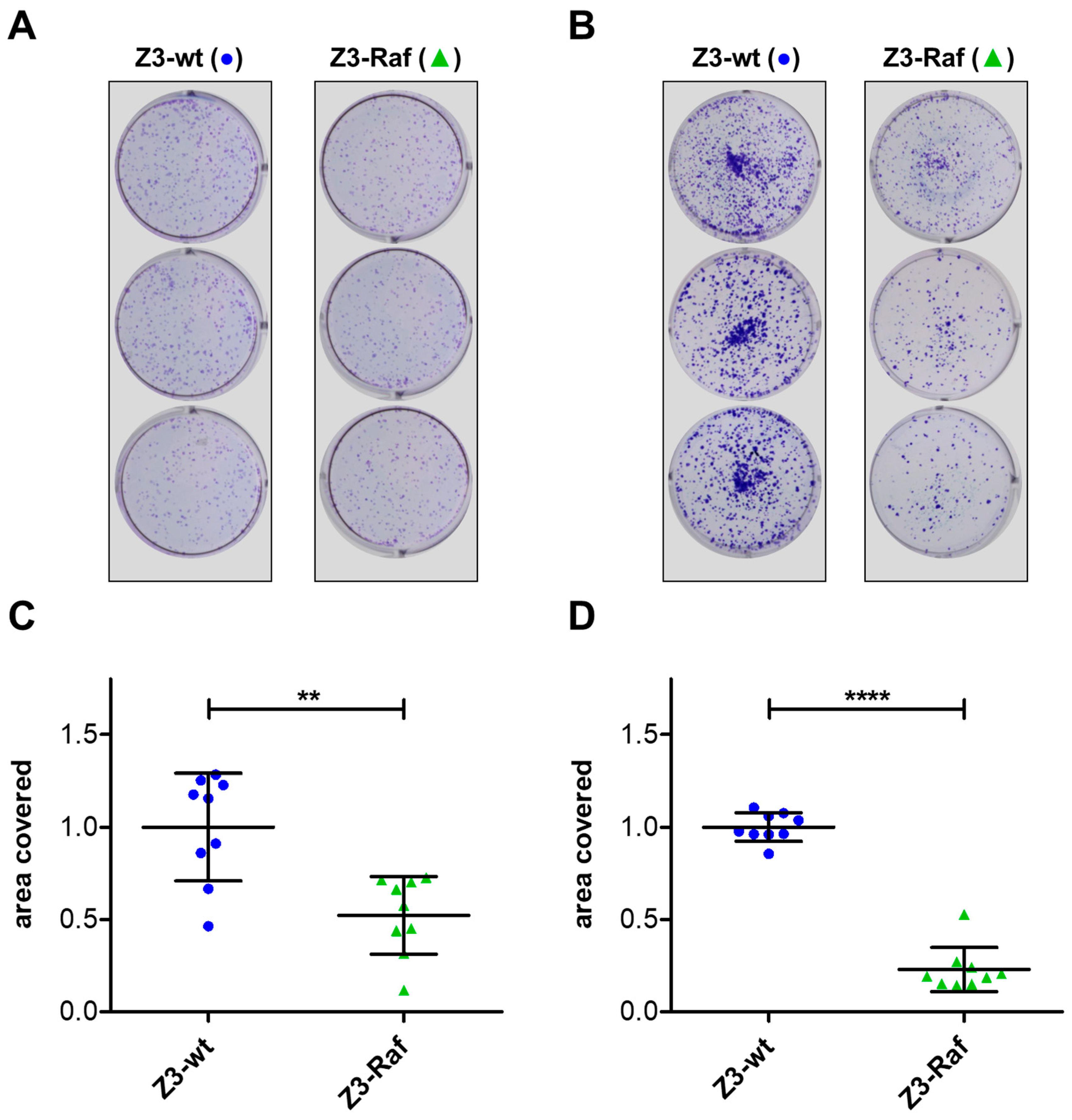
2.6. Colony Formation and Growth Following Affibody Injection
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Cloning of Z3-Affibody Constructs
4.3. Purification of Recombinant TcA and BC-Z3-Affibodies
4.4. Purification of Z3-Affibodies
4.5. Surface Plasmon Resonance Measurements
4.6. Pull-Down with Magnetic Beads
4.7. Pull-Down with GST-Glutathione Matrix
4.8. In Vitro Injection Assays
4.9. Colony Forming Assays
4.10. AP-1 Luciferase Reporter Assays
4.11. EGF Stimulation Assays
4.12. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Al Shaer, D.; Al Musaimi, O.; Albericio, F.; de la Torre, B.G. 2021 FDA TIDES (Peptides and Oligonucleotides) Harvest. Pharmaceuticals 2022, 15, 222. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, N.J.; Hinner, M.J. Getting across the cell membrane: An overview for small molecules, peptides, and proteins. Methods Mol. Biol. 2015, 1266, 29–53. [Google Scholar] [CrossRef] [PubMed]
- Beilhartz, G.L.; Sugiman-Marangos, S.N.; Melnyk, R.A. Repurposing bacterial toxins for intracellular delivery of therapeutic proteins. Biochem. Pharmacol. 2017, 142, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Bowen, D.; Rocheleau, T.A.; Blackburn, M.; Andreev, O.; Golubeva, E.; Bhartia, R.; Ffrench-Constant, R.H. Insecticidal toxins from the bacterium Photorhabdus luminescens. Science 1998, 280, 2129–2132. [Google Scholar] [CrossRef] [PubMed]
- Sergeant, M.; Jarrett, P.; Ousley, M.; Morgan, J.A. Interactions of insecticidal toxin gene products from Xenorhabdus nematophilus PMFI296. Appl. Environ. Microbiol. 2003, 69, 3344–3349. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jiang, F.; Li, N.; Wang, X.; Cheng, J.; Huang, Y.; Yang, Y.; Yang, J.; Cai, B.; Wang, Y.-P.; Jin, Q.; et al. Cryo-EM Structure and Assembly of an Extracellular Contractile Injection System. Cell 2019, 177, 370–383.e15. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Shen, J.; Cheng, J.; Wang, X.; Yang, J.; Li, N.; Gao, N.; Jin, Q. N-terminal signal peptides facilitate the engineering of PVC complex as a potent protein delivery system. Sci. Adv. 2022, 8, eabm2343. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cornelis, G.R. Type III secretion: A bacterial device for close combat with cells of their eukaryotic host. Philos. Trans. R. Soc. Lond 2000, 355, 681–693. [Google Scholar] [CrossRef]
- Ffrench-Constant, R.; Waterfield, N. An ABC guide to the bacterial toxin complexes. Adv. Appl. Microbiol. 2006, 58, 169–183. [Google Scholar] [PubMed]
- Roderer, D.; Raunser, S. Tc Toxin Complexes: Assembly, Membrane Permeation, and Protein Translocation. Annu. Rev. Microbiol. 2019, 73, 247–265. [Google Scholar] [CrossRef] [PubMed]
- Waterfield, N.; Hares, M.; Yang, G.; Dowling, A.; Ffrench-Constant, R. Potentiation and cellular phenotypes of the insecticidal Toxin complexes of Photorhabdus bacteria. Cell. Microbiol. 2005, 7, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Gatsogiannis, C.; Lang, A.E.; Meusch, D.; Pfaumann, V.; Hofnagel, O.; Benz, R.; Aktories, K.; Raunser, S. A syringe-like injection mechanism in Photorhabdus luminescens toxins. Nature 2013, 495, 520–523. [Google Scholar] [CrossRef] [PubMed]
- Busby, J.N.; Panjikar, S.; Landsberg, M.J.; Hurst, M.R.; Lott, J.S. The BC component of ABC toxins is an RHS-repeat-containing protein encapsulation device. Nature 2013, 501, 547–550. [Google Scholar] [CrossRef] [PubMed]
- Meusch, D.; Gatsogiannis, C.; Efremov, R.G.; Lang, A.E.; Hofnagel, O.; Vetter, I.R.; Aktories, K.; Raunser, S. Mechanism of Tc toxin action revealed in molecular detail. Nature 2014, 508, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Sheets, J.; Aktories, K. Insecticidal Toxin Complexes from Photorhabdus luminescens. Curr. Top. Microbiol. Immunol. 2017, 402, 3–23. [Google Scholar] [CrossRef] [PubMed]
- Gatsogiannis, C.; Merino, F.; Prumbaum, D.; Roderer, D.; Leidreiter, F.; Meusch, D.; Raunser, S. Membrane insertion of a Tc toxin in near-atomic detail. Nat. Struct. Mol. Biol. 2016, 23, 884–890. [Google Scholar] [CrossRef] [PubMed]
- Lang, A.E.; Schmidt, G.; Schlosser, A.; Hey, T.D.; Larrinua, I.M.; Sheets, J.J.; Mannherz, H.G.; Aktories, K. Photorhabdus luminescens toxins ADP-ribosylate actin and RhoA to force actin clustering. Science 2010, 327, 1139–1142. [Google Scholar] [CrossRef] [PubMed]
- Lang, A.E.; Schmidt, G.; Sheets, J.J.; Aktories, K. Targeting of the actin cytoskeleton by insecticidal toxins from Photorhabdus luminescens. Naunyn Schmiedebergs Arch. Pharmacol. 2011, 383, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Lang, A.E.; Qu, Z.; Schwan, C.; Silvan, U.; Unger, A.; Schoenenberger, C.A.; Aktories, K.; Mannherz, H.G. Actin ADP-ribosylation at Threonine148 by Photorhabdus luminescens toxin TccC3 induces aggregation of intracellular F-actin. Cell. Microbiol. 2017, 19, e12636. [Google Scholar] [CrossRef] [PubMed]
- Ng Ang, A.P.; Ebner, J.K.; Plessner, M.; Aktories, K.; Schmidt, G. Engineering Photorhabdus luminescens toxin complex (PTC) into a recombinant injection nanomachine. Life Sci. Alliance 2019, 2, e201900485. [Google Scholar] [CrossRef] [PubMed]
- Roderer, D.; Schubert, E.; Sitsel, O.; Raunser, S. Towards the application of Tc toxins as a universal protein translocation system. Nat. Commun. 2019, 10, 5263. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nilsson, B.; Moks, T.; Jansson, B.; Abrahmsen, L.; Elmblad, A.; Holmgren, E.; Henrichson, C.; Jones, T.A.; Uhlén, M. A synthetic IgG-binding domain based on staphylococcal protein A. Protein Eng. 1987, 1, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Hogbom, M.; Eklund, M.; Nygren, P.A.; Nordlund, P. Structural basis for recognition by an in vitro evolved affibody. Proc. Natl. Acad. Sci. USA 2003, 100, 3191–3196. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nord, K.; Nilsson, J.; Nilsson, B.; Uhlen, M.; Nygren, P.A. A combinatorial library of an alpha-helical bacterial receptor domain. Protein. Eng. 1995, 8, 601–608. [Google Scholar] [CrossRef] [PubMed]
- Nord, K.; Gunneriusson, E.; Ringdahl, J.; Stahl, S.; Uhlen, M.; Nygren, P.A. Binding proteins selected from combinatorial libraries of an alpha-helical bacterial receptor domain. Nat. Biotechnol. 1997, 15, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Nygren, P.A. Alternative binding proteins: Affibody binding proteins developed from a small three-helix bundle scaffold. FEBS J. 2008, 275, 2668–2676. [Google Scholar] [CrossRef] [PubMed]
- Lofblom, J.; Feldwisch, J.; Tolmachev, V.; Carlsson, J.; Stahl, S.; Frejd, F.Y. Affibody molecules: Engineered proteins for therapeutic, diagnostic and biotechnological applications. FEBS Lett. 2010, 584, 2670–2680. [Google Scholar] [CrossRef] [PubMed]
- Orlova, A.; Magnusson, M.; Eriksson, T.L.; Nilsson, M.; Larsson, B.; Hoiden-Guthenberg, I.; Widström, C.; Carlsson, J.; Tolmachev, V.; Ståhl, S.; et al. Tumor imaging using a picomolar affinity HER2 binding affibody molecule. Cancer Res. 2006, 66, 4339–4348. [Google Scholar] [CrossRef] [PubMed]
- Malm, M.; Kronqvist, N.; Lindberg, H.; Gudmundsdotter, L.; Bass, T.; Frejd, F.Y.; Höidén-Guthenberg, I.; Varasteh, Z.; Orlova, A.; Tolmachev, V.; et al. Inhibiting HER3-mediated tumor cell growth with affibody molecules engineered to low picomolar affinity by position-directed error-prone PCR-like diversification. PLoS ONE 2013, 8, e62791. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lindberg, H.; Hard, T.; Lofblom, J.; Stahl, S. A truncated and dimeric format of an Affibody library on bacteria enables FACS-mediated isolation of amyloid-beta aggregation inhibitors with subnanomolar affinity. Biotechnol. J. 2015, 10, 1707–1718. [Google Scholar] [CrossRef] [PubMed]
- Stahl, S.; Graslund, T.; Eriksson Karlstrom, A.; Frejd, F.Y.; Nygren, P.A.; Lofblom, J. Affibody Molecules in Biotechnological and Medical Applications. Trends Biotechnol. 2017, 35, 691–712. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, J.; Sandberg, D.; Sandstrom, M.; Wennborg, A.; Feldwisch, J.; Tolmachev, V.; Åström, G.; Lubberink, M.; Garske-Román, U.; Carlsson, J.; et al. First-in-human molecular imaging of HER2 expression in breast cancer metastases using the 111In-ABY-025 affibody molecule. J. Nucl. Med. 2014, 55, 730–735. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Wallberg, H.; Grafstrom, J.; Lu, L.; Thorell, J.O.; Hagg Olofsson, M.; Linder, S.; Johansson, K.; Tegnebratt, T.; Arnér, E.S.; et al. Preclinical PET imaging of EGFR levels: Pairing a targeting with a non-targeting Sel-tagged Affibody-based tracer to estimate the specific uptake. EJNMMI Res. 2016, 6, 58. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rosestedt, M.; Andersson, K.G.; Mitran, B.; Tolmachev, V.; Lofblom, J.; Orlova, A.; Ståhl, S. Affibody-mediated PET imaging of HER3 expression in malignant tumours. Sci. Rep. 2015, 5, 15226. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Strand, J.; Varasteh, Z.; Eriksson, O.; Abrahmsen, L.; Orlova, A.; Tolmachev, V. Gallium-68-labeled affibody molecule for PET imaging of PDGFRbeta expression in vivo. Mol. Pharm. 2014, 11, 3957–3964. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lundberg, E.; Vernet, E.; Larsson, B.; Hoiden-Guthenberg, I.; Graslund, T. Selection of affibody molecules to the ligand-binding site of the insulin-like growth factor-1 receptor. Biotechnol. Appl. Biochem. 2010, 55, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Lindborg, M.; Cortez, E.; Hoiden-Guthenberg, I.; Gunneriusson, E.; von Hage, E.; Syud, F.; Morrison, M.; Abrahmsén, L.; Herne, N.; Pietras, K.; et al. Engineered high-affinity affibody molecules targeting platelet-derived growth factor receptor beta in vivo. J. Mol. Biol. 2011, 407, 298–315. [Google Scholar] [CrossRef] [PubMed]
- Kronqvist, N.; Malm, M.; Gostring, L.; Gunneriusson, E.; Nilsson, M.; Hoiden Guthenberg, I.; Gedda, L.; Frejd, F.Y.; Ståhl, S.; Löfblom, J. Combining phage and staphylococcal surface display for generation of ErbB3-specific Affibody molecules. Protein. Eng. Des. Sel. 2011, 24, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Fleetwood, F.; Klint, S.; Hanze, M.; Gunneriusson, E.; Frejd, F.Y.; Stahl, S.; Löfblom, J. Simultaneous targeting of two ligand-binding sites on VEGFR2 using biparatopic Affibody molecules results in dramatically improved affinity. Sci. Rep. 2014, 4, 7518. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, H. 177Lu-CHX-A″-DTPA-ABD-Affibody (ZHER2:342)2. In Molecular Imaging and Contrast Agent Database (MICAD); National Center for Biotechnology Information (US): Bethesda, MD, USA, 2004. [Google Scholar]
- Grimm, S.; Lundberg, E.; Yu, F.; Shibasaki, S.; Vernet, E.; Skogs, M.; Nygren, P.Å.; Gräslund, T. Selection and characterisation of affibody molecules inhibiting the interaction between Ras and Raf in vitro. New Biotechnol. 2010, 27, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Grimm, S.; Salahshour, S.; Nygren, P.A. Monitored whole gene in vitro evolution of an anti-hRaf-1 affibody molecule towards increased binding affinity. New Biotechnol. 2012, 29, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Shibasaki, S.; Karasaki, M.; Graslund, T.; Nygren, P.A.; Sano, H.; Iwasaki, T. Inhibitory effects of H-Ras/Raf-1-binding affibody molecules on synovial cell function. AMB Express 2014, 4, 82. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liao, X.; Rabideau, A.E.; Pentelute, B.L. Delivery of antibody mimics into mammalian cells via anthrax toxin protective antigen. Chembiochem 2014, 15, 2458–2466. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kalthoff, H.; Schmiegel, W.; Roeder, C.; Kasche, D.; Schmidt, A.; Lauer, G.; Thiele, H.G.; Honold, G.; Pantel, K.; Riethmüller, G. p53 and K-RAS alterations in pancreatic epithelial cell lesions. Oncogene 1993, 8, 289–298. [Google Scholar]
- Sha, F.; Salzman, G.; Gupta, A.; Koide, S. Monobodies and other synthetic binding proteins for expanding protein science. Protein. Sci. 2017, 26, 910–924. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ng’ang’a, P.N.; Folz, J.; Kucher, S.; Roderer, D.; Xu, Y.; Sitsel, O.; Belyy, A.; Prumbaum, D.; Kühnemuth, R.; Assafa, T.E. Multistate kinetics of the syringe-like injection mechanism of Tc toxins. Sci. Adv. 2025, 11, eadr2019. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kusama, T.; Mukai, M.; Iwasaki, T.; Tatsuta, M.; Matsumoto, Y.; Akedo, H.; Nakamura, H. Inhibition of epidermal growth factor-induced RhoA translocation and invasion of human pancreatic cancer cells by 3-hydroxy-3-methylglutaryl-coenzyme a reductase inhibitors. Cancer Res. 2001, 61, 4885–4891. [Google Scholar]
- Leidreiter, F.; Roderer, D.; Meusch, D.; Gatsogiannis, C.; Benz, R.; Raunser, S. Common architecture of Tc toxins from human and insect pathogenic bacteria. Sci. Adv. 2019, 5, eaax6497. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Roderer, D.; Brocker, F.; Sitsel, O.; Kaplonek, P.; Leidreiter, F.; Seeberger, P.H.; Raunser, S. Glycan-dependent cell adhesion mechanism of Tc toxins. Nat. Commun. 2020, 11, 2694. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ng’ang’a, P.N.; Siukstaite, L.; Lang, A.E.; Bakker, H.; Romer, W.; Aktories, K.; Schmidt, G. Involvement of N-glycans in binding of Photorhabdus luminescens Tc toxin. Cell. Microbiol. 2021, 23, e13326. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Viswanatha, R.; Sitsel, O.; Roderer, D.; Zhao, H.; Ashwood, C.; Voelcker, C.; Tian, S.; Raunser, S.; Perrimon, N.; et al. CRISPR screens in Drosophila cells identify Vsg as a Tc toxin receptor. Nature 2022, 610, 349–355. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Müller, T.; Gieß, S.; Maier, F.; Hofacker, L.; Stenger, L.; Parker, L.; Grosse, R.; Schmidt, G. Injection of Affibodies by a Self-Organizing Bacterial Syringe to Interfere with Intracellular Signaling. Toxins 2025, 17, 448. https://doi.org/10.3390/toxins17090448
Müller T, Gieß S, Maier F, Hofacker L, Stenger L, Parker L, Grosse R, Schmidt G. Injection of Affibodies by a Self-Organizing Bacterial Syringe to Interfere with Intracellular Signaling. Toxins. 2025; 17(9):448. https://doi.org/10.3390/toxins17090448
Chicago/Turabian StyleMüller, Thomas, Sophie Gieß, Fanny Maier, Lara Hofacker, Luca Stenger, Larissa Parker, Robert Grosse, and Gudula Schmidt. 2025. "Injection of Affibodies by a Self-Organizing Bacterial Syringe to Interfere with Intracellular Signaling" Toxins 17, no. 9: 448. https://doi.org/10.3390/toxins17090448
APA StyleMüller, T., Gieß, S., Maier, F., Hofacker, L., Stenger, L., Parker, L., Grosse, R., & Schmidt, G. (2025). Injection of Affibodies by a Self-Organizing Bacterial Syringe to Interfere with Intracellular Signaling. Toxins, 17(9), 448. https://doi.org/10.3390/toxins17090448





