Biocatalytic Detoxification of Ochratoxins A/B by a Fungal Dye-Decolorizing Peroxidase: Mechanistic Insights and Toxicity Assessment
Abstract
1. Introduction
2. Results and Discussion
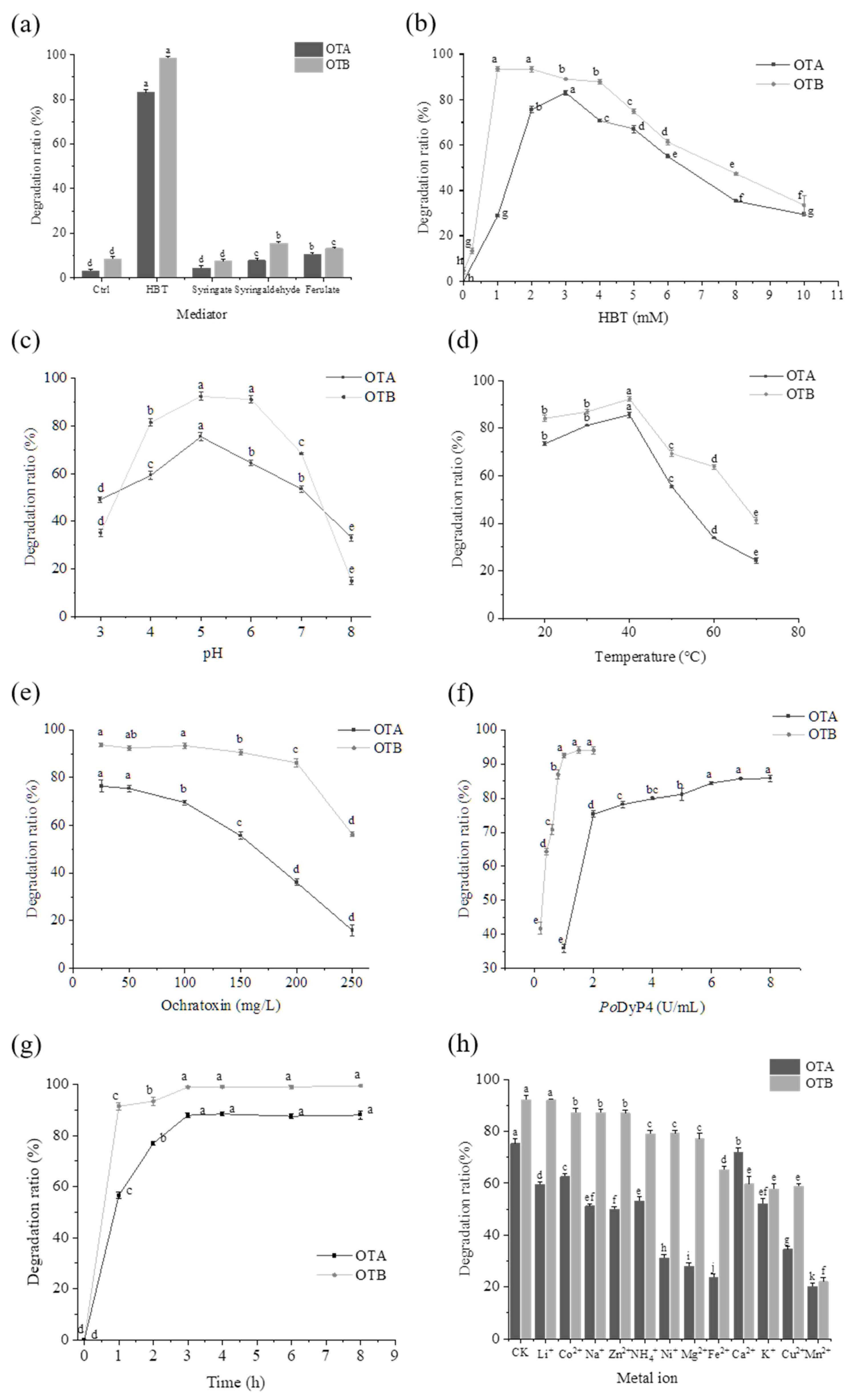
2.1. Degradation Properties of Ochratoxin A and B with PoDyP4
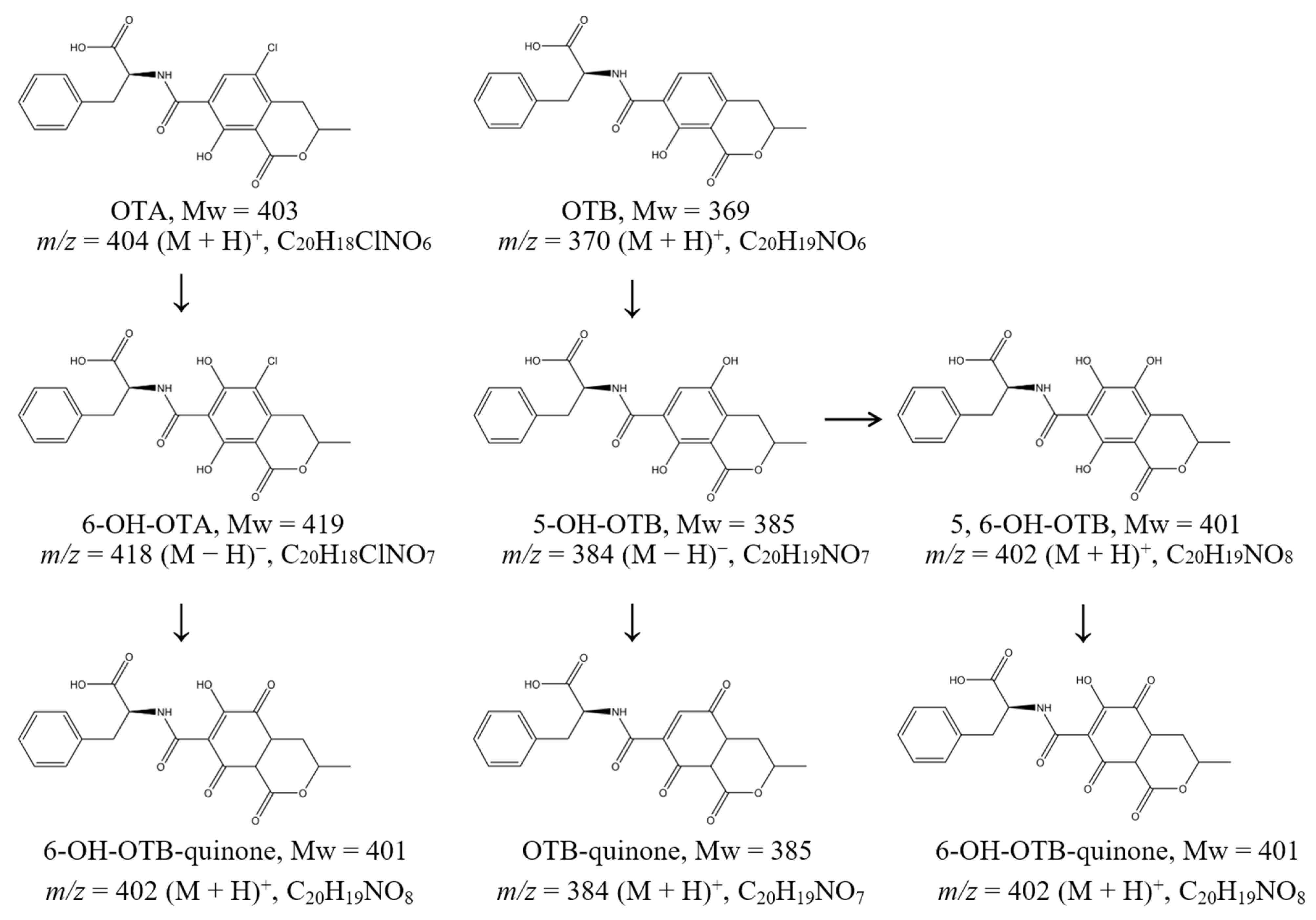
2.2. Degradation Products of Ochratoxin A and B and Their Pathways
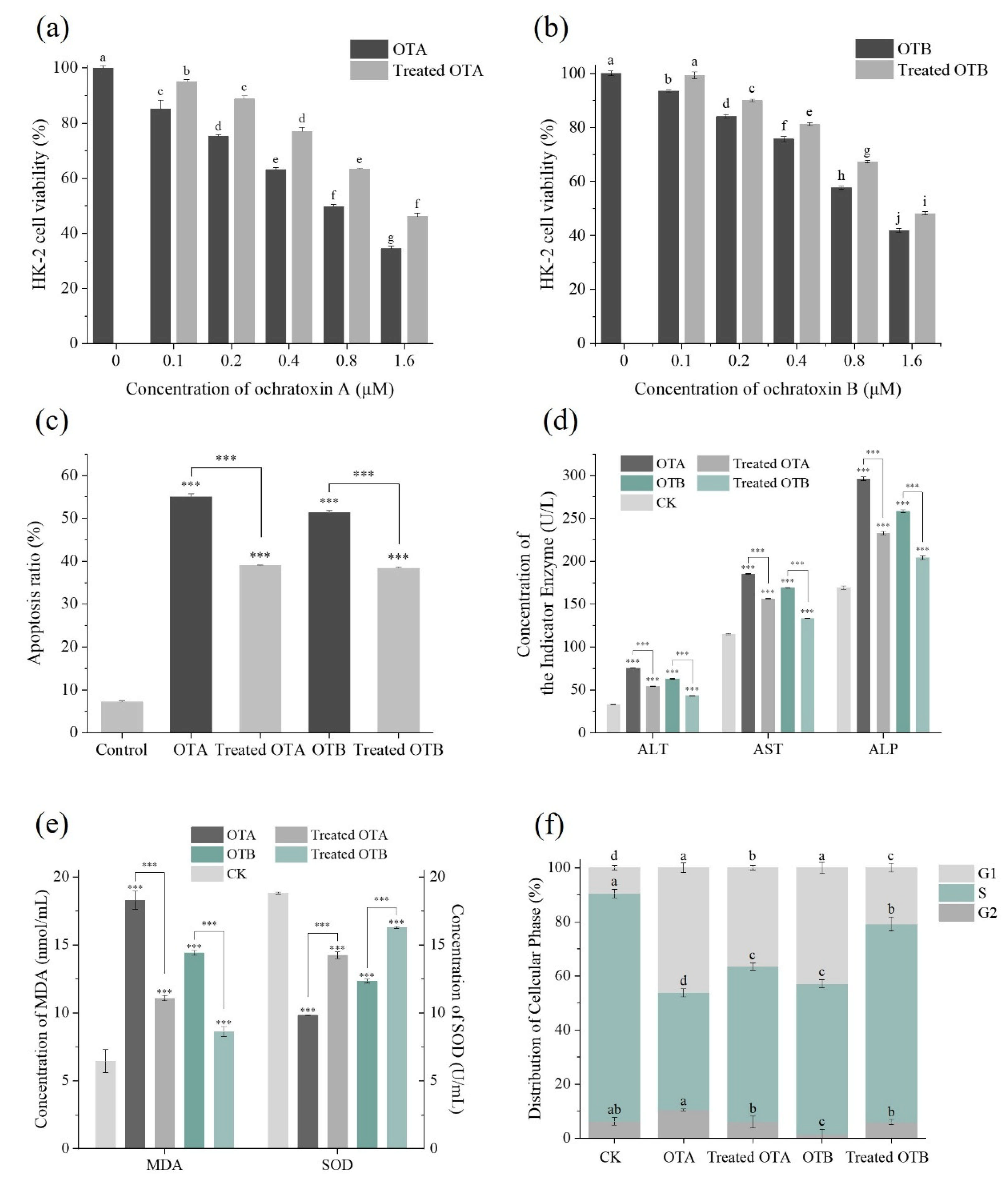
2.3. The Assessment of Cytotoxicity of Degradation Products on HK-2 Cells
2.4. Molecular Binding Mode of PoDyP4 with Ochratoxin A and B
3. Conclusions
4. Materials and Methods
4.1. Substrates and Chemicals
4.2. Enzymatic Degradation of Ochratoxin A and B by PoDyP4
4.3. Detection and Identification of Ochratoxin A and B and Their Degradation Products
4.4. HK-2 Cell Viability and Apoptosis Assay
4.5. Biochemical Index Analysis of HK-2 Cells
4.6. Molecular Docking of PoDyP4 with Ochratoxin A and B
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eskola, M.; Kos, G.; Elliott, C.T.; Hajslova, J.; Mayar, S.; Krska, R. Worldwide contamination of food-crops with mycotoxins: Validity of the widely cited ‘FAO estimate’ of 25. Crit. Rev. Food Sci. Nutr. 2020, 60, 2773–2789. [Google Scholar] [CrossRef]
- De Santis, B.; Debegnach, F.; Toscano, P.; Crisci, A.; Battilani, P.; Brera, C. Overall Exposure of European Adult Population to Mycotoxins by Statistically Modelled Biomonitoring Data. Toxins 2021, 13, 695. [Google Scholar] [CrossRef]
- Ganesan, A.R.; Mohan, K.; Karthick Rajan, D.; Pillay, A.A.; Palanisami, T.; Sathishkumar, P.; Conterno, L. Distribution, toxicity, interactive effects, and detection of ochratoxin and deoxynivalenol in food: A review. Food Chem. 2022, 378, 131978. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Mahato, D.K.; Sharma, B.; Borah, R.; Haque, S.; Mahmud, M.M.C.; Shah, A.K.; Rawal, D.; Bora, H.; Bui, S. Ochratoxins in food and feed: Occurrence and its impact on human health and management strategies. Toxicon 2020, 187, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Banahene, J.C.M.; Ofosu, I.W.; Odai, B.T.; Lutterodt, H.E.; Agyemang, P.A.; Ellis, W.O. Ochratoxin A in food commodities: A review of occurrence, toxicity, and management strategies. Heliyon 2024, 10, e39313. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, Q.; Wang, S.; Cai, R.; Yuan, Y.; Yue, T.; Wang, Z. Bio-control on the contamination of Ochratoxin A in food: Current research and future prospects. Curr. Res. Food Sci. 2022, 5, 1539–1549. [Google Scholar] [CrossRef]
- Sun, H.; He, Z.; Xiong, D.; Long, M. Mechanisms by which microbial enzymes degrade four mycotoxins and application in animal production: A review. Anim. Nutr. 2023, 15, 256–274. [Google Scholar] [CrossRef]
- Obafemi, B.A.; Adedara, I.A.; Rocha, J.B.T. Neurotoxicity of ochratoxin A: Molecular mechanisms and neurotherapeutic strategies. Toxicology 2023, 497–498, 153630. [Google Scholar] [CrossRef]
- GB 2761-2017; National Food Safety Standard: Maximum Levels of Mycotoxins in Foods. National Health Commission of the People’s Republic of China: Beijing, China, 2017.
- GB 13078-2017; Hygienic Standard for Feeds. Standardization Administration of the People’s Republic of China: Beijing, China, 2017.
- Wang, Y.; Wang, L.; Wu, F.; Liu, F.; Wang, Q.; Zhang, X.; Selvaraj, J.N.; Zhao, Y.; Xing, F.; Yin, W.B.; et al. A Consensus Ochratoxin A Biosynthetic Pathway: Insights from the Genome Sequence of Aspergillus ochraceus and a Comparative Genomic Analysis. Appl. Environ. Microbiol. 2018, 84, e01009-18. [Google Scholar] [CrossRef]
- Heussner, A.H.; Bingle, L.E. Comparative Ochratoxin Toxicity: A Review of the Available Data. Toxins 2015, 7, 4253–4282. [Google Scholar] [CrossRef]
- Wang, G.; Li, E.; Gallo, A.; Perrone, G.; Varga, E.; Ma, J.; Yang, B.; Tai, B.; Xing, F. Impact of environmental factors on ochratoxin A: From natural occurrence to control strategy. Environ. Pollut. 2023, 317, 120767. [Google Scholar] [CrossRef]
- Magan, N.; Hope, R.; Cairns, V.; Aldred, D. Post-Harvest Fungal Ecology: Impact of Fungal Growth and Mycotoxin Accumulation in Stored Grain. Eur. J. Plant Pathol. 2003, 109, 723–730. [Google Scholar] [CrossRef]
- Mwabulili, F.; Xie, Y.; Li, Q.; Sun, S.; Yang, Y.; Ma, W. Research progress of ochratoxin a bio-detoxification. Toxicon 2023, 222, 107005. [Google Scholar] [CrossRef] [PubMed]
- Taheur, F.B.; Fedhila, K.; Chaieb, K.; Kouidhi, B.; Bakhrouf, A.; Abrunhosa, L. Adsorption of aflatoxin B1, zearalenone and ochratoxin A by microorganisms isolated from Kefir grains. Int. J. Food Microbiol. 2017, 251, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Khoury, R.E.; Mathieu, F.; Atoui, A.; Kawtharani, H.; Khoury, A.E.; Afif, C.; Maroun, R.G.; Khoury, A.E. Ability of Soil Isolated Actinobacterial Strains to Prevent, Bind and Biodegrade Ochratoxin A. Toxins 2017, 9, 222. [Google Scholar] [CrossRef]
- Wang, L.; Hua, X.; Shi, J.; Jing, N.; Ji, T.; Lv, B.; Liu, L.; Chen, Y. Ochratoxin A: Occurrence and recent advances in detoxification. Toxicon 2022, 210, 11–18. [Google Scholar] [CrossRef]
- Chen, W.; Li, C.; Zhang, B.; Zhou, Z.; Shen, Y.; Liao, X.; Yang, J.; Wang, Y.; Li, X.; Li, Y.; et al. Advances in Biodetoxification of Ochratoxin A—A Review of the Past Five Decades. Front. Microbiol. 2018, 9, 1386. [Google Scholar] [CrossRef]
- Liuzzi, V.C.; Fanelli, F.; Tristezza, M.; Haidukowski, M.; Picardi, E.; Manzari, C.; Lionetti, C.; Grieco, F.; Logrieco, A.F.; Thon, M.R.; et al. Transcriptional Analysis of Acinetobacter sp. neg1 Capable of Degrading Ochratoxin A. Front. Microbiol. 2016, 7, 2162. [Google Scholar] [CrossRef]
- Pitout, M.J. The hydrolysis of ochratoxin A by some proteolytic enzymes. Biochem. Pharmacol. 1969, 18, 485–491. [Google Scholar] [CrossRef]
- Wu, Q.; Dohnal, V.; Huang, L.; Kuca, K.; Wang, X.; Chen, G.; Yuan, Z. Metabolic pathways of ochratoxin A. Curr. Drug Metab. 2011, 12, 1–10. [Google Scholar] [CrossRef]
- Sanchez-Arroyo, A.; Plaza-Vinuesa, L.; de Las Rivas, B.; Mancheno, J.M.; Munoz, R. Aspergillus niger Ochratoxinase Is a Highly Specific, Metal-Dependent Amidohydrolase Suitable for OTA Biodetoxification in Food and Feed. J. Agric. Food Chem. 2024, 72, 18658–18669. [Google Scholar] [CrossRef] [PubMed]
- Liers, C.; Aranda, E.; Strittmatter, E.; Piontek, K.; Plattner, D.A.; Zorn, H.; Ullrich, R.; Hofrichter, M. Phenol oxidation by DyP-type peroxidases in comparison to fungal and plant peroxidases. J. Mol. Catal. B Enzym. 2014, 103, 41–46. [Google Scholar] [CrossRef]
- Ding, S.; Lin, C.; Xiao, Q.; Feng, F.; Wang, J.; Zhang, X.; Yang, S.; Li, L.; Li, F. Effective degradation of zearalenone by dye-decolorizing peroxidases from Pleurotus ostreatus and its metabolic pathway and toxicity analysis. Sci. Total Environ. 2024, 908, 168500. [Google Scholar] [CrossRef] [PubMed]
- Loi, M.; Renaud, J.B.; Rosini, E.; Pollegioni, L.; Vignali, E.; Haidukowski, M.; Sumarah, M.W.; Logrieco, A.F.; Mule, G. Enzymatic transformation of aflatoxin B(1) by Rh_DypB peroxidase and characterization of the reaction products. Chemosphere 2020, 250, 126296. [Google Scholar] [CrossRef] [PubMed]
- Nobre, C.; Gonzalez, A.; Losoya, C.; Teixeira, J.A.; Belmares, R.; Abrunhosa, L. Detoxification of ochratoxin A and zearalenone by Pleurotus ostreatus during in vitro gastrointestinal digestion. Food Chem. 2022, 384, 132525. [Google Scholar] [CrossRef]
- Santos, J.; Castro, T.; Venancio, A.; Silva, C. Degradation of ochratoxins A and B by lipases: A kinetic study unraveled by molecular modeling. Heliyon 2023, 9, e19921. [Google Scholar] [CrossRef]
- Luo, H.; Wang, G.; Chen, N.; Fang, Z.; Xiao, Y.; Zhang, M.; Gerelt, K.; Qian, Y.; Lai, R.; Zhou, Y. A Superefficient Ochratoxin A Hydrolase with Promising Potential for Industrial Applications. Appl. Environ. Microbiol. 2022, 88, e0196421. [Google Scholar] [CrossRef]
- Ozawa, S.; Ojiro, R.; Tang, Q.; Zou, X.; Jin, M.; Yoshida, T.; Shibutani, M. In vitro and in vivo induction of ochratoxin A exposure-related micronucleus formation in rat proximal tubular epithelial cells and expression profiling of chromosomal instability-related genes. Food Chem. Toxicol. 2024, 185, 114486. [Google Scholar] [CrossRef]
- Erceg, S.; Mateo, E.M.; Zipancic, I.; Rodriguez Jimenez, F.J.; Perez Arago, M.A.; Jimenez, M.; Soria, J.M.; Garcia-Esparza, M.A. Assessment of Toxic Effects of Ochratoxin A in Human Embryonic Stem Cells. Toxins 2019, 11, 217. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Q.; Xu, Z.; Guo, X.; Wu, S. Association between short-term exposure to ambient particulate air pollution and biomarkers of oxidative stress: A meta-analysis. Environ. Res. 2020, 191, 110105. [Google Scholar] [CrossRef]
- Shi, M.H.; Wu, Y.; Li, L.; Cai, Y.F.; Liu, M.; Gao, X.H.; Chen, H.D. Meta-analysis of the association between vitiligo and the level of superoxide dismutase or malondialdehyde. Clin. Exp. Dermatol. 2017, 42, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Fleury, C.; Mignotte, B.; Vayssiere, J.L. Mitochondrial reactive oxygen species in cell death signaling. Biochimie 2002, 84, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, W.; Zhu, N.; Mei, J.; Peng, Y.; Song, F.; Ding, S.; Li, F.; Zhou, X. Biocatalytic Detoxification of Ochratoxins A/B by a Fungal Dye-Decolorizing Peroxidase: Mechanistic Insights and Toxicity Assessment. Toxins 2025, 17, 438. https://doi.org/10.3390/toxins17090438
Xia W, Zhu N, Mei J, Peng Y, Song F, Ding S, Li F, Zhou X. Biocatalytic Detoxification of Ochratoxins A/B by a Fungal Dye-Decolorizing Peroxidase: Mechanistic Insights and Toxicity Assessment. Toxins. 2025; 17(9):438. https://doi.org/10.3390/toxins17090438
Chicago/Turabian StyleXia, Wenjing, Nianqing Zhu, Jie Mei, Yueqin Peng, Fanglin Song, Shuai Ding, Fei Li, and Xue Zhou. 2025. "Biocatalytic Detoxification of Ochratoxins A/B by a Fungal Dye-Decolorizing Peroxidase: Mechanistic Insights and Toxicity Assessment" Toxins 17, no. 9: 438. https://doi.org/10.3390/toxins17090438
APA StyleXia, W., Zhu, N., Mei, J., Peng, Y., Song, F., Ding, S., Li, F., & Zhou, X. (2025). Biocatalytic Detoxification of Ochratoxins A/B by a Fungal Dye-Decolorizing Peroxidase: Mechanistic Insights and Toxicity Assessment. Toxins, 17(9), 438. https://doi.org/10.3390/toxins17090438




