Abstract
Bacterial diseases of Pleurotus pulmonarius, caused by diverse pathogens and associated with a range of symptoms, reduce its commercial value and lead to substantial economic losses. While most research has focused on Pseudomonas tolaasii and its non-volatile toxin tolaasin, little is known about other bacterial pathogens and their volatile metabolites. In this study, two bacterial pathogens were isolated from symptomatic P. pulmonarius fruiting bodies in Guangxi, China, and identified as Ewingella americana and Cedecea neteri. Using headspace solid-phase microextraction coupled with gas chromatography–mass spectrometry (HS-SPME-GC-MS), we identified 16 volatile organic compounds (VOCs) produced by these two species, seven of which exhibited toxicity-inducing sunken lesions, discoloration, and inhibition of mycelial growth. Symptom severity was quantified by colorimetric analysis. Among the toxic VOCs, 2,4-di-tert-butylphenol was the most potent, inducing sunken lesions and slight discoloration at concentrations as low as 0.5 mg/mL, and causing significant inhibition of mycelial growth at 5 μg/L. The remaining VOCs also caused varying degrees of sunken lesions, yellowing or browning, and suppression of mycelial growth. This study is the first to demonstrate the pathogenic potential of VOCs produced by bacterial pathogens in P. pulmonarius, underscoring their role as important virulence factors and providing a foundation for further investigation into their mechanisms and control strategies.
Keywords:
Pleurotus pulmonarius; bacterial disease; Ewingella americana; Cedecea neteri; volatile organic compound; toxicity Key Contribution:
This is the first study to demonstrate the toxicity of Volatile Organic Compounds (VOCs) produced by bacterial pathogens Ewingella americana and Cedecea neteri in Pleurotus pulmonarius, underscoring their role as important virulence factors.
1. Introduction
Pleurotus pulmonarius is an important edible and medicinal fungus that has attracted much attention due to its unique nutritional composition and health effects [1]. However, it is prone to disease in high-temperature and high-humidity environments, with yellow blotch and brown blotch caused by Pseudomonas bacteria as the major diseases. Currently, research on bacterial diseases of edible mushrooms mainly focuses on Agaricus bisporus [2] and Pleurotus ostreatus [3]. Pseudomonas, represented by Pseudomonas tolaasii, has become one of the most extensively studied pathogenic bacteria due to its widespread distribution, high pathogenicity, and diverse toxin mechanisms [4]. This bacterium can cause a series of important diseases, such as gingeri blotch disease in A. bisporus [5] and brown rot disease in P. ostreatus [6] and Pleurotus citrinipileatus [7]. Related research covers the molecular structure, biosynthesis pathways, mechanisms of action, and control measures of its toxin, Tolaasin. In addition, P. fluorescens [8], Pseudomonas migulae [9] and others have also been proven to cause rot or spot lesions on the fruiting bodies of various edible fungi, and Pseudomonas is a central topic in the study of bacterial diseases of edible fungi.
However, research on bacterial diseases of P. pulmonarius remains limited, particularly regarding pathogenic mechanisms, epidemiology, and control strategies. Current studies indicate that Ewingella americana and Cedecea neteri exhibit strong pathogenicity across multiple edible fungi. C. neteri produces phenylacetic acid and p-hydroxybenzoic acid, which induce brown spots on both mature and immature caps of Agaricus bisporus [10]. C. neteri has also been associated with yellow rot in P. pulmonarius [11], yellow sticky disease in Flammulina velutipes [12], and decay symptoms in P. pulmonarius [13]. E. americana is known to cause stipe necrosis in Pleurotus spp. [14] and brown rot in Naematelia aurantialba [15], Lentinula edodes [16], Pleurotus eryngii [17], and F. velutipes [18]. These infections significantly disrupt cell structure and metabolic functions, leading to reduced edibility and commercial value. Although we have successfully isolated Ewingella americana and Cedecea neteri from diseased tissues and confirmed their pathogenicity, their virulence factors, pathogenic mechanisms, and interactions with other microorganisms remain poorly understood. Currently, there is no systematic research on their transmission modes or toxic metabolites in P. pulmonarius, which significantly hinders both our understanding of disease complexity and the development of effective control strategies.
In the study of toxins related to bacterial diseases in edible fungi, non-volatile toxins such as lipopeptides (e.g., tolaasin) have received a great deal of attention due to their direct effects on cell membranes, their role in pore formation, and their ability to induce cell lysis [19]. Furthermore, small-molecule toxins, including monoacetylphloroglucinol (MAPG), p-hydroxybenzoic acid (PHBA), and phenylacetic acid (PAA), have also been shown to contribute to the development of mushroom brown blotch [2,10]. These toxins have therefore become important targets for the development of control measures.
However, studies into the toxicity, pathogenic mechanisms, and related effects of pathogen-derived volatile organic compounds (VOCs) on edible fungi remain limited. To date, only one study has reported that VOCs produced by Ps. tolaasii exhibit toxicity toward P. ostreatus and P. eryngii. Under non-sealed conditions, the VOCs produced by Ps. tolaasii inhibit mycelial growth in both P. ostreatus and P. eryngii, with a maximum inhibition rate of 90%. These VOCs also cause browning and softening of the fruiting body tissue of A. bisporus and P. ostreatus. GC-MS analysis revealed that Ps. tolaasii produces VOCs, including methanethiol, dimethyl disulfide, and 1-undecene, which are toxic to mushrooms [20]. VOCs produced by endofungal bacteria (Pseudomonas sp. Bi1 and De1, Bacillus sp. De3, Pantoea sp. Ma3) have been reported to exhibit antagonistic activity against Ps. tolaasii [21]. These compounds significantly reduced brown blotch on mushroom caps and inhibited the growth of Ps. tolaasii to varying degrees. Therefore, further research on the volatile toxins released by P. pulmonarius pathogens, particularly whether E. americana and C. neteri possess characteristic VOCs and their biological functions, will provide a new scientific basis for the rapid detection and green prevention and control of edible mushroom diseases.
Based on the above analysis, we isolated and identified E. americana and C. neteri as the primary bacterial pathogens responsible for diseases in P. pulmonarius cultivation facilities in Guangxi, China. In view of the current research gap concerning P. pulmonarius diseases, especially the limited understanding of these two atypical edible mushroom pathogens, and the significant lack of research on related toxins, particularly volatile toxins, this study aims to systematically elucidate their pathogenic features and VOC profiles. The findings are expected to provide both theoretical insights and practical guidance for elucidating the pathogenic mechanisms and virulence factors involved in bacterial diseases of P. pulmonarius, as well as for developing effective early warning systems and control strategies.
2. Results
2.1. Pathogen Isolation and Pathogenicity Test
Two distinct symptoms were observed on P. pulmonarius fruiting bodies collected from Nanning, Hechi, and Baise in Guangxi, China: yellow-brown spots and yellow blotches (Figure 1a,b). These symptoms resulted in a disease incidence of approximately 5–10%, significantly reducing both yield and commercial value. A total of 30 bacterial strains were isolated from symptomatic tissues, and pathogenicity tests confirmed 24 strains were pathogenic to P. pulmonarius. Among these, 13 pathogenic strains (KD1-1, KD1-2, KD1-6, KD2-1, KD2-3, KD3-4, KD3-5, KD3-7, KD4-4, ST1-2, ST2-4, ST3-2, ST3-18) were isolated from fruiting bodies showing yellow-brown spot, while the remaining 11 strains (KD3-13, KD5-1, KD5-2, KD5-4, KD5-8, KD7-3, XC1-2, XC1-5, XC2-5, XC2-9, XC2-10) were obtained from fruiting bodies with yellow blotch lesions.
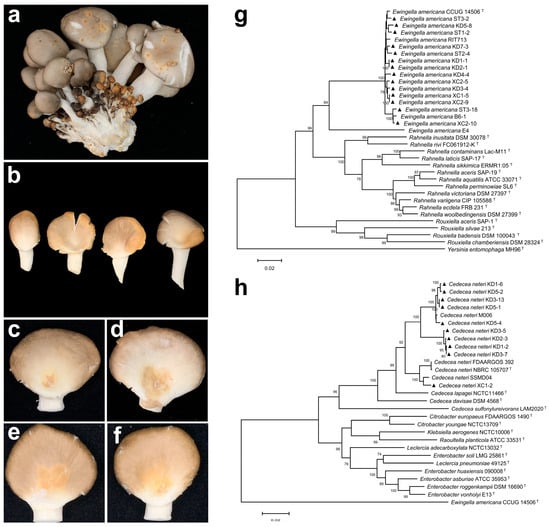
Figure 1.
Symptoms of natural infection of blight disease and yellow rot disease in P. pulmonarius, pathogenicity testing, and multi-locus sequence analysis of the pathogens. (a) Yellow–brown spots on naturally infected fruiting bodies. (b) Yellow blotch on naturally infected fruiting bodies. (c) Symptoms 48 h after stab inoculation with E. americana suspension. (d) Symptoms 48 h after droplet inoculation with E. americana suspension. (e) Symptoms 48 h after stab inoculation with C. neteri suspension. (f) Symptoms 48 h after droplet inoculation with C. neteri suspension. (g) Phylogenetic tree of inter-species relationships within the Yersiniaceae family, based on concatenated sequences of 16S rRNA (1–1154 bp), atpD (1155–1942 bp), dnaJ (1943–2643 bp), tuf (2644–3277 bp), and gyrB (3278–4338 bp). (h) Phylogenetic tree of C. neteri and closely related species based on concatenated sequences of 16S rRNA (1–1333 bp), atpD (1334–2221 bp), dnaJ (2222–2933 bp), tuf (2934–3687 bp), and gyrB (3688–4777 bp). The tree was constructed using the Maximum Likelihood method (ML). ▲ indicates pathogenic strains obtained in this study. The superscript ‘T’ marks sequences from type strains.
Strains KD1-1, KD2-1, KD3-4, KD4-4, KD5-8, KD7-3, ST1-2, ST2-4, ST3-2, ST3-18, XC1-5, XC2-5, XC2-9, and XC2-10 consistently induced characteristic symptoms: stab inoculation resulted in yellow sunken spots on the caps (Figure 1c), while droplet inoculation produced expanding lesions with increasingly yellow sunken areas over time (Figure 1d). In contrast, strains KD1-2, KD1-6, KD2-3, KD3-5, KD3-7, KD3-13, KD5-1, KD5-2, KD5-4, and XC1-2 induced distinct symptoms: stab inoculation caused yellow blotches, and droplet inoculation led to yellow blotch lesions without prominent sunken areas (Figure 1f).
2.2. Pathogen Identification
All pathogenic strains formed ivory-colored, round, convex, smooth and opaque cultures on nutrient agar (NA) medium. These strains were Gram-negative and did not produce fluorescent pigments on King’s B medium (Figure S1).
Based on multi-locus sequence analysis (MLSA), strains KD1-1, KD2-1, KD3-4, KD4-4, KD5-8, KD7-3, ST1-2, ST2-4, ST3-2, ST3-18, XC1-5, XC2-5, XC2-9, and XC2-10 were clustered with E. americana (Figure 1g). All strains were catalase-positive and oxidase-negative. Esculin and salicin were hydrolyzed, whereas gelatin, arginine, and urea were not. Glucose, lactose, and mannitol were utilized, but sucrose, arabinose, and rhamnose were not. Nitrate reduction tested positive (Table S1). These characteristics are consistent with those of the E. americana type strain ATCC 33852 [22]. Therefore, the 14 strains were identified as E. americana based on MLSA, morphological traits, and physio-biochemical profiles.
The phylogenetic tree clustered the strains KD1-2, KD1-6, KD2-3, KD3-5, KD3-7, KD3-13, KD5-1, KD5-2, KD5-4 and XC1-2 with C. neteri (Figure 1h). These strains exhibited consistent physio-biochemical characteristics: they were catalase-positive and oxidase-negative; capable of hydrolyzing esculin, salicin, and urea, but not gelatin or arginine; utilized glucose, lactose, sucrose, mannitol, and salicin, but not arabinose or rhamnose; and tested positive for nitrate reduction (Table S2). These traits are consistent with those of the type strain C. neteri ATCC 33855. Based on MLSA, morphological characteristics, and physio-biochemical profiles, these 10 strains were identified as C. neteri.
2.3. Effect of VOCs from E. americana and C. neteri on P. pulmonarius
In the dual-culture assay, VOCs produced by E. americana and C. neteri strains significantly inhibited the growth of P. pulmonarius strain TaiXiu 57 mycelium (Figure 2a,c). The mycelial growth curves (Figure 2d) showed that inhibition began at 36 h of co-culture and nearly halted growth by 60 h. These results indicate that VOCs from the two pathogenic strains reached peak inhibitory effects at 60 h, effectively suppressing the growth of P. pulmonarius mycelium.
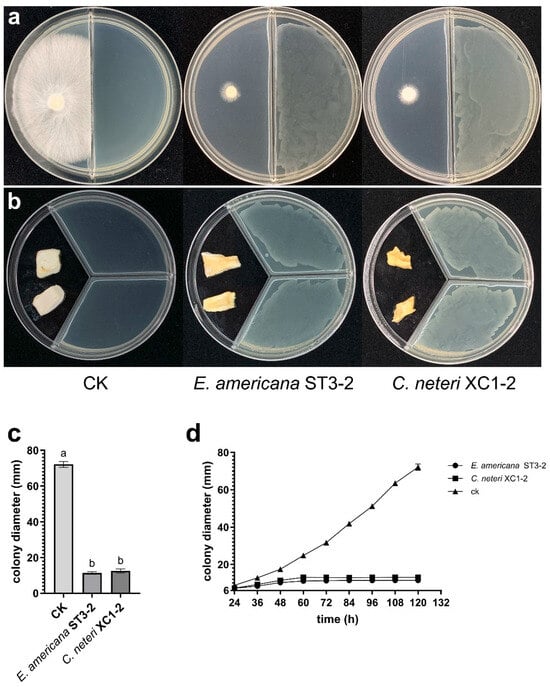
Figure 2.
Effects of VOCs from E. americana and C. neteri on mycelial growth and tissue block morphology of P. pulmonarius. (a) Inhibition of P. pulmonarius mycelial growth by VOCs from two pathogenic strains after 120 h of co-culture; (b) Effect of VOCs from two pathogenic strains on P. pulmonarius tissue blocks after 72 h of exposure; (c) Colony diameters of P. pulmonarius after 120 h of exposure to VOCs. Different letters (a, b) above the bars indicate significant differences (p < 0.05).; (d) Mycelial growth curve of P. pulmonarius under VOCs exposure from 24 to 120 h.
The effects of VOCs produced by E. americana and C. neteri strains on P. pulmonarius tissue blocks were also investigated. After 72 h of exposure, VOCs from both pathogenic strains induced yellow rot symptoms in the tissue blocks, accompanied by a foul odor (Figure 2b). Among the two pathogens, C. neteri caused more severe symptoms, resulting in water-soaked soft rot. In contrast, the control tissue blocks showed no discoloration, and mycelial growth was observed on surface.
2.4. GC-MS Analysis of VOCs from E. americana and C. neteri on P. pulmonarius
As shown in Table 1, E. americana ST3-2 produced 9 VOCs, classified into four categories: alcohols (isoamyl alcohol, 2-ethylhexan-1-ol, 2-phenylethanol, and 2-undecanol), esters (isoamyl acetate), ketones (2-heptanone, 2-nonanone, and 2-undecanone), and phenols (2,4-di-tert-butylphenol). The three most abundant compounds were 2-nonanone (14.72%), 2,4-di-tert-butylphenol (11.93%), and isoamyl acetate (10.50%).

Table 1.
Volatile organic compounds (VOCs) identified from E. americana and C. neteri by GC-MS.
C. neteri XC1-2 produced 15 VOCs, including six ketones (2-heptanone, 2-nonanone, 2-decanone, 2-undecanone, 2-dodecanone, and 2-tridecanone), five alcohols (2-ethylhexan-1-ol, 2-heptanol, 2-nonanol, 2-phenylethanol, and 2-undecanol), one ester (isoamyl acetate), one phenol (2,4-di-tert-butylphenol), one sulfur compound (dimethyl trisulfide), and one aromatic compound (1,3-di-tert-butylbenzene). The predominant VOC was 2-undecanone, accounting for 32.71% of the total. Other compounds, such as dimethyl trisulfide, 1,3-di-tert-butylbenzene, 2-ethylhexan-1-ol, 2-heptanol and 2-dodecanone, were present at levels below 1%.
The total ion chromatograms (TICs) and mass spectrum of the GC-MS analyses are provided in Figures S2–S4.
2.5. Toxicity of VOCs on P. pulmonarius
From the 16 identified VOCs, seven compounds were selected based on their ability to induce severe discoloration in P. pulmonarius fruiting bodies at 4 mg/mL (Figure S5). The chemical structures of these VOCs are presented in Figure 3a–g. The toxicity of VOCs produced by C. neteri and E. americana was then evaluated on P. pulmonarius fruiting body and mycelial growth. Among these seven VOCs, significant differences in phytotoxic effects and concentration-dependent symptoms were observed.
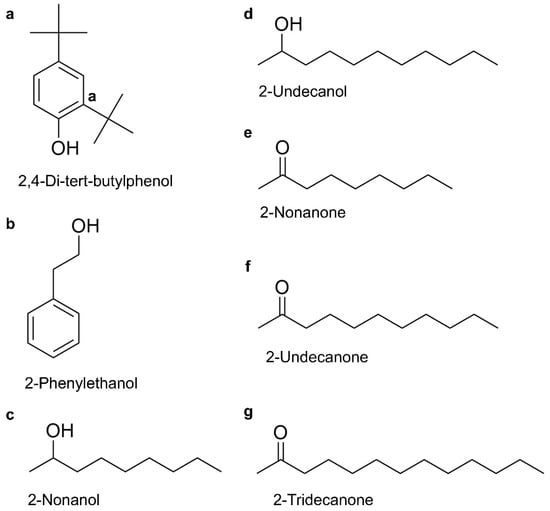
Figure 3.
The chemical structures of seven toxic VOCs produced by E. americana and C. neteri; (a) 2,4-di-tert-butylphenol; (b) 2-phenylethanol; (c) 2-nonanol; (d) 2-undecanol; (e) 2-nonanone; (f) 2-undecanone; (g) 2-tridecanone.
All tested VOCs induced varying degrees of tissue sinking and discoloration on P. pulmonarius, with severity increasing at higher concentrations (Figure 4a–g). 2,4-DTBP exhibited moderate toxicity even at low concentrations. At 0.5 mg/mL, it induced apparent sunken lesions rather than discoloration, with both symptoms worsening as the concentration increased. At 4 mg/mL, 2-tridecanone caused large crater-like sunken lesions with slight browning. Both 2-undecanone and 2-nonanone induced sunken and discoloration at concentrations ≥ 2 mg/mL. In particular, 2-undecanone caused increasingly severe browning at the inoculation site with rising concentrations, whereas 2-nonanone only induced noticeable discoloration at the highest concentration. Likewise, 2-phenylethanol caused only mild discoloration at 1–2 mg/mL but led to significant yellowing at 4 mg/mL. For 2-undecanol and 2-nonanol, mild symptoms were observed at 0.5 mg/mL. However, the toxicity of 2-undecanol increased with concentration, causing noticeable yellowing at 1 mg/mL. At 4 mg/mL, both alcohols induced pronounced discoloration and sunken lesions.
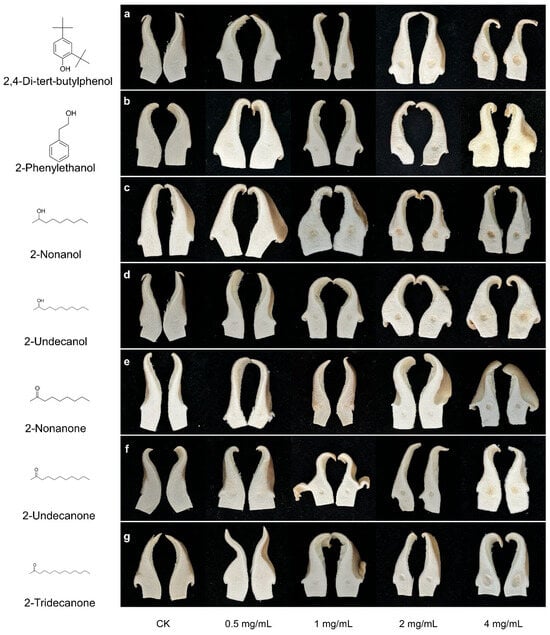
Figure 4.
Symptoms of P. pulnomarius induced by different concentration of seven toxic VOCs. (a) 2,4-di-tert-butylphenol; (b) 2-phenylethanol; (c) 2-nonanol; (d) 2-undecanol; (e) 2-nonanone; (f) 2-undecanone; (g) 2-tridecanone. The control was a sterile 4% dimethyl sulfoxide aqueous solution used to dissolve the test compounds.
To quantify discoloration patterns in fruiting bodies, colorimetric analysis was conducted across the full concentration range (0–4 mg/mL). The seven VOCs induced distinct, concentration-dependent color changes in P. pulmonarius (Figure 5a–g). 2,4-DTBP triggered early and broad-spectrum discoloration, characterized by strong increases in redness (a: +99.69%, +5.32) and yellowness (b: +39.88%, +6.02), along with the most pronounced reduction in lightness (L: −32.20%, −27.98), indicating intense browning. In contrast, 2-phenylethanol produced a gradual yellowing trend, with b values rising from +13.19% (+1.80) at 0.5 mg/mL to +56.04% (+7.65) at 4 mg/mL, exceeding its redshift (a: +49.54%, +2.69). The modest decrease in L (−8.87%, −7.67) supports yellowing as the dominant effect. Similarly, 2-undecanol acted as a potent redshift agent, showing early and strong increases in a (+81.53%, +4.37 at 4 mg/mL), accompanied by moderate changes in b and L, indicative of combined reddening and browning. Both 2-nonanone and 2-undecanone exhibited.
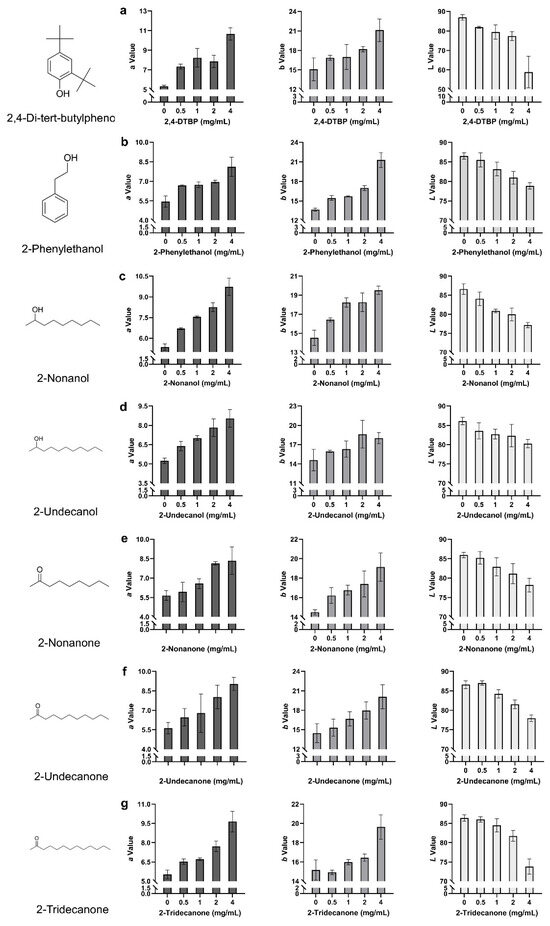
Figure 5.
Colorimetric changes in P. pulmonarius fruiting bodies following topical application of seven toxic VOCs. (a) 2,4-di-tert-butylphenol; (b) 2-phenylethanol; (c) 2-nonanol; (d) 2-undecanol; (e) 2-nonanone; (f) 2-undecanone; (g) 2-tridecanone. Each panel shows the changes in L (lightness), a (red–green), and b (yellow–blue) values in response to individual VOC treatment. Threshold-dependent responses, with minimal changes at low concentrations but sharp increases in a and b at 4 mg/mL, suggesting critical concentration-triggered discoloration. Additionally, 2-tridecanone induced a steady loss of lightness (~3.5% per concentration step) and a consistent rise in a (+74.32%, +4.11), while b increased moderately (+29.42%, +4.46), indicating a predominantly browning effect. Lastly, 2-nonanol showed a distinct red-dominant profile (a: +62.92%, +3.30 at 4 mg/mL) with a relatively muted b response, suggesting suppression of yellowing pathways at high concentrations. These chromatic trends were consistent with the visible symptoms on fruiting bodies.
To further evaluate the antifungal effects of VOCs, we examined their impact on P. pulmonarius mycelial growth using a distinct concentration range (Figure 6a–g; Table 2). All VOCs exhibited concentration-dependent inhibition, but their efficacies varied considerably. 2,4-DTBP displayed the highest potency, causing nearly complete mycelial suppression at only 5 μg/L. Among the other compounds, 2-undecanol was the most effective, achieving 97.76% inhibition at 25 μg/L and complete suppression at higher concentrations, followed by 2-nonanol, which fully inhibited growth at 50 μg/L. 2-phenylethanol showed moderate activity, reaching complete inhibition at 100 μg/L. In contrast, 2-undecanone, 2-tridecanone, and 2-nonanone required higher concentrations for comparable effects; 2-undecanone achieved complete suppression at 200 μg/L, 2-tridecanone reached 86.88% inhibition at 100 μg/L, while 2-nonanone was the least effective, causing only 66.32% inhibition even at 100 μg/L.
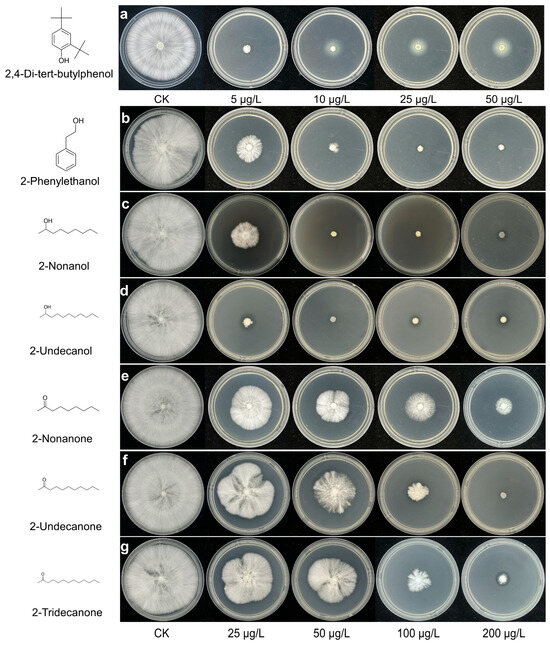
Figure 6.
Concentration-dependent inhibition of P. pulmonarius mycelial growth by seven toxic VOCs. (a) 2,4-di-tert-butylphenol; (b) 2-phenylethanol; (c) 2-nonanol; (d) 2-undecanol; (e) 2-nonanone; (f) 2-undecanone; (g) 2-tridecanone. Each panel shows the changes in L (lightness), a (red–green), and b (yellow–blue) values in response to individual VOC treatment. For 2,4-DTBP, lower concentrations (5–50 μg/L) were used due to its high potency, while the other six compounds (2-undecanol, 2-nonanol, 2-phenylethanol, 2-nonanone, 2-undecanone, and 2-tridecanone) were tested at 25–200 μg/L. Inhibition was calculated relative to the untreated control. Each panel shows the concentration–response curves for each VOC. Due to its high potency, 2,4-DTBP was tested at lower concentrations (5–50 μg/L), while the other compounds were tested at 25–200 μg/L. Inhibition was calculated relative to the untreated control. Each panel shows agar plates treated with increasing concentrations of the respective VOC. A white halo appears around the central colony in panel a (2,4-DTBP) at 10, 25, and 50 μg/L, caused by condensation of 2,4-DTBP on the agar surface rather than mycelial growth. This was not observed for the other VOCs.

Table 2.
Inhibition rates of P. pulmonarius mycelial growth by seven toxic VOCs at various concentrations.
3. Discussion
Building on our isolation and identification results, we found that E. americana and C. neteri could be simultaneously isolated from P. pulmonarius fruiting bodies exhibiting distinct disease symptoms. These symptoms were consistent with those previously described [11,23], suggesting a potential synergistic role of the two bacterial pathogens in disease development. E. americana was first identified as a pathogen of A. bisporus in 1995 [24], and was later reported to infect Lentinula edodes and Pleurotus ostreatus in Spain [25]. However, its association with P. pulmonarius has only been recognized in recent years. In contrast, C. neteri was not known to infect edible mushrooms until 2017, when it was found to cause disease in Flammulina velutipes [12]; prior to that, it had previously been known only as a human-associated pathogen. The emergence of both bacteria as P. pulmonarius pathogens may be linked to the rapid expansion of its cultivation, highlighting the need to clarify their pathogenic mechanisms and develop effective control strategies.
Previous studies on toxins produced by pathogens of edible fungi have primarily focused on non-volatile compounds, such as tolaasin, monoacetylphloroglucinol (MAPG), p-hydroxybenzoic acid (PHBA), and phenylacetic acid (PAA) [2,10,26]. In contrast, studies on the toxicity and pathogenicity of VOCs remain limited. VOCs produced by Ps. tolaasii following infection of P. ostreatus were reported to cause browning and decay [27]. Similarly, VOCs from Trichoderma pleuroticola, the pathogen of Auricularia auricula, were found to inhibit its mycelial growth, although specific toxicities were not identified [19]. In a broader context, VOCs have also been shown to exhibit toxicity in plant–pathogen interactions. For example, isoamyl alcohol, 2-phenylethanol, 4-ethylphenol, and 4-ethyl-2-methoxyphenol, produced by Valsa mali, the causal agent of apple canker, have been reported to exhibit toxicity against apple branches [28]. Although this study was conducted on plants rather than mushrooms, it nevertheless provides indirect evidence supporting the notion that VOCs from pathogens may be detrimental to their host organisms.
Compared to previously reported toxins, purified tolaasin exhibits stronger toxicity at low concentrations, inducing brown lesions on the fruiting bodies of A. bisporus and P. ostreatus at 0.06 mg/mL and 0.125 mg/mL, respectively [29,30]. MAPG, PHBA, and PAA also caused browning on A. bisporus at concentrations of 0.25 mg/mL, 0.5 mg/mL, and 1 mg/mL, respectively [2,10]. In contrast, 2,4-DTBP primarily caused sunken lesions on the fruiting bodies of P. pulmonarius at concentrations of 0.5 mg/mL or lower, with slight discoloration observed. In contrast to its effect on fruiting bodies, 2,4-DTBP exhibited stronger toxicity than tolaasin in inhibiting mycelial growth. At a concentration of 5 μg/L, 2,4-DTBP almost completely inhibited the growth of P. pulmonarius mycelium, while the minimum inhibitory concentrations of tolaasin for A. bisporus and P. ostreatus were 8 μg/mL and 16 μg/mL, respectively [26].
At a concentration of 4 mg/mL, 2,4-DTBP caused enlarged yellowish-brown sunken lesions at inoculation sites, which closely resembled the symptoms caused by direct inoculation with E. americana suspension and were similar to symptoms of P. pulmonarius blight disease [23]. As a lipophilic phenol identified, 2,4-DTBP exhibiting diverse biological activities such as cytotoxicity, antimicrobial effects, and phytotoxicity across a wide range of organisms [31]. It has been shown that 2,4-DTBP binds to β-tubulin of Fusarium oxysporum, disrupting cytoskeletal stability and inhibiting hyphal growth [32]. Transcriptomic analysis revealed that 2,4-DTBP compromises the integrity of the cell wall and membrane structures in Ustilaginoidea virens [33]. These findings collectively indicate that 2,4-DTBP toxicity is closely linked to the disruption of cell structural integrity, providing a molecular explanation for the formation of sunken lesions observed in P. pulmonarius fruiting bodies. Nevertheless, the precise molecular mechanisms of 2,4-DTBP toxicity in P. pulmonarius remain to be fully elucidated and warrant further investigation.
Among the other six VOCs, 2-tridecanone exhibited similar effects to 2,4-DTBP at 4 mg/mL, inducing both browning and severe sunken lesions. High concentrations of 2-nonanone and 2-undecanone also resulted in discoloration and sunken of fruiting body to varying extents. Additionally, 2-nonanol, 2-undecanol, and 2-phenylethanol caused notable yellow lesions of fruiting body at 1 mg/mL, 2 mg/mL, and 4 mg/mL, respectively. The presence of multiple VOC toxins capable of inducing discoloration suggests that these compounds may act synergistically. The term “volatoxins” was first coined by Bennett et al., who defined fungal volatoxins as volatile metabolites emitted by fungi that exert toxic effects on other organisms at physiological or moderately elevated concentrations (typically 2–10 times above ambient levels) [34]. While their definition was limited to fungal sources, the concept of volatoxins can be more broadly applied. In the present study, the bacterial VOCs that exhibited toxicity against P. pulmonarius also fulfill this criterion and can therefore be considered volatoxins.
Interestingly, the seven toxic VOCs identified in this study also exhibited antifungal activity against pathogenic microorganisms. For example, 2-undecanone produced by Pythium oligandrum caused hyphal contraction and lysis of cell membranes and organelles in Pythium myriotylum [35]; 2-nonanone from Pseudomonas sp. AN3A02 significantly reduced Botrytis cinerea infection rates on blueberries [36]; 2-tridecanone from P. fluorescens PMFe01 exhibited long-range inhibition of Legionella pneumophila [37]; 2-phenylethanol inhibited B. cinerea by disrupting cell membrane integrity, disturbing redox balance, suppressing antioxidant enzyme activity, and inducing lipid peroxidation [38]. Similar mechanisms were reported for 2-undecanol against Rhizopus stolonifer [39], and 2-nonanol was shown to inhibit Alternaria solani and B. cinerea [40]. These studies collectively highlight that many VOCs target cell wall/membrane structures and disrupt redox balance, consistent with the action mode of 2,4-DTBP.
In this study, we identified 16 VOCs. Both the diversity and abundance of the detected compounds were lower than those reported in other HS-SPME-GC-MS studies [41,42], and notably, no aldehydes were detected. This discrepancy may be attributed to the type of SPME fiber coating used and the polarity of the GC column. For example, a DVB/CAR/PDMS fiber combined with a polar DB-Wax column has been shown to be more effective for detecting aldehydes [43]. Additionally, factors such as fiber derivatization, GC-MS detection limits for aldehydes, and microbial differences in metabolism could contribute to the absence of aldehyde detection [44,45,46]. It is noteworthy that in our study, GC-MS chromatograms of each pathogen displayed multiple high-intensity column bleed peaks (silicon-containing peaks) that persisted throughout the analysis. GC-MS analysis of both pathogens revealed extensive column bleed peaks appearing throughout the chromatograms, potentially interfering with VOC detection. Several peaks identified as ethyl iso-allocates, which are labeled as non-microbial in origin (“Microorganism: No”) by the mVOC 4.0 platform, were still detected even after column replacement. These limitations suggest that not all VOCs produced by E. americana and C. neteri were detected, and that certain key compounds may have been obscured or interfered with by column bleed peaks. As noted [47], increased column bleed interferes with detection and causes data bias, while contamination of ion sources or optical components reduces absolute instrument sensitivity, further complicating compound identification and quantification. Therefore, future studies should consider employing more stable, low-bleed chromatographic systems and higher-resolution mass spectrometry to more accurately resolve the authentic VOC profiles produced by microorganisms.
4. Conclusions
In this study, we isolated and identified two bacterial pathogens of P. pulmonarius in Guangxi—E. americana and C. neteri—from samples showing distinct disease symptoms. Notably, this is the first report of E. americana as a pathogen of P. pulmonarius in Guangxi. Together, these two strains appear to be the major bacterial pathogens affecting P. pulmonarius cultivation in this region.
Using HS-SPME-GC-MS, we identified 16 volatile organic compounds (VOCs) produced by the pathogens, seven of which—including three ketones, three alcohols, and one phenolic compound—exhibited toxic effects. Among them, 2,4-DTBP showed the highest toxicity, causing significant tissue damage, discoloration, and inhibition of mycelial growth. The remaining VOCs also induced varying degrees of fruiting body discoloration and mycelial suppression. Colorimetric analysis quantitatively confirmed symptom progression, supporting the visual observations.
This study is the first to demonstrate the pathogenic potential of bacterial VOCs against P. pulmonarius. Our findings highlight the role of VOCs as important virulence factors, expanding understanding of mushroom-bacteria interactions and laying a foundation for future research into their mechanisms and control strategies.
5. Materials and Methods
5.1. Pathogen Isolation
To isolate the pathogen, tissues samples (5 × 5 × 5 mm) were sterilized in 75% ethanol, and triple-rinsed with sterile deionized water (SDW) (HHitech, Shanghai, China), homogenized in a microcentrifuge tube with 1 mL SDW, then serially diluted from 10−1 to 10−7. Each dilution (150 μL) was spread-plated on Luria–Bertani (LB) medium (Solarbio, Beijing, China) , and then incubated at 28 °C for 24 h. Colonies with different colors, shapes, and sizes were selected and purified two to three times on LB agar plates using streaking methods to obtain pure cultures.
5.2. Pathogen Analysis
Single bacterial colonies were inoculated into centrifuge tubes containing 15 mL of LB liquid medium and cultured with shaking at 150 rpm for 18 h at 28 °C. Two milliliters of the cultured bacterial suspension were transferred to a sterile EP tube, and cells were collected by centrifugation at 12,000 rpm for 1 min. The resulting cell pellet was washed and centrifuged with deionized water three times. And the final pellet was resuspended in SDW to prepare a bacterial suspension of 2 McFarland (≈6 × 108 CFU/mL).
For the pathogenicity test, P. pulmonarius fruiting bodies with similar size and complete-looking were selected. A total of six fruiting bodies were used for each pathogen treatment, with three subjected to wound inoculation and three to droplet inoculation, each receiving 30 μL of the suspension. The inoculated mushrooms were placed in an incubator maintained at 25 °C and 80–90% relative humidity. Pathogenicity was assessed 1 to 3 days after inoculation. Bacteria were re-isolated from symptomatic tissues to compare their morphological and molecular characteristics with those of the original inoculum, thereby fulfilling Koch’s postulates.
5.3. Pathogenicity Identification
Genetic identification of pathogens was performed using a multi-gene approach. Genus-level classification was first achieved by sequencing the 16S rRNA gene. Subsequently, four conserved housekeeping genes were amplified using previously reported primer pairs [10,48,49,50] (Table S3). PCR was conducted in a total volume of 30 μL, comprising 15 μL of 2× Rapid Taq Master Mix, 1 μL each of forward and reverse primers, 1 μL of genomic DNA, and 12 μL of ddH2O. The thermal cycling program included an initial denaturation at 95 °C for 3 min; followed by 32 cycles of denaturation at 95 °C for 30 s, annealing at 44–60 °C for 30 s, and extension at 72 °C for 30 s; with a final extension step at 72 °C for 10 min. PCR products were separated on 1% agarose gel, purified, and sequenced by BGI Genomics Co., Ltd. (Shenzhen, China).
The obtained sequences were analyzed via BLAST (v2.16.0) against the NCBI GenBank database and have been deposited under the accession numbers listed in Table S2. Representative type strain sequences were retrieved from GenBank and aligned using MUSCLE implemented in MEGA X (v10.2.6). The aligned sequences were then concatenated, and a phylogenetic tree was constructed using the maximum likelihood (ML) method with 1000 bootstrap replicates.
For morphological identification, purified colonies were inoculated onto NA medium using a three-zone method and cultured for 24 h. Colony morphology and color were observed under a stereomicroscope (SMZ 745T, Nikon, Tokyo, Japan). Colonies were also inoculated into King’s B plates and observe fluorescence after 48 h. Bacterial cells were harvested into sterile EP tubes, prepared as suspensions, and Gram-stained using a commercial kit, then observed under 100× oil immersion microscopy (ECLIPSE 80i, Nikon, Japan).
Physiological and biochemical characterization was conducted using reagents from Hangzhou Microbial Reagent Co., (Hangzhou, China) including assays for oxidase, catalase, gelatinase, urease, arginine dihydrolase, glucose, lactose, sucrose, arabinose, rhamnose utilization, aesculin, salicin, mannitol metabolism, and nitrate reduction. Pathogens were identified by integrating morphological, physiological, biochemical, and multi-locus sequence analysis (MLSA) results.
5.4. Bioassay of VOCs by E. americana and C. neteri In Vitro
To evaluate the impact of pathogen-derived volatile organic compounds (VOCs) on the mycelial growth of P. pulmonarius strain Taixiu 57, a two-compartment petri dish confrontation assay was conducted, and growth curves were plotted. Pathogen strains were activated on LA medium and cultured in LB medium at 28 °C with shaking at 150 rpm for 12 h. The bacterial suspension was diluted with ultrapure water to 0.5 McFarland concentration (≈1.5 × 108 CFU/mL). Ten milliliters of LA and PDA medium were added into separate compartment. After solidification, 100 μL of diluted bacterial suspension was spread onto the LA side and air-dried. A 6-mm mycelial block of P. pulmonarius (cultured for 96 h) was placed onto the PDA side. The dish was incubated at 28 °C for 120 h, and colony diameters were recorded every 12 h from 24 h using the cross-intersection method.
Bioactivity of VOCs on P. pulmonarius fruiting body tissue was performed using a three-compartment petri dish confrontation method [20]. 6 mL of LA medium were poured into two compartments. After air-drying, 60 μL of 0.5 McFarland bacterial suspension was applied on these compartments evenly. Internal tissue blocks excised from surface-sterilized P. pulmonarius fruitbodies were placed in the medium-free compartment. The dish was incubated at 28 °C for 72 h, and changes in the tissue blocks were observed.
5.5. HS-SPME-GC-MS Analysis of VOCs
Pathogenic bacterial strains were inoculated into flasks containing 250 mL of LB liquid medium and shaken for 60 h and equilibrated at 300 rpm and 35 °C for 30 min. Meanwhile, an SPME fiber that coated with DVB/CAR/PDMS (57328-U, Supelco, St. Louis, MO, USA), was conditioned at 270 °C for 30 min in the injection port of a gas chromatograph. The fiber was then inserted into the flask headspace for VOC extraction for 30 min under stirring.
The fiber was desorbed for 5 min in the GC injection port at 250 °C in splitless mode. Helium served as the carrier gas at 1 mL/min. GC-MS analysis was conducted on a GC-MS system (TSQ9000, Thermo, Waltham, MA, USA) with a TG-5SliMS column (Thermo, USA). The GC-MS temperature programs were optimized for E. americana and C. neteri, respectively. For E. americana, 35 °C for 5 min, ramped at 8 °C/min to 180 °C, then 20 °C/min to 250 °C, held for 5 min; for C. neteri, 40 °C for 5 min, ramped at 6 °C/min to 120 °C, then 12 °C/min to 200 °C, and 20 °C/min to 260 °C, held for 5 min. Mass spectrometry parameters included a scan range of 30–400 m/z, 0.2 s/full scan. SPME fibers reconditioned at 270 °C for 20 min after each run.
Chromatograms were processed using Thermo Xcalibur (v4.2.47) software. Compounds were identified by matching spectra against the NIST 20 database via NIST MS Search (v2.4) and validated in mVOC 4.0 (https://bioinformatics.charite.de/mvoc/. Accessed on 12 March 2025). A compound was considered microbial VOCs if they had >50% similarity in the database and were labeled “Microorganism: yes” in mVOC 4.0 platform [51].
5.6. Toxicity Bioassays of Individual VOCs on P. pulmonarius
To ensure solubility consistency during dilution, all VOC standards, which were purchased from Adamas-beta (Shanghai, China), were dissolved in 50% DMSO to 100 mg/mL stock solutions, diluted to 0.25–4 mg/mL with 2% DMSO, and filtered through a 22-μm micro membrane filtration. 20 μL of each VOC concentration was applied to cut surfaces of halved P. pulmonarius fruitbodies and the control was treated with 20 μL of 4% DMSO solution [10]. The fruit bodies were placed in petri dishes and sealed with parafilm, then incubated at 20 °C and 80% humidity for 48 h. Color differences were measured via colorimeter to calculate the toxicity of VOCs. VOCs causing spots or depressions were selected for mycelial growth assays. PDA plates were inoculated with P. pulmonarius mycelial and exposed to VOCs via filter paper affixed to dish lids, with doses corresponding to 25–200 μg/L (5–50 μg/L for 2,4-DTBP). Plates were sealed and incubated at 28 °C for 120 h, with colony diameters recorded by the cross method.
5.7. Statistical Analysis
Statistical analysis was performed using GraphPad Prism 10.4.1 with Student’s t-test and ANOVA followed by Tukey’s HSD test; p < 0.05 was considered significant.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/toxins17090449/s1, Figure S1: Morphological characteristics of pathogens; Figure S2: The total ion chromatogram (TIC) obtained from GC-MS analysis of the Ewingella americana ST3-2; Figure S3: The total ion chromatogram (TIC) obtained from GC-MS analysis of the Cedecea neteri XC1-2; Figure S4: Experimental and reference mass spectra of seven selected VOCs; Figure S5: Toxicity of 16 identified VOCs in P. pulmonarius fruiting bodies at 4 mg/mL; Table S1: Primers for sequence amplification in the MLSA of this study; Table S2: GenBank accession numbers of the sequences amplified in this study; Table S3: Physiological and biochemical characteristic of Ewingella americana and Cedecea neteri.
Author Contributions
Conceptualization, B.L. and Z.W.; methodology, Z.W.; software, Z.W., J.Q. and Y.N.; validation, Z.W., J.Q., Y.N. and L.W.; formal analysis, Z.W.; investigation, Z.W. and B.L.; resources, Z.W. and B.L.; data curation, Z.W.; writing—original draft preparation, Z.W. and Y.W.; writing—review and editing, B.L., Z.W. and Y.W.; visualization, Z.W., Y.W., J.Q. and Y.N.; supervision, B.L.; funding acquisition, B.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Key Research and Development Program of Guangxi [AB18221047] and the Guangxi Mushroom Science and Technology Vanguard [GNKM202504-2].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article and Supplementary Materials. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chen, L.L.; Xu, S.M.; Chen, J.Q.; Zhang, M.; Qiu, M.M.; Jiang, Y.J.; Chen, B.Z. Transcriptome Sequencing Analysis of the Effects of 1-MCP and Ethephon Treatments on the Storage Quality of Pleurotus pulmonarius. Sci. Hortic. 2024, 338, 113721. [Google Scholar] [CrossRef]
- Huang, Z.X.; Liang, X.S.; Wang, Y.F.; Mo, M.Q.; Qiu, Y.; Liu, B. Ginger Blotches on Agaricus bisporus Due to Monoacetylphloroglucinol Production by the Pathogen Pseudomonas ‘gingeri’. Pest Manag. Sci. 2023, 79, 5197–5207. [Google Scholar] [CrossRef]
- Rakhmonov, U.; Soatov, T. Harmful Competitors and Diseases of Pleurotus ostreatus and Their Control Measures. E3S Web Conf. 2023, 389, 03101. [Google Scholar] [CrossRef]
- Taparia, T.; Hendrix, E.; Hendriks, M.; Krijger, M.; de Boer, W.; van Der Wolf, J. Comparative Studies on the Disease Prevalence and Population Dynamics of Ginger Blotch and Brown Blotch Pathogens of Button Mushrooms. Plant Dis. 2021, 105, 542–547. [Google Scholar] [CrossRef]
- Huang, Z.X.; Nie, Y.L.; Huang, Y.Y.; Liu, L.Z.; Liu, B. Elucidating the Role of Monoacetylphloroglucinol in the Pathogenicity of Pseudomonas ‘gingeri’ against Agaricus bisporus. Pest Manag. Sci. 2024, 80, 3526–3539. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.Z.; Liu, C.G.; Shen, N.; Wu, Y.Z.; Bian, Y.B.; Xiao, Y. Differential Analyses of Morphology and Transcription from Oyster Mushroom Pleurotus ostreatus Response to Brown Blotch Disease. Sci. Hortic. 2024, 331, 113141. [Google Scholar] [CrossRef]
- Liu, Z.H.; Zhao, Y.T.; Sossah, F.L.; Okorley, B.A.; Amoako, D.G.; Liu, P.B.; Sheng, H.Y.; Li, D.; Li, Y. Characterization, Pathogenicity, Phylogeny, and Comparative Genomic Analysis of Pseudomonas tolaasii Strains Isolated from Various Mushrooms in China. Phytopathology 2022, 112, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Bruno, G.L.; De Corato, U.; Rana, G.L.; De Luca, P.; Pipoli, V.; Lops, R.; Scarola, L.; Mannerucci, F.; Piscitelli, L.; Cariddi, C. Suppressiveness of White Vinegar and Steam-Exploded Liquid Waste against the Causal Agents of Pleurotus eryngii Yellowing. Crop Prot. 2015, 70, 61–69. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, R.P.; Guo, M.P.; Shen, N.; Chuaoen, P.; Qiu, K.X.; Bian, Y.B.; Xiao, Y. Serial Transcriptional Changes of Flammulina filiformis (Winter Mushroom) Mycelia Infected by Pseudomonas migulae. Sci. Hortic. 2022, 297, 110965. [Google Scholar] [CrossRef]
- Huang, Z.X.; Huang, Y.Y.; Nie, Y.L.; Liu, B. Biological Characteristics of Two Pathogens Causing Brown Blotch in Agaricus bisporus and the Toxin Identification of Cedecea neteri. Phytopathol. Res. 2024, 6, 21. [Google Scholar] [CrossRef]
- Liu, Z.L.; Zhou, S.Y.; Zhang, W.L.; Wu, S.J.; Chen, X.F.; Wang, X.G.; Hu, H.J.; Chen, Q. First Report of Cedecea neteri Causing Yellow Rot Disease in Pleurotus pulmonarius in China. Plant Dis. 2021, 105, 1189. [Google Scholar] [CrossRef]
- Yan, J.J.; Liu, Y.Y.; Wang, R.Q.; Mukhtar, I.; Liu, F.; Lin, Z.Y.; Jiang, Y.J.; Xie, B.G. First Report of Cedecea neteri Causing Yellow Sticky Disease in Flammulina velutipes in China. Plant Dis. 2019, 103, 1014–1015. [Google Scholar] [CrossRef]
- Sugendra Kumar, V. Antimicrobial Activity of Silver Nanoparticles for the Control of Microbial Spoilage of Grey Oyster Mushroom (Pleurotus pulmonarius). Ph.D. Dissertation, Universiti Malaya, Kuala Lumpur, Malaysia, 2021. Available online: https://www.proquest.com/dissertations-theses/antimicrobial-activity-silver-nanoparticles/docview/3143984806/se-2 (accessed on 10 June 2025).
- Bellettini, M.B.; Bellettini, S.; Fiorda, F.A.; Pedro, A.C.; Bach, F.; Fabela-Morón, M.F.; Hoffmann-Ribani, R. Diseases and Pests Noxious to Pleurotus spp. Mushroom Crops. Rev. Argent. Microbiol. 2018, 50, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, Q.; Zhang, Z.X.; Dong, C.H. First Report of Bacterial Brown Rot Disease Caused by Ewingella americana on Cultivated Naematelia aurantialba in China. Plant Dis. 2023, 107, 2513. [Google Scholar] [CrossRef]
- Na, W.J.; Luo, H.; Yu, J.M. First Report of Bacterial Brown Rot Disease on Shiitake Mushroom (Lentinula edodes) Caused by Ewingella americana in Korea. J. Plant Pathol. 2021, 103, 1325–1326. [Google Scholar] [CrossRef]
- Kim, Y.N.; Lee, D.H.; Yu, J.M. Brown Rot Caused by Ewingella americana on King Oyster Mushroom (Pleurotus eryngii) in Korea. J. Plant Pathol. 2023, 105, 327. [Google Scholar] [CrossRef]
- Liu, Z.H.; Sossah, F.L.; Li, Y.; Fu, Y.P. First Report of Ewingella americana Causing Bacterial Brown Rot Disease on Cultivated Needle Mushroom (Flammulina velutipes) in China. Plant Dis. 2018, 102, 2633. [Google Scholar] [CrossRef]
- Dang, H.; Kong, Q.Q.; Winchester, W.; Wan, X.; Lei, Y.; Zhang, H.S.; Zhao, Y.; Liu, X.Y.; Xu, B.B.; Zhang, B.S.; et al. Isolation, Identification, and Pathogenic Effects of Trichoderma spp. from Auricularia auricula. Adv. Compos. Hybrid Mater. 2023, 6, 96. [Google Scholar] [CrossRef]
- Lo Cantore, P.; Giorgio, A.; Iacobellis, N.S. Bioactivity of Volatile Organic Compounds Produced by Pseudomonas tolaasii. Front. Microbiol. 2015, 6, 1082. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, S.; Harighi, B.; Mojarrab, M.; Azizi, A. Response of Pseudomonas tolaasii, the Causal Agent of Mushroom Brown Blotch Disease, to the Volatile Compounds Produced by Endofungal Bacteria. BioControl 2021, 66, 421–432. [Google Scholar] [CrossRef]
- Müller, H.E.; Fanning, G.R.; Brenner, D.J. Isolation of Ewingella americana from Mollusks. Curr. Microbiol. 1995, 31, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Lu, N.; Yan, J.; Wang, W.K. Identification and Pollution Source Analysis of Pleurotus pulmonarius Blight Disease Caused by Ewingella americana. Acta Edulis Fungi 2023, 30, 92–101. [Google Scholar] [CrossRef]
- Inglis, P.W.; Peberdy, J.F. Isolation of Ewingella americana from the Cultivated Mushroom, Agaricus bisporus. Curr. Microbiol. 1996, 33, 334–337. [Google Scholar] [CrossRef]
- Reyes, J.E.; Venturini, M.E.; Oria, R.; Blanco, D. Prevalence of Ewingella americana in Retail Fresh Cultivated Mushrooms (Agaricus bisporus, Lentinula edodes, and Pleurotus ostreatus) in Zaragoza (Spain). FEMS Microbiol. Ecol. 2004, 47, 291–296. [Google Scholar] [CrossRef]
- Lo Cantore, P.; Lazzaroni, S.; Coraiola, M.; Dalla Serra, M.; Cafarchia, C.; Evidente, A.; Iacobellis, N.S. Biological Characterization of White Line-Inducing Principle (WLIP) Produced by Pseudomonas reactans NCPPB1311. Mol. Plant Microbe Interact. 2006, 19, 1113–1120. [Google Scholar] [CrossRef]
- Shirata, A. Production of Volatile Components by Pseudomonas tolaasii and Their Toxic Activity. Jpn. J. Phytopathol. 1996, 62, 185–193. [Google Scholar] [CrossRef]
- Li, S.N.; Chen, Q.; Wang, H.; Huang, L.L. Volatile Metabolites and Toxin Activity of Valsa mali. Acta Agric. Boreali-Occident. Sin. 2018, 27, 692–698. [Google Scholar] [CrossRef]
- Soler-Rivas, C.; Arpin, N.; Olivier, J.-M.; Wichers, H.J. The Effects of Tolaasin, the Toxin Produced by Pseudomonas tolaasii, on Tyrosinase Activities and the Induction of Browning in Agaricus bisporus Fruiting Bodies. Physiol. Mol. Plant Pathol. 1999, 55, 21–28. [Google Scholar] [CrossRef]
- Shirata, A.; Sugaya, K.; Takasugi, M.; Monde, K. Isolation and Biological Activity of Toxins Produced by a Japanese Strain of Pseudomonas tolaasii, the Pathogen of Bacterial Rot of Cultivated Oyster Mushroom. Jpn. J. Phytopathol. 1995, 61, 493–502. [Google Scholar] [CrossRef][Green Version]
- Zhao, F.Q.; Wang, P.; Lucardi, R.D.; Su, Z.S.; Li, S.Y. Natural Sources and Bioactivities of 2,4-Di-Tert-Butylphenol and Its Analogs. Toxins 2020, 12, 35. [Google Scholar] [CrossRef]
- Dharni, S.; Sanchita; Maurya, A.; Samad, A.; Srivastava, S.K.; Sharma, A.; Patra, D.D. Purification, Characterization, and In Vitro Activity of 2,4-Di-tert-butylphenol from Pseudomonas monteilii PsF84: Conformational and Molecular Docking Studies. J. Agric. Food Chem. 2014, 62, 6138–6146. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.; Yu, Y.N.; Hu, Z.; Qian, S.A.; Zhao, Z.H.; Meng, J.J.; Zheng, S.M.; Huang, Q.W.; Zhang, Z.Q.; Nie, D.X.; et al. Antifungal Activity and Action Mechanisms of 2,4-Di-tert-butylphenol against Ustilaginoidea virens. J. Agric. Food Chem. 2023, 71, 17723–17732. [Google Scholar] [CrossRef]
- Bennett, J.W.; Inamdar, A.A. Are Some Fungal Volatile Organic Compounds (VOCs) Mycotoxins? Toxins 2015, 7, 3785–3804. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, T.M.M.; Zhou, D.M.; Haider, M.S.; Hussain, S.; Wang, N.; Chen, S.Q.; Zhao, Y.S.; Wen, X.; Feng, H.; Wang, X.Y.; et al. Volatile Organic Compounds from Pythium oligandrum Play a Role in Its Parasitism on Plant-Pathogenic Pythium myriotylum. Appl. Environ. Microbiol. 2023, 89, e0203622. [Google Scholar] [CrossRef]
- Ulloa, P.A.; Valencia, A.L.; Olivares, D.; Poblete-Morales, M.; Silva-Moreno, E.; Defilippi, B.G. Antifungal Effect of Volatile Organic Compounds (VOCs) Released from Antarctic Bacteria under Postharvest Conditions. Food Packag. Shelf Life 2023, 39, 101160. [Google Scholar] [CrossRef]
- Corre, M.-H.; Mercier, A.; Bouteiller, M.; Khalil, A.; Ginevra, C.; Depayras, S.; Dupont, C.; Rouxel, M.; Gallique, M.; Grac, L.; et al. Bacterial Long-Range Warfare: Aerial Killing of Legionella pneumophila by Pseudomonas fluorescens. Microbiol. Spectr. 2021, 9, e0040421. [Google Scholar] [CrossRef]
- Zou, X.R.; Wei, Y.Y.; Jiang, S.; Xu, F.; Wang, H.F.; Zhan, P.P.; Shao, X.F. ROS Stress and Cell Membrane Disruption Are the Main Antifungal Mechanisms of 2-Phenylethanol Against Botrytis cinerea. J. Agric. Food Chem. 2022, 70, 14468–14479. [Google Scholar] [CrossRef]
- Gomaa, A.E.-R.F.; Tao, J.; Liang, S.-Y.; Liu, J.-Q.; Yang, S.; Shi, X.-Q.; El-Sayed, M.H.; Xing, K.; Qin, S. Biocontrol of Postharvest Soft Rot Caused by Rhizopus stolonifer in Sweet Potatoes Using Volatile Organic Compounds from Actinomycete Nocardiopsis dassonvillei MI-S24. Food Control 2025, 111, 111359. [Google Scholar] [CrossRef]
- Ren, L.; Zhou, J.B.; Yin, H.; Qin, N.; Yao, F.T.; Ma, D.L.; Zhao, X.J. Antifungal Activity and Control Efficiency of Endophytic Bacillus velezensis ZJ1 Strain and Its Volatile Compounds Against Alternaria solani and Botrytis cinerea. J. Plant Pathol. 2022, 104, 575–589. [Google Scholar] [CrossRef]
- Delgado, N.; Olivera, M.; Cádiz, F.; Bravo, G.; Montenegro, I.; Madrid, A.; Fuentealba, C.; Pedreschi, R.; Salgado, E.; Besoain, X. Volatile Organic Compounds (VOCs) Produced by Gluconobacter cerinus and Hanseniaspora osmophila Displaying Control Effect against Table Grape-Rot Pathogens. Antibiotics 2021, 10, 663. [Google Scholar] [CrossRef]
- Rey-Serra, P.; Mnejja, M.; Monfort, A. Inheritance of Esters and Other Volatile Compounds Responsible for the Fruity Aroma in Strawberry. Front. Plant Sci. 2022, 13, 959155. [Google Scholar] [CrossRef]
- Krüger, R.L.; Dallago, R.M.; Nascimento Filho, I.; Di Luccio, M. Study of Odor Compounds in Gaseous Effluents Generated During Production of Poultry Feather and Viscera Meal Using Headspace Solid Phase Microextraction. Environ. Monit. Assess. 2009, 158, 355–363. [Google Scholar] [CrossRef]
- Martos, P.A.; Pawliszyn, J. Sampling and Determination of Formaldehyde Using Solid-Phase Microextraction with On-Fiber Derivatization. Anal. Chem. 1998, 70, 2311–2320. [Google Scholar] [CrossRef]
- Rodigast, M.; Mutzel, A.; Iinuma, Y.; Haferkorn, S.; Herrmann, H. Characterisation and Optimisation of a Method for the Detection and Quantification of Atmospherically Relevant Carbonyl Compounds in Aqueous Medium. Atmos. Meas. Tech. Discuss. 2015, 8, 2409–2416. [Google Scholar] [CrossRef]
- Yu, X.; Sun, Y.R.; Shen, X.; Li, W.C.; Cai, H.Y.; Guo, S.; Sun, Z.H. Effect of Different Isolation Sources of Lactococcus lactis subsp. lactis on Volatile Metabolites in Fermented Milk. Food Chem. 2024, 21, 101224. [Google Scholar] [CrossRef]
- Han, T.-L.; Yang, Y.; Zhang, H.; Law, K.P. Analytical Challenges of Untargeted GC-MS-Based Metabolomics and the Critical Issues in Selecting the Data Processing Strategy. F1000Research 2017, 6, 967. [Google Scholar] [CrossRef]
- Galkiewicz, J.P.; Kellogg, C.A. Cross-kingdom amplification using bacteria-specific primers: Complications for studies of coral microbial ecology. Appl. Environ. Microbiol. 2008, 74, 7828–7831. [Google Scholar] [CrossRef]
- Pham, H.N.; Ohkusu, K.; Mishima, N.; Noda, M.; Shah, M.M.; Sun, X.; Hayashi, M.; Ezaki, T. Phylogeny and species identification of the family Enterobacteriaceae based on dnaJ sequences. Diagn. Microbiol. Infect. Dis. 2007, 58, 153–161. [Google Scholar] [CrossRef]
- Yamamoto, S.; Harayama, S. PCR amplification and direct sequencing of gyrB genes with universal primers and their application to the detection and taxonomic analysis of Pseudomonas putida strains. Appl. Environ. Microbiol. 1995, 61, 1104–1109. [Google Scholar] [CrossRef]
- Kemmler, E.; Lemfack, M.C.; Goede, A.; Gallo, K.; Toguem, S.M.T.; Ahmed, W.; Millberg, I.; Preissner, S.; Piechulla, B.; Preissner, R. mVOC 4.0: A Database of Microbial Volatiles. Nucleic Acids Res. 2025, 53, D1692–D1696. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).