Application of Graphitized Multi-Walled Carbon Nanotubes Combined with Orbitrap High-Resolution Mass Spectrometry for the Rapid Detection of Ten Toxins in Wild Mushrooms
Abstract
1. Introduction
2. Results
2.1. Selection of Liquid Chromatography Conditions
2.2. Optimization of Mass Spectrometry Conditions
2.3. Optimization of Purification Sorbent Amount
2.4. Linearity, Limits of Detection and Quantification
2.5. Evaluation of Matrix Effect
2.6. Method Recovery and Precision
2.7. Analysis of Real Samples
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Instruments and Equipment
5.2. Materials and Reagents
5.3. Solution Preparation
5.3.1. Preparation of Standard Solutions
5.3.2. Methanol Aqueous Solution, 60%
5.3.3. Mixed Aqueous Solution of 0.1% Formic Acid and 0.01% Ammonia
5.4. Sample Pretreatment
5.5. Spiking Experiments
5.6. Matrix Effect
5.7. Instrumentation Conditions
5.7.1. Chromatographic Conditions
5.7.2. Mass Spectrometry Conditions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xu, X.-M.; Cai, Z.-X.; Zhang, J.-S.; Chen, Q.; Huang, B.-F.; Ren, Y.-P. Screening of polypeptide toxins as adulteration markers in the food containing wild edible mushroom by liquid chromatography-triple quadrupole mass spectrometry. Food Control 2017, 71, 393–402. [Google Scholar] [CrossRef]
- Barbosa, I.; Domingues, C.; Barbosa, R.M.; Ramos, F. Amanitins in wild mushrooms: The development of HPLC-UV-EC and HPLC-DAD-MS methods for food safety purposes. Foods 2022, 11, 3929. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Zhu, J.; Marchioni, E.; Zhao, M.; Li, H.; Zhou, L. Discrimination of wild edible and poisonous fungi with similar appearance (Cantharellus albovenosus vs. Omphalotus olearius) based on lipidomics using UHPLC-HR-AM/MS/MS. Food Chem. 2025, 466, 142189. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Xie, R.; Wang, N.; Zhang, J.; Sun, X.; Wang, H.; Tan, J.; Chen, A. Rapid identification of Amanita citrinoannulata poisoning using colorimetric and real-time fluorescence and loop-mediated isothermal amplification (LAMP) based on the nuclear ITS region. Food Chem. Mol. Sci. 2022, 4, 100082. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, S.; Wang, C.; Shi, J.; He, J.; Chen, J.; Liang, L.; Jiang, F. Polymerase chain reaction-based methods for the rapid identification of Amanita exitialis. Food Chem. 2024, 448, 139086. [Google Scholar] [CrossRef]
- Zhang, S.; Fan, M.; Zhang, Y.; Li, S.; Lu, C.; Zhou, J.; Zou, L. Establishment and validation of a nomogram model for prediction of clinical outcomes in patients with amanita phalloides poisoning. Heliyon 2024, 10, e37320. [Google Scholar] [CrossRef]
- He, Z.; Li, X.; Feng, M.; Wang, X.; Wang, Y.; Chen, J. Wild mushroom poisoning: A case study of amatoxin-containing mushrooms and implications for public health. Toxicon 2024, 240, 107639. [Google Scholar] [CrossRef]
- Su, H.; Jiang, Z.-H.; Hsu, Y.-W.; Wang, Y.-C.; Chen, Y.-Y.; Wu, D.-C.; Shiea, J.; Lee, C.-W. Rapid identification of mushroom toxins by direct electrospray probe mass spectrometry for emergency care. Anal. Chim. Acta 2024, 1296, 342343. [Google Scholar] [CrossRef]
- Shenoy, A.G.; Sadani, K.; Nag, P. Optic fiber sensors with tunable sensitivities for rapid detection of amatoxins in water and mushroom derived agro-products. Food Res. Int. 2025, 203, 115885. [Google Scholar] [CrossRef]
- Garcia, J.; Costa, V.M.; Carvalho, A.; Baptista, P.; de Pinho, P.G.; de Lourdes Bastos, M.; Carvalho, F. Amanita phalloides poisoning: Mechanisms of toxicity and treatment. Food Chem. Toxicol. 2015, 86, 41–55. [Google Scholar] [CrossRef]
- Wu, Z.; Dai, J.; Fan, J.; Ding, C.; Zhao, W.; Yu, C.; Yao, Q.; Sun, J.; Li, H.; Sun, C. Determination of protein-bound α-amanitin in mouse plasma: A potential new indicator of poisoning with the mushroom toxin α-amanitin. Toxicon 2023, 226, 107067. [Google Scholar] [CrossRef]
- Li, Y.-H.; Xu, F.; Zhao, W.-L.; Tang, X.-F.; Liu, F.; Bo, C.-M. Designing boron-doped carbon dot-functionalized COFs for fluorescence screening and liquid chromatography tandem mass spectrometry detection of toxins. J. Chromatogr. A 2025, 1739, 465515. [Google Scholar] [CrossRef]
- Yilmaz, I.; Akata, I.; Horoz, E.; Kaya, E. Lepiota castanea mushroom growing in Turkiye does not contain phallotoxins and amatoxins. Toxicon 2024, 243, 107736. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhao, L.; Tian, E.; Zheng, F.; Zhao, X.; Zhou, C. Ultrasensitive and visual identification of lethal mushroom Russula subnigricans by LAMP-based CRISPR/Cas12a assay. Microchem. J. 2025, 211, 113100. [Google Scholar] [CrossRef]
- Patra, A.; Mukherjee, A.K. Mushroom mycetism—A neglected and challenging medical emergency in the Indian subcontinent: A road map for its prevention and treatment. Toxicon 2022, 217, 56–77. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Peng, Z.; Zhang, J.; Song, Y.; Yu, C.; Han, Q. Truncated DNA aptamer for rapid fluorometric detection of the lethal toxin α-amanitin. Food Biosci. 2023, 55, 103064. [Google Scholar] [CrossRef]
- Barbosa, I.; Domingues, C.; Ramos, F.; Barbosa, R.M. Analytical methods for amatoxins: A comprehensive review. J. Pharm. Biomed. Anal. 2023, 232, 115421. [Google Scholar] [CrossRef]
- Maurer, H.H.; Schmitt, C.J.; Weber, A.A.; Kraemer, T. Validated electrospray liquid chromatographic–mass spectrometric assay for the determination of the mushroom toxins α-and β-amanitin in urine after immunoaffinity extraction. J. Chromatogr. B Biomed. Sci. Appl. 2000, 748, 125–135. [Google Scholar] [CrossRef]
- Lin, L.-Y.; Tong, Y.-L.; Lu, Y.-Q. The characteristics of liver injury induced by Amanita and clinical value of α-amanitin detection. Hepatobiliary Pancreat. Dis. Int. 2022, 21, 257–266. [Google Scholar] [CrossRef]
- Lei, S.; Shi, P.; Wu, W.; Xia, B.; Fu, X.; Wan, Y.; Zhou, Y. Extensive screening of cyclopeptide toxins in mushrooms by ultra-high-performance liquid chromatography coupled with quadrupole-Orbitrap mass spectrometry. Food Chem. 2020, 329, 127146. [Google Scholar] [CrossRef]
- Dong, M.; Si, W.; Jiang, K.; Nie, D.; Wu, Y.; Zhao, Z.; De Saeger, S.; Han, Z. Multi-walled carbon nanotubes as solid-phase extraction sorbents for simultaneous determination of type A trichothecenes in maize, wheat and rice by ultra-high performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2015, 1423, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Shan, Y.; Li, J.; Chen, Y.; Li, D.; Luo, Y.; Pang, Y.; Chen, D. Hydroxyl-functionalized multi-walled carbon nanotube-coated pipette tips for extraction and determination of illegally adulterated androgenic steroids. Microchem. J. 2025, 212, 113301. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, Y.; Li, H.; Zhou, S.; Chen, D.; Zhang, Y.; Yao, Q.; Sun, C. A simple and high-throughput analysis of amatoxins and phallotoxins in human plasma, serum and urine using UPLC-MS/MS combined with PRiME HLB μElution platform. Toxins 2016, 8, 128. [Google Scholar] [CrossRef] [PubMed]
- Bambauer, T.P.; Wagmann, L.; Weber, A.A.; Meyer, M.R. Analysis of α-and β-amanitin in human plasma at subnanogram per milliliter levels by reversed phase ultra-high performance liquid chromatography coupled to orbitrap mass spectrometry. Toxins 2020, 12, 671. [Google Scholar] [CrossRef]
- Ok, H.J.; Park, E.Y.; Shin, Y.; Kim, J.H.; Song, M.H.; Lee, J.H. Development of Simultaneous Analytical Method of Three Polypeptide Toxins α-Amanitin, β-Amanitin and Phalloidin in Poisonous Mushrooms and Human Serum Using UHPLC–MS/MS. J. Mass Spectrom. 2025, 60, e5145. [Google Scholar] [CrossRef]
- Jiang, Y.; Lang, L.; Zhang, J.; Ji, Y.; Zhang, C.; Yang, R.; Li, X. Amanita peptide toxin analysis: An innovative UPLC–MS/MS method utilizing Fe3O4-based magnetic adsorbent. J. Food Compos. Anal. 2024, 131, 106246. [Google Scholar] [CrossRef]
- Zhou, R.; Zhang, X.; Wen, Y.; Huo, W.; Mu, Y.; Liu, H.; Zhang, C.; Li, J. One-step purification using MWCNTs@ chitosan composite adsorbents for effective matrix interference removal in determination of 49 Pesticides in aquatic products. J. Hazard. Mater. 2025, 496, 139295. [Google Scholar] [CrossRef]
- Du, Y.; Jin, W.; Yang, S.; Jia, Y.; Li, X.; Li, J.; Zhang, M.; Zhang, Y. Determination of bisphenol analogues in bottled water using deep eutectic solvent and magnetic multi-walled carbon nanotubes followed by ultra-high performance liquid chromatography coupled with tandem mass spectrometry. J. Chromatogr. A 2024, 1738, 465479. [Google Scholar] [CrossRef]
- Sun, X.; Luo, J.; Lu, Q.; Li, C.; Zhao, Z.; An, F.; Yang, M. Application of hydroxylated multi-walled carbon nanotubes as depigmentation agent in the determination of multiple pesticide residues in Lonicerae japonicae flower buds. Microchem. J. 2022, 177, 107280. [Google Scholar] [CrossRef]
- Zhu, B.; Xu, X.; Luo, J.; Jin, S.; Chen, W.; Liu, Z.; Tian, C. Simultaneous determination of 131 pesticides in tea by on-line GPC-GC–MS/MS using graphitized multi-walled carbon nanotubes as dispersive solid phase extraction sorbent. Food Chem. 2019, 276, 202–208. [Google Scholar] [CrossRef]
- Wang, S.-L.; Pang, X.-Q.; Cao, J.; Cao, W.; Xu, J.-J.; Zhu, Q.-Y.; Zhang, Q.-Y.; Peng, L.-Q. Effervescence and graphitized multi-walled carbon nanotubes assisted microextraction for natural antioxidants by ultra high performance liquid chromatography with electrochemical detection and quadrupole time-of-flight tandem mass spectrometry. J. Chromatogr. A 2015, 1418, 12–20. [Google Scholar] [CrossRef]
- Xiao, Z.; Xing, Y.; Zhu, J.; Liu, Y.; Wang, J.; Liu, Q.; Huang, M.; Zhong, G. An effective pretreatment technique based on multi-walled carbon nanotubes to reduce the matrix effect in plasma samples analyzed by a new type probe electrospray ionization method. Anal. Chim. Acta 2023, 1263, 341268. [Google Scholar] [CrossRef]
- Kim, M.-A.; Kim, D.-Y.; Yun, C.-I.; Kim, Y.-J. Validation, measurement uncertainty, and application of HPLC and LC-MS/MS analysis methods for determining organic acids in processed food products. J. Food Compos. Anal. 2025, 141, 107335. [Google Scholar] [CrossRef]
- Liu, S.; Xiao, P.; Xu, J.; Yang, D.; Chen, M.; Zhou, C. Deep eutectic solvent-modified multi-walled carbon nanotubes as QuEChERS adsorbents coupled with gas chromatography-mass spectrometry for the determination of pesticide residues in Lycium barbarum. J. Food Compos. Anal. 2025, 141, 107350. [Google Scholar] [CrossRef]
- Qin, J.; Tong, K.; Chang, Q.; Xie, Y.; Wu, X.; Fan, C.; Zhang, H.; Shi, Z.; Chen, H. Simultaneous determination of PAHs and pesticides in milk based on improved QuEChERS combined with GC-MS/MS. J. Food Compos. Anal. 2025, 141, 107336. [Google Scholar] [CrossRef]
- Bever, C.S.; Adams, C.A.; Hnasko, R.M.; Cheng, L.W.; Stanker, L.H. Lateral flow immunoassay (LFIA) for the detection of lethal amatoxins from mushrooms. PLoS ONE 2020, 15, e0231781. [Google Scholar] [CrossRef]
- Zhang, Y.-Z.; Yan, Y.-Y.; Li, H.-J.; Fan, Y.-G.; Xu, F. Toxin screening of Pseudosperma umbrinellum (Agaricals, Basidiomycota): First report of phalloidin in Inocybaceae mushroom. Toxicon 2022, 217, 155–161. [Google Scholar] [CrossRef]
- Yoshioka, N.; Akamatsu, S.; Mitsuhashi, T.; Todo, C.; Asano, M.; Ueno, Y. A simple method for the simultaneous determination of mushroom toxins by liquid chromatography–time-of-flight mass spectrometry. Forensic Toxicol. 2014, 32, 89–96. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, J.; Li, H.; Sun, C. Simultaneous Determination of Multi-Class Mushroom Toxins in Mushroom and Biological Liquid Samples Using LC-MS/MS. Separations 2024, 11, 183. [Google Scholar] [CrossRef]
- Li, J.; Fang, J.; Liang, L.; Zhang, Y.; Li, H.; Wang, D.; Wu, S.; Li, C.; Li, C.; Dong, F. A combined strategy of multi-toxin detection and ITS identification for accurate and efficient inspection of poisonous mushrooms. J. Food Compos. Anal. 2025, 144, 107680. [Google Scholar] [CrossRef]
- Ren, X.; Yao, Y.; Wu, X.; Wu, L.; Guo, H.; Jiang, N. Halloysite nanotubes modified by hyperbranched polyamideamine as a novel QuEChERS adsorbent for the determination of 16 pesticide residues in chive, cowpea and cabbage by GC–MS/MS. Microchem. J. 2025, 212, 113460. [Google Scholar] [CrossRef]
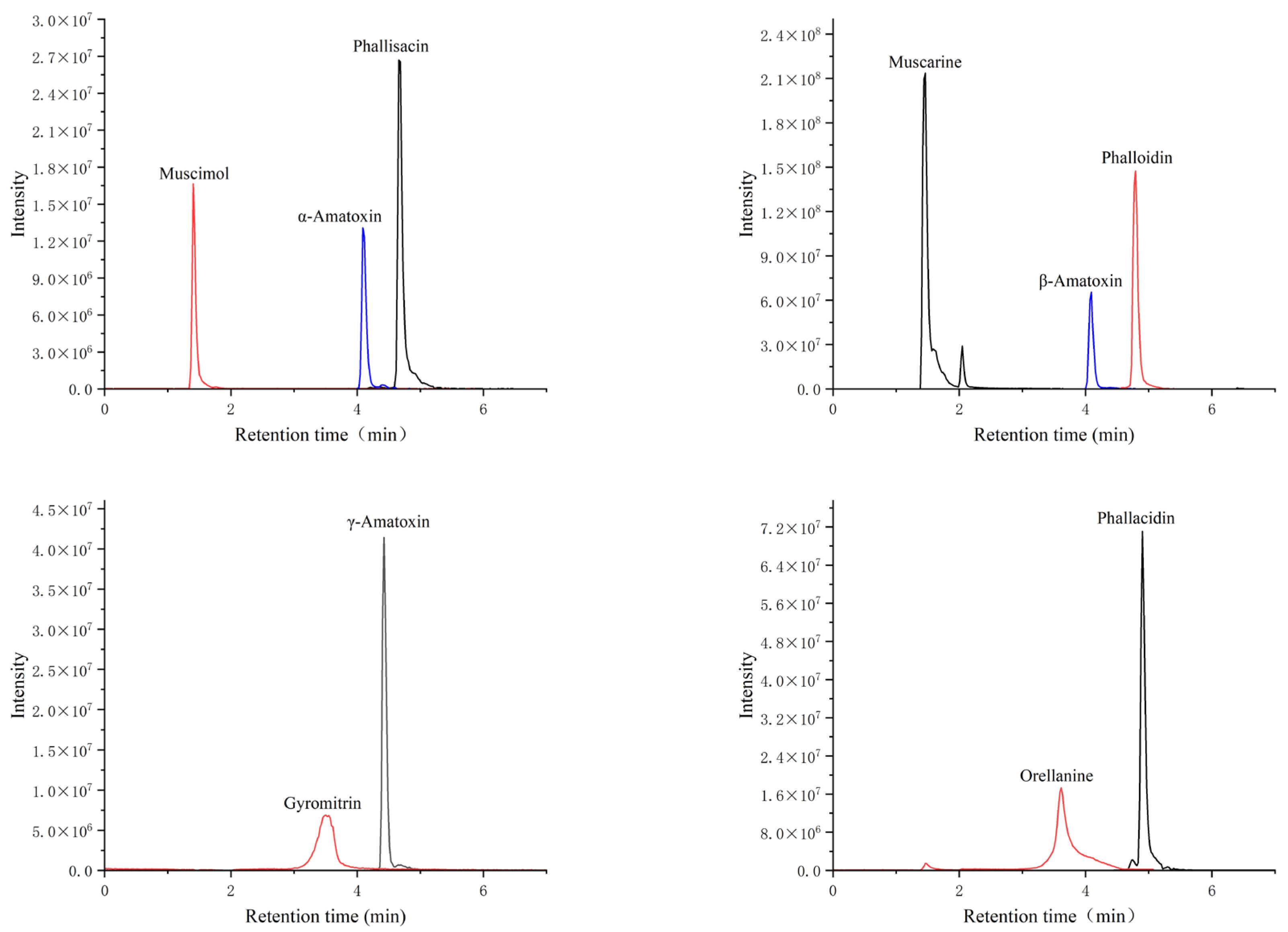

| Compound | Molecular Formula | Retention Time | Precursor Ion (m/z) | Production (m/z) | |||
|---|---|---|---|---|---|---|---|
| Theoretical Value | Measured Value | Mass Error (10−6) | Fragment 1 | Fragment 2 | |||
| α-Amatoxin | C39H54N10O14S | 4.11 | 917.34689 | 917.34753 | 0.70222 | 899.33533 | 201.07072 |
| β-Amatoxin | C39H53N9O15S | 4.09 | 918.33091 | 918.33081 | 0.10830 | 900.31924 | 205.04417 |
| γ-Amatoxin | C39H54N10O13S | 4.42 | 901.35198 | 901.35211 | 0.14624 | 883.34033 | 205.04390 |
| Phalloidin | C35H48N8O11S | 4.79 | 789.3236 | 789.32336 | 0.29866 | 86.06004 | 157.07602 |
| Phallacidin | C37H50N8O13S | 4.90 | 847.32908 | 847.32892 | 0.19065 | 811.30795 | 157.07602 |
| Phallisacin | C37H50N8O14S | 4.61 | 863.32400 | 863.32465 | 0.74827 | 130.06513 | 157.07602 |
| Orellanine | C10H8N2O6 | 3.62 | 253.04551 | 253.04546 | 0.21367 | 191.04530 | 219.04015 |
| Gyromitrin | C4H8N2O | 3.53 | 101.07094 | 101.07087 | 0.69807 | 58.02874 | 42.03383 |
| Muscarine | C9H20NO2 | 1.74 | 174.14886 | 174.14882 | 0.23560 | 97.06479 | 57.03349 |
| Muscimol | C4H6N2O2 | 1.41 | 115.0502 | 115.05016 | 0.38557 | 98.02365 | 67.01784 |
| Compound | Linear Range (mg·L−1) | Regression Equation | R2 | LOD (mg·kg−1) | LOQ (mg·kg−1) |
|---|---|---|---|---|---|
| α-Amatoxin | 0.02–1 | y = 2.711 × 104x − 2.384 × 102 | 0.9985 | 0.02 | 0.06 |
| β-Amatoxin | 0.01–1 | y = 1.246 × 105x − 6.919 × 103 | 0.9969 | 0.015 | 0.045 |
| γ-Amatoxin | 0.02–1 | y = 6.410 × 104x − 2.007 × 104 | 0.9954 | 0.02 | 0.06 |
| Phalloidin | 0.01–1 | y = 7.517 × 104x − 8.747 × 103 | 0.9937 | 0.015 | 0.045 |
| Phallacidin | 0.02–1 | y = 2.876 × 104x + 6.787 × 103 | 0.9989 | 0.02 | 0.06 |
| Phallisacin | 0.02–1 | y = 2.778 × 104x + 6.403 × 103 | 0.9986 | 0.02 | 0.06 |
| Orellanine | 0.2–5 | y = 5.197 × 103 x + 2.597 × 103 | 0.9940 | 0.2 | 0.6 |
| Gyromitrin | 0.05–5 | y = 3.199 × 104 x − 2.026 × 103 | 0.9936 | 0.1 | 0.3 |
| Muscarine | 0.005–1 | y = 2.655 × 105x − 9.613 × 103 | 0.9952 | 0.005 | 0.015 |
| Muscimol | 0.2–5 | y = 3.891 × 103x + 2.709 × 103 | 0.9977 | 0.2 | 0.6 |
| Compound | Pure Solvent | R2 | Sample Extract with Treatment | R2 | MF (%) |
|---|---|---|---|---|---|
| Y = K1X + b1 | Y = K2X + b2 | ||||
| α-Amatoxin | Y = 1.021 × 104X − 7.615 × 104 | 0.9996 | Y = 9.307 × 103X + 3.454 × 103 | 0.9977 | 91.16 |
| β-Amatoxin | Y = 4.579 × 104X − 4.430 × 103 | 0.9992 | Y = 5.076 × 104X − 4.429 × 103 | 0.9928 | 110.85 |
| γ-Amatoxin | Y = 2.605 × 104X − 3.062 × 103 | 0.9987 | Y = 2.337 × 104X + 3.673 × 103 | 0.9993 | 89.71 |
| Phalloidin | Y = 6.766 × 104X − 7.205 × 103 | 0.9993 | Y = 7.073 × 104X + 1.012 × 103 | 0.9992 | 104.54 |
| Phallacidin | Y = 1.419 × 104X − 3.109 × 103 | 0.9907 | Y = 1.143 × 104X − 5.932 × 104 | 0.9924 | 80.55 |
| Phallisacin | Y = 3.586 × 104X + 8.618 × 103 | 0.9965 | Y = 3.812 × 104X − 6.792 × 103 | 0.9971 | 106.30 |
| Orellanine | Y = 5.953 × 103X + 8.633 × 103 | 0.9945 | Y = 5.770 × 103X + 3.611 × 103 | 0.9970 | 96.93 |
| Gyromitrin | Y = 4.084 × 104X − 1.963 × 104 | 0.9992 | Y = 4.519 × 104X + 5.8261 × 103 | 0.9983 | 110.65 |
| Muscarine | Y = 4.092 × 105X − 4.213 × 104 | 0.9919 | Y = 3.794 × 105X + 8.356 × 103 | 0.9990 | 92.72 |
| Muscimol | Y = 4.590 × 103X − 1.483 × 103 | 0.9902 | Y = 5.458 × 103X + 9.195 × 103 | 0.9932 | 118.91 |
| Compound | Added/(mg·kg−1) | Recovery/% | RSD/% (n = 6) |
|---|---|---|---|
| α-Amatoxin | 0.05, 0.15, 0.5 | 70.67, 82.57, 87.29 | 18.11, 9.91, 8.45 |
| β-Amatoxin | 0.05, 0.15, 0.5 | 117.60, 104.72, 106.31 | 11.12, 5.75, 7.13 |
| γ-Amatoxin | 0.05, 0.15, 0.5 | 106.87, 103.65, 93.46 | 8.72, 5.76, 6.04 |
| Phalloidin | 0.05, 0.15, 0.5 | 75.16, 86.47, 97.76 | 10.81, 7.33, 7.18 |
| Phallacidin | 0.05, 0.15, 0.5 | 76.82, 91.68, 101.83 | 12.01, 7.48, 5.23 |
| Phallisacin | 0.05, 0.15, 0.5 | 90.68, 95.81, 102.85 | 6.83, 7.25, 5.14 |
| Orellanine | 0.5, 1, 2 | 72.86, 82.11, 90.79 | 8.15, 5.82, 2.13 |
| Gyromitrin | 0.5, 1, 2 | 93.21, 104.84, 96.05 | 7.10, 3.83, 6.70 |
| Muscarine | 0.05, 0.15, 0.5 | 91.89, 109.44, 115.37 | 4.84, 5.57, 2.55 |
| Muscimol | 0.5, 1, 2 | 87.35, 108.14, 97.94 | 9.01, 4.90, 4.36 |
| Sample Serial Number | Detection Results (mg/kg) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| α-Amatoxin | β-Amatoxin | γ-Amatoxin | Phalloidin | Phallacidin | Phallisacin | Orellanine | Gyromitrin | Muscarine | Muscimol | |
| 1 | 113.68 | 250.29 | 55.49 | 61.18 | ND | ND | ND | ND | ND | ND |
| 2 | 281.47 | 50.07 | 3.11 | 445.10 | 2.74 | ND | ND | ND | ND | ND |
| 3 | ND | ND | ND | 0.42 | 0.032 | ND | ND | ND | ND | ND |
| 4 | ND | ND | ND | ND | ND | ND | 1.24 | ND | ND | ND |
| 5 | ND | ND | ND | ND | ND | ND | ND | ND | 0.035 | ND |
| 6 | ND | ND | ND | ND | ND | ND | ND | ND | 0.82 | ND |
| 7 | ND | ND | ND | ND | ND | ND | ND | ND | 0.54 | ND |
| 8 | ND | ND | ND | ND | ND | ND | ND | ND | 0.57 | ND |
| 9 | ND | ND | ND | ND | ND | ND | ND | ND | ND | 55.05 |
| 10 | ND | ND | ND | 1.77 | ND | ND | ND | ND | ND | 119.55 |
| Method | Retention Time (min) | Number of Detected Toxins | LOD/(µg kg−1) | Purification Material | Matrix Effects (%) | Sample Purification Cost (USD) | Processing Time | References |
|---|---|---|---|---|---|---|---|---|
| HPLC-UV-EC | 18.1 | 2 | 24–64 | HLB-SPE | NA | 3 | 3 h | [2] |
| UHPLC-Q-Orbitrap MS | 13.5 | 4 | 8–20 | NA | 48–101 | NA | 1 h | [20] |
| LC-QqQLIT-MS/MS | 15 | 2 | 100–1000 | QuCHERS-PP column | NA | 2.8 | 2 h | [37] |
| LC-TOF-MS/MS | 22.3 | 9 | 9.8–4900 | HLB-SPE | NA | 3 | 2 h | [38] |
| UHPLC-MS/MS | 8 | 12 | 5–100 | NA | 38–66 | NA | 1 h | [39] |
| UHPLC-Q Orbitrap HRMS | 9 | 10 | 5–200 | G-MWCNTs | 80–119 | 1.8 | 1 h | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; Liu, Y.; Li, S.; Li, R.; Zhang, Y.; Zhao, H. Application of Graphitized Multi-Walled Carbon Nanotubes Combined with Orbitrap High-Resolution Mass Spectrometry for the Rapid Detection of Ten Toxins in Wild Mushrooms. Toxins 2025, 17, 445. https://doi.org/10.3390/toxins17090445
Zhang B, Liu Y, Li S, Li R, Zhang Y, Zhao H. Application of Graphitized Multi-Walled Carbon Nanotubes Combined with Orbitrap High-Resolution Mass Spectrometry for the Rapid Detection of Ten Toxins in Wild Mushrooms. Toxins. 2025; 17(9):445. https://doi.org/10.3390/toxins17090445
Chicago/Turabian StyleZhang, Bo, Yang Liu, Shengnan Li, Ruonan Li, Yunhui Zhang, and Hua Zhao. 2025. "Application of Graphitized Multi-Walled Carbon Nanotubes Combined with Orbitrap High-Resolution Mass Spectrometry for the Rapid Detection of Ten Toxins in Wild Mushrooms" Toxins 17, no. 9: 445. https://doi.org/10.3390/toxins17090445
APA StyleZhang, B., Liu, Y., Li, S., Li, R., Zhang, Y., & Zhao, H. (2025). Application of Graphitized Multi-Walled Carbon Nanotubes Combined with Orbitrap High-Resolution Mass Spectrometry for the Rapid Detection of Ten Toxins in Wild Mushrooms. Toxins, 17(9), 445. https://doi.org/10.3390/toxins17090445





