Histopathological Assessment and Oxidative Biomarker Analysis of Wild Boar Tissues Affected by Ochratoxin A Contamination in the Campania Region, Southern Italy
Abstract
1. Introduction
2. Results
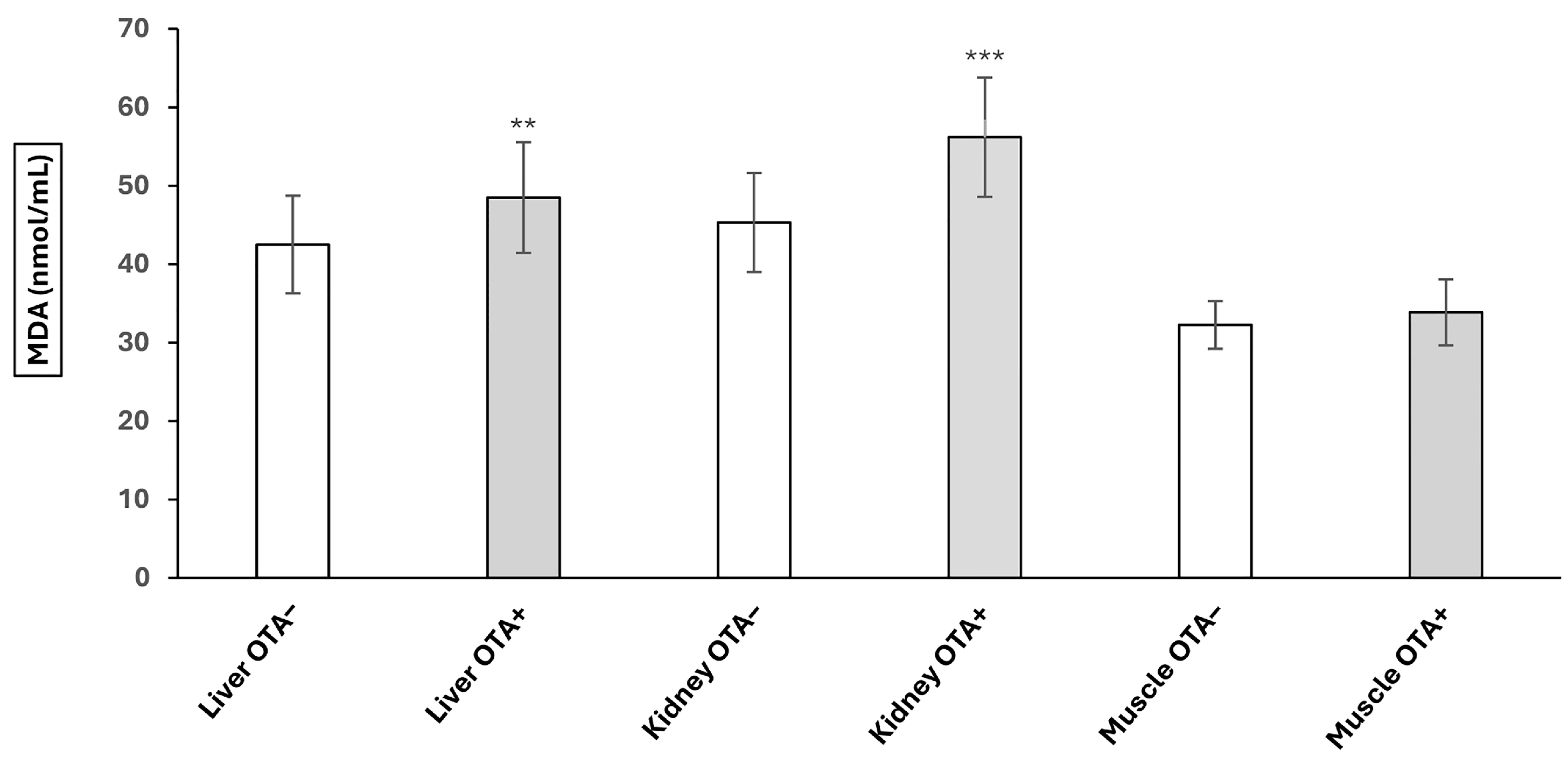
2.1. The Effect of OTA on Lipid Peroxidation
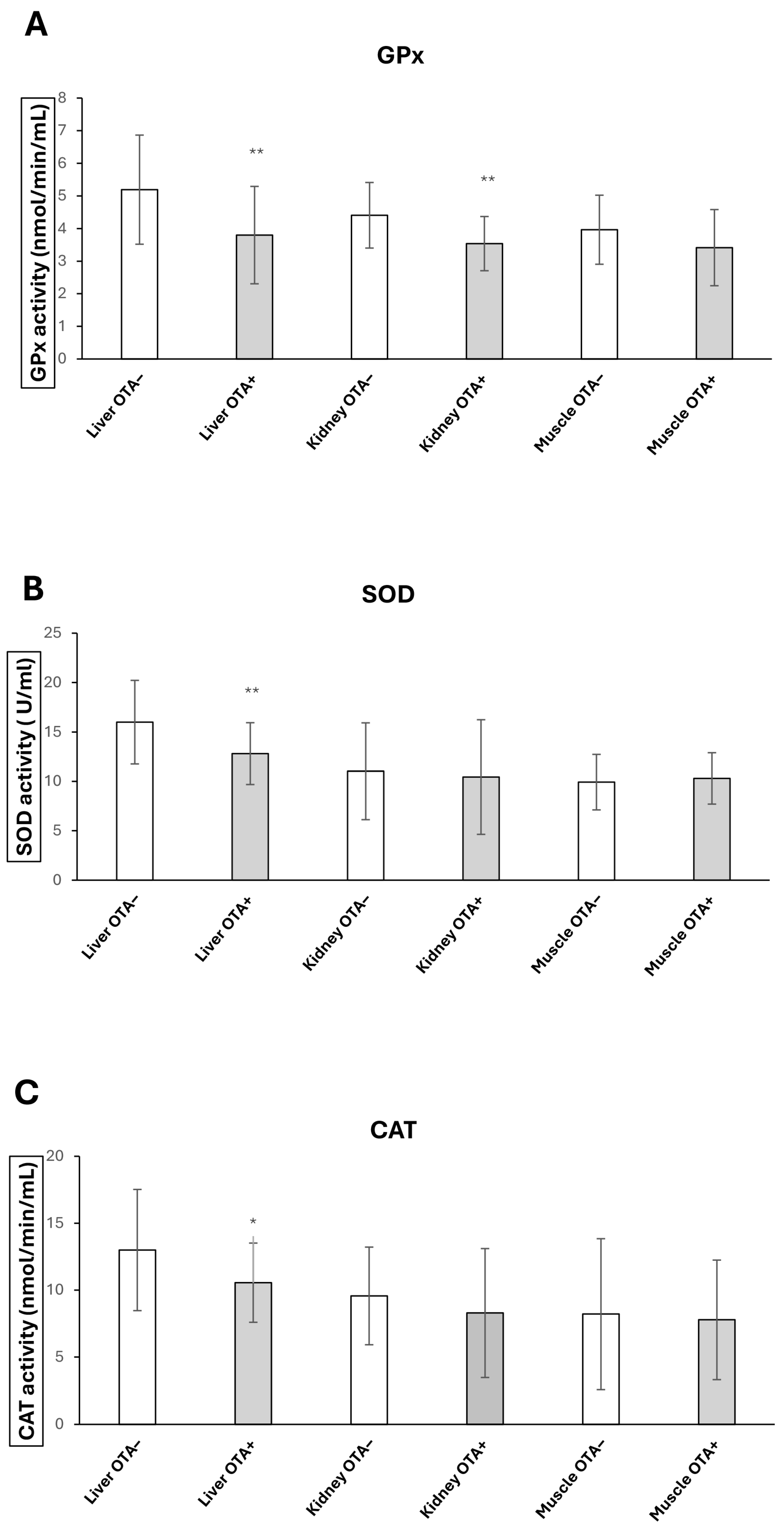
2.2. GPx, CAT and SOD Activities Changes upon OTA Exposure
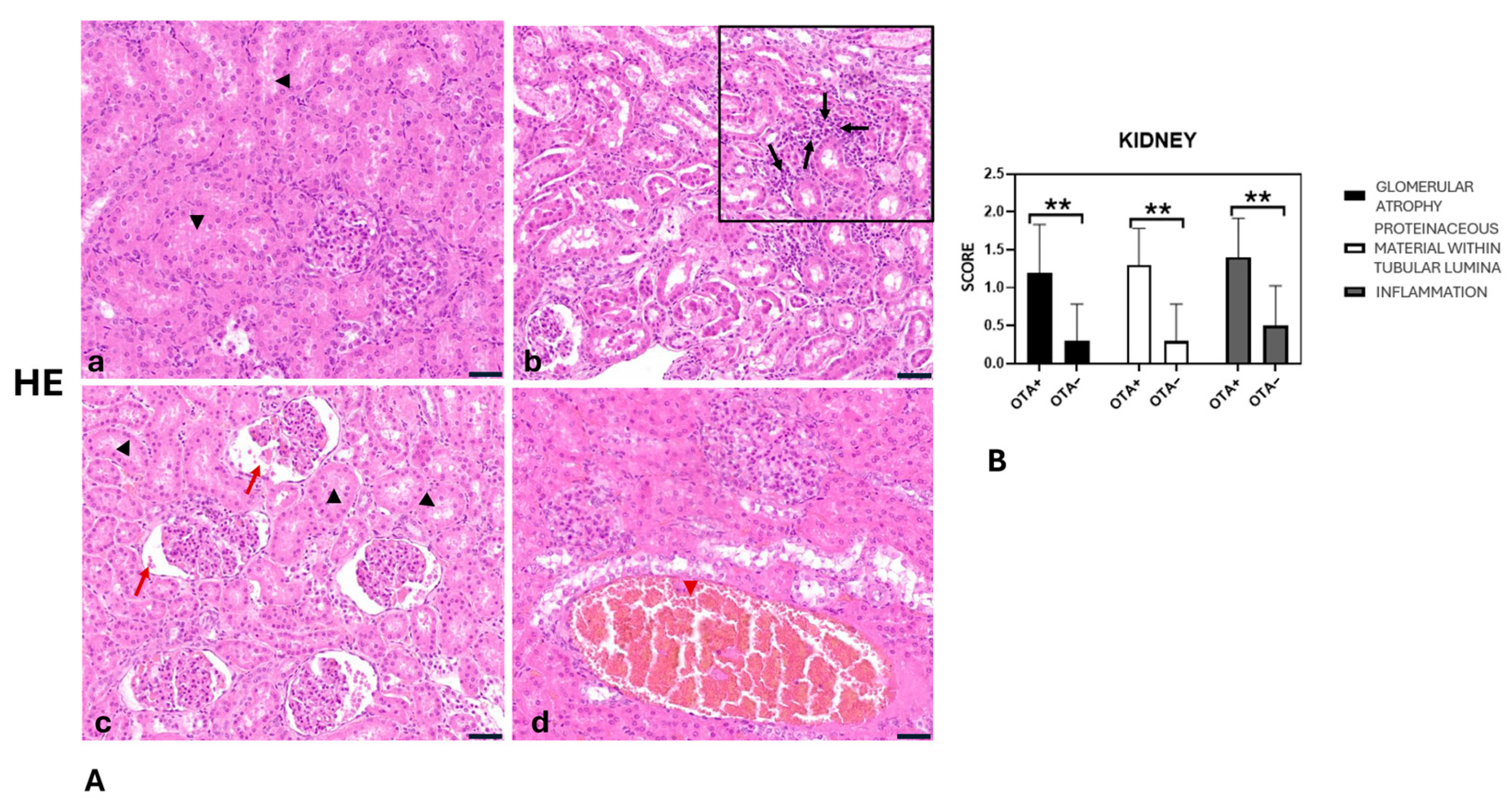
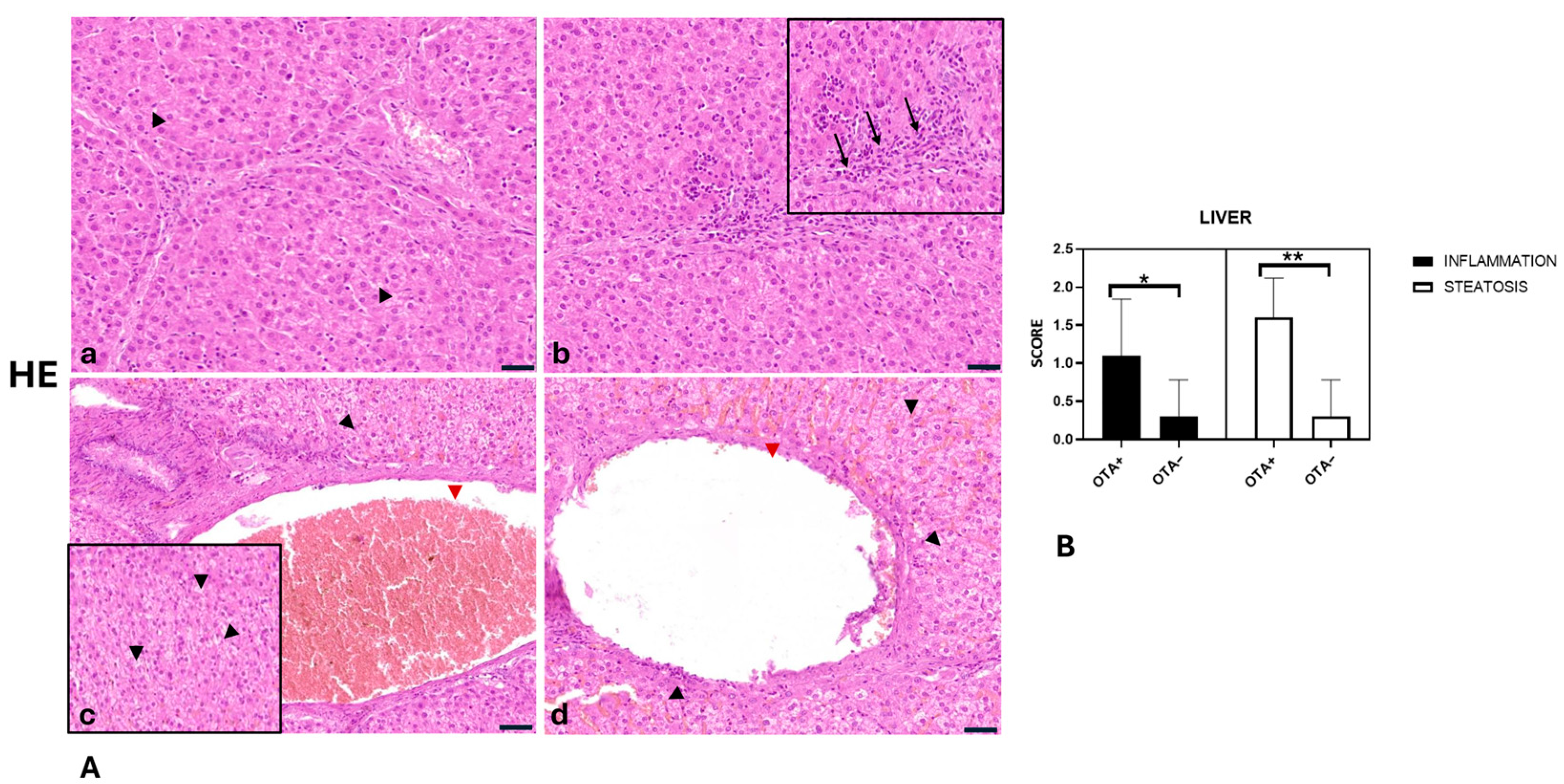
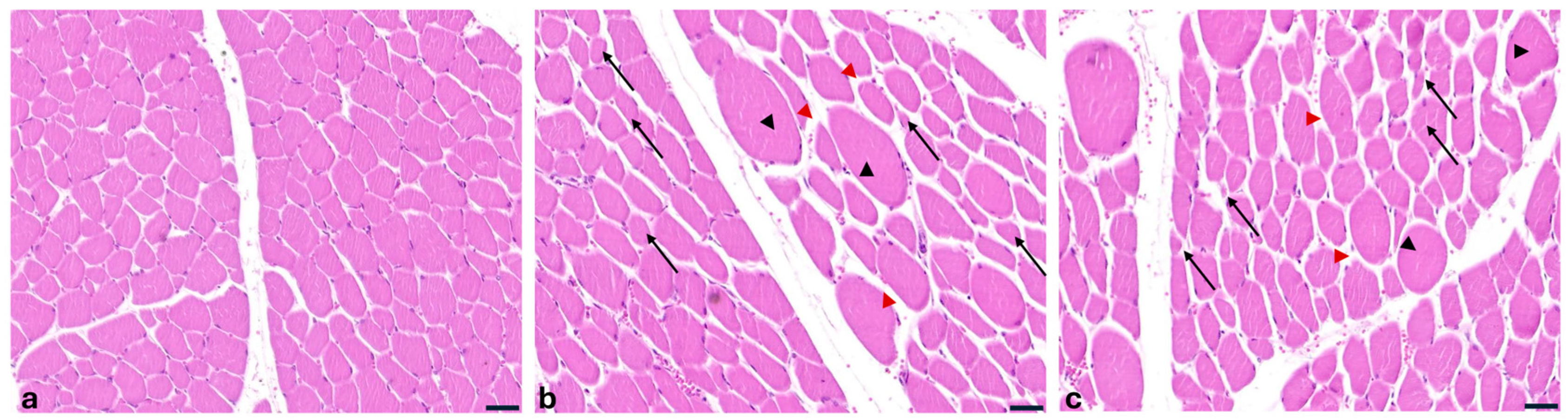
2.3. Histopathological Findings of Liver, Kidneys and Muscles
- Glomerular atrophy was significantly greater in the OTA+ group compared with the OTA− group (p = 0.0080).
- Proteinaceous material in tubular lumina was significantly more abundant in the OTA+ group than in the OTA− group (p = 0.0013).
- Kidney inflammation was significantly higher in the OTA+ group compared with the OTA− group (p = 0.0050).
- Liver inflammation was significantly greater in the OTA+ group compared with the OTA− group (p = 0.0270).
- Liver steatosis was significantly more pronounced in the OTA+ group compared with the OTA− group (p = 0.0004).
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Ethics Statement
5.2. Study Area: Wild Boar Population Density in Avellino, Italy
5.3. Sampling of Free-Ranging Wild Boar
5.4. Analysis of Malondialdehyde (MDA) and Antioxidant Enzyme Activities
5.5. Histopathological Examinations
5.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| OTA | Ochratoxin A |
| MDA | Malondialdehyde |
| GPx | Glutathione peroxidase |
| SOD | Superoxide dismutase |
| CAT | Catalase |
| WHO | World Health Organisation |
| MRLs | Maximum residue limits |
References
- Mesfin, A.; Lachat, C.; Vidal, A.; Croubels, S.; Haesaert, G.; Ndemera, M.; Okoth, S.; Belachew, T.; Boevre, M.; De Saeger, S.; et al. Essential descriptors for mycotoxin contamination data in food and feed. Food Res. Int. 2022, 152, 110883. [Google Scholar] [CrossRef] [PubMed]
- Hao, W.; Li, A.; Wang, J.; An, G.; Guan, S. Mycotoxin Contamination of Feeds and Raw Materials in China in Year 2021. Front. Vet. Sci. 2022, 9, 929904. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Malir, F.; Ostry, V.; Pfohl-Leszkowicz, A.; Malir, J.; Toman, J. Ochratoxin A: 50 Years of Research. Toxins 2016, 8, 191. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Heussner, A.H.; Bingle, L.E. Comparative Ochratoxin Toxicity: A Review of the Available Data. Toxins 2015, 7, 4253–4282. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wu, F.; Groopman, J.D.; Pestka, J.J. Public health impacts of foodborne mycotoxins. Annu. Rev. Food Sci. Technol. 2014, 5, 351–372. [Google Scholar] [CrossRef] [PubMed]
- Bui-Klimke, T.R.; Wu, F. Ochratoxin A and human health risk: A review of the evidence. Crit. Rev. Food Sci. Nutr. 2015, 55, 1860–1869. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- van Egmond, H.P.; Schothorst, R.C.; Jonker, M.A. Regulations relating to mycotoxins in food: Perspectives in a global and European context. Anal. Bioanal. Chem. 2007, 389, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Kőszegi, T.; Poór, M. Ochratoxin A: Molecular Interactions, Mechanisms of Toxicity and Prevention at the Molecular Level. Toxins 2016, 8, 111. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rhouati, A.; Yang, C.; Hayat, A.; Marty, J.L. Aptamers: A promising tool for ochratoxin A detection in food analysis. Toxins 2013, 5, 1988–2008. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- IARC. Ochratoxin A. IARC Monogr. Eval. Carcinog. Risks Hum. 1993, 56, 489–521. [Google Scholar]
- Liu, W.-C.; Pushparaj, K.; Meyyazhagan, A.; Arumugam, V.A.; Pappuswamy, M.; Bhotla, H.K.; Baskaran, R.; Issara, U.; Balasubramanian, B.; Khaneghah, A.M. Ochratoxin A as an alarming health threat for livestock and human: A review on molecular interactions, mechanism of toxicity, detection, detoxification, and dietary prophylaxis. Toxicon 2022, 213, 59–75. [Google Scholar] [CrossRef] [PubMed]
- Reddy, L.; Bhoola, K. Ochratoxins-food contaminants: Impact on human health. Toxins 2010, 2, 771–779. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Prior, M.G.; Sisodia, C.S.; O’Neil, J.B. Acute oral ochratoxicosis in day-old White Leghorns, turkeys and Japanese Quail. Poult. Sci. 1976, 55, 786–790. [Google Scholar] [CrossRef] [PubMed]
- Kuiper-Goodman, T.; Hilts, C.; Billiard, S.M.; Kiparissis, Y.; Richard, I.D.; Hayward, S. Health risk assessment of ochratoxin A for all age-sex strata in a market economy. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess 2010, 27, 212–240. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stefanaki, I.; Foufa, E.; Tsatsou-Dritsa, A.; Dais, P. Ochratoxin A concentrations in Greek domestic wines and dried vine fruits. Food Addit. Contam. 2003, 20, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Lyon, C.J.; Ying, B.; Hu, T. Climate change, its impact on emerging infectious diseases and new technologies to combat the challenge. Emerg. Microbes Infect. 2024, 13, 2356143. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Commission Regulation (EU) 2022/1370 of 5 August 2022 Amending Regulation (EC) No 1881/2006 as Regards Maximum Levels of Ochratoxin A in Certain foodstuffs. Official Journal of the European Union (OJ L 206). 8 August 2022, pp. 11–14. Available online: https://eur-lex.europa.eu/eli/reg/2022/1370/oj/eng (accessed on 22 August 2025).
- Hing, S.; Narayan, E.J.; Andrew Thompson, R.C.; Godfrey, S.S. The relationship between physiological stress and wildlife disease: Consequences for health and conservation. Wildl. Res. 2016, 43, 51–60. [Google Scholar] [CrossRef]
- Armorini, S.; Altafini, A.; Zaghini, A.; Roncada, P. Ochratoxin A in artisan salami produced in Veneto (Italy). Food Addit. Contam. Part B Surveill. 2016, 9, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Barrios-García, M.N.; Gonzalez-Polo, M.; Simberloff, D.; Classen, A.T. Wild boar rooting impacts soil function differently in different plant community types. Biol. Invasions 2023, 25, 583–592. [Google Scholar] [CrossRef]
- Valente, A.M.; Acevedo, P.; Figueiredo, A.M.; Fonseca, C.; Torres, R.T. Overabundant wild ungulate populations in Europe: Management with consideration of socio-ecological consequences. Mamm. Rev. 2020, 50, 353–366. [Google Scholar] [CrossRef]
- Limonciel, A.; Jennings, P. A review of the evidence that ochratoxin A is an Nrf2 inhibitor: Implications for nephrotoxicity and renal carcinogenicity. Toxins 2014, 6, 371–379. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Longobardi, C.; Ferrara, G.; Andretta, E.; Montagnaro, S.; Damiano, S.; Ciarcia, R. Ochratoxin A and Kidney Oxidative Stress: The Role of Nutraceuticals in Veterinary Medicine—A Review. Toxins 2022, 14, 398. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ramyaa, P.; Krishnaswamy, R.; Padma, V.V. Quercetin modulates OTA-induced oxidative stress and redox signalling in HepG2 cells-up regulation of Nrf2 expression and down regulation of NF-κB and COX-2. Biochim. Biophys. Acta 2014, 1840, 681–692. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhang, S.; Lan, H.; Zheng, X. Ochratoxin A (OTA) causes intestinal aging damage through the NLRP3 signaling pathway mediated by calcium overload and oxidative stress. Environ. Sci. Pollut. Res. Int. 2024, 31, 27864–27882. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, M.; Costantini, D. Biomarkers of oxidative status: Missing tools in conservation physiology. Conserv. Physiol. 2014, 2, cou014. [Google Scholar] [CrossRef] [PubMed]
- Costantini, D.; Helfenstein, F. Editorial: Oxidative Stress and Signal Honesty. Front. Ecol. Evol. 2017, 5, 32. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef]
- Stoev, S.D. New Evidences about the Carcinogenic Effects of Ochratoxin A and Possible Prevention by Target Feed Additives. Toxins 2022, 14, 380. [Google Scholar] [CrossRef] [PubMed]
- Khoi, C.S.; Chen, J.H.; Lin, T.Y.; Chiang, C.K.; Hung, K.Y. Ochratoxin A-Induced Nephrotoxicity: Up-to-Date Evidence. Int. J. Mol. Sci. 2021, 22, 11237. [Google Scholar] [CrossRef]
- Więckowska, M.; Cichon, N.; Szelenberger, R.; Gorniak, L.; Bijak, M. Ochratoxin A and Its Role in Cancer Development: A Comprehensive Review. Cancers 2024, 16, 3473. [Google Scholar] [CrossRef]
- Damiano, S.; Longobardi, C.; De Marchi, L.; Piscopo, N.; Meucci, V.; Lenzi, A.; Ciarcia, R. Detection of Ochratoxin A in Tissues of Wild Boars (Sus scrofa) from Southern Italy. Toxins 2025, 17, 74. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kozlov, A.V.; Javadov, S.; Sommer, N. Cellular ROS and Antioxidants: Physiological and Pathological Role. Antioxidants 2024, 13, 602. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cordiano, R.; Di Gioacchino, M.; Mangifesta, R.; Panzera, C.; Gangemi, S.; Minciullo, P.L. Malondialdehyde as a Potential Oxidative Stress Marker for Allergy-Oriented Diseases: An Update. Molecules 2023, 28, 5979. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Son, Y.; Lee, H.J.; Ryu, D.; Kim, J.R.; Kim, H.Y. Ochratoxin A induces hepatic and renal toxicity in mice through increased oxidative stress, mitochondrial damage, and multiple cell death mechanisms. Arch. Toxicol. 2024, 98, 2281–2295. [Google Scholar] [CrossRef] [PubMed]
- Ighodaro, O.M.; Akinloye, O.A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM); Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; Del Mazo, J.; Grasl-Kraupp, B.; Hogstrand, C.; Hoogenboom, L.R.; Leblanc, J.C.; et al. Risks for animal health related to the presence of ochratoxin A (OTA) in feed. EFSA J. 2023, 21, e08375. [Google Scholar] [CrossRef]
- Novi, S.; Vestuto, V.; Campiglia, P.; Tecce, N.; Bertamino, A.; Tecce, M.F. Anti-Angiogenic Effects of Natural Compounds in Diet-Associated Hepatic Inflammation. Nutrients 2023, 15, 2748. [Google Scholar] [CrossRef]
- Yang, W.S.; SriRamaratnam, R.; Welsch, M.E.; Shimada, K.; Skouta, R.; Viswanathan, V.S.; Cheah, J.H.; Clemons, P.A.; Shamji, A.F.; Clish, C.B.; et al. Regulation of ferroptotic cancer cell death by GPX4. Cell 2014, 156, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Qin, S.; Zheng, Y.; Xia, C.; Zhang, P.; Zhang, L.; Yao, J.; Yi, Y.; Deng, L. T-2 Toxin Induces Ferroptosis by Increasing Lipid Reactive Oxygen Species (ROS) and Downregulating Solute Carrier Family 7 Member 11 (SLC7A11). J. Agric. Food Chem. 2021, 69, 15716–15727. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cao, F.; Yin, H.L.; Huang, Z.-J.; Lin, Z.-T.; Mao, N.; Sun, B.; Wang, G. Ferroptosis: Past, present and future. Cell Death Dis. 2020, 11, 88. [Google Scholar] [CrossRef]
- Loboda, A.; Stachurska, A.; Sobczak, M.; Podkalicka, P.; Mucha, O.; Jozkowicz, A.; Dulak, J. Nrf2 deficiency exacerbates ochratoxin A-induced toxicity in vitro and in vivo. Toxicology 2017, 389, 42–52. [Google Scholar] [CrossRef]
- Longobardi, C.; Damiano, S.; Fabroni, S.; Montagnaro, S.; Russo, V.; Vaccaro, E.; Giordano, A.; Florio, S.; Ciarcia, R. Red Orange and Lemon Extract Ameliorates the Renal Oxidative Stress and Inflammation Induced by Ochratoxin A through the Modulation of Nrf2. Toxins 2024, 16, 151. [Google Scholar] [CrossRef]
- Simarro Doorten, A.Y.; Bull, S.; van der Doelen, M.A.; Fink-Gremmels, J. Metabolism-mediated cytotoxicity of ochratoxin A. Toxicol. Vitr. Int. J. Public Assoc. BIBRA 2004, 18, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Popescu, R.G.; Bulgaru, C.; Untea, A.; Vlassa, M.; Filip, M.; Hermenean, A.; Marin, D.; Țăranu, I.; Georgescu, S.E.; Dinischiotu, A. The Effectiveness of Dietary Byproduct Antioxidants on Induced CYP Genes Expression and Histological Alteration in Piglets Liver and Kidney Fed with Aflatoxin B1 and Ochratoxin A. Toxins 2021, 13, 148. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kuhn, M.; Hassan, R.; González, D.; Myllys, M.; Hobloss, Z.; Degen, G.H.; Humpf, H.U.; Hengstler, J.G.; Cramer, B.; Ghallab, A. Role of albumin in the metabolism and excretion of ochratoxin A. Mycotoxin Res. 2024, 40, 433–445. [Google Scholar] [CrossRef]
- Brown, J.L.; Rosa-Caldwell, M.E.; Lee, D.E.; Blackwell, T.A.; Brown, L.A.; Perry, R.A.; Haynie, W.S.; Hardee, J.P.; Carson, J.A.; Wiggs, M.P.; et al. Mitochondrial degeneration precedes the development of muscle atrophy in progression of cancer cachexia in tumour-bearing mice. J. Cachexia Sarcopenia Muscle 2017, 8, 926–938. [Google Scholar] [CrossRef]
- Pfohl-Leszkowicz, A.; Manderville, R.A. Ochratoxin A: An overview on toxicity and carcinogenicity in animals and humans. Mol. Nutr. Food Res. 2007, 51, 61–99. [Google Scholar] [CrossRef]
- Gassó, D.; Vicente, J.; Mentaberre, G.; Soriguer, R.; Rodríguez, R.J.; Navarro-González, N.; Tvarijonaviciute, A.; Lavín, S.; Fernández-Llario, P.; Segalés, J.; et al. Oxidative Stress in Wild Boars Naturally and Experimentally Infected with Mycobacterium bovis. PLoS ONE 2016, 11, e0163971. [Google Scholar] [CrossRef]
- Ferrara, G.; Longobardi, C.; D’Ambrosi, F.; Amoroso, M.G.; D’Alessio, N.; Damiano, S.; Ciarcia, R.; Iovane, V.; Iovane, G.; Pagnini, U.; et al. Aujeszky’s Disease in South-Italian Wild Boars (Sus scrofa): A Serological Survey. Animals 2021, 11, 3298. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Damiano, S.; Longobardi, C.; Ferrara, G.; Piscopo, N.; Riccio, L.; Russo, V.; Meucci, V.; De Marchi, L.; Esposito, L.; Florio, S.; et al. Oxidative Status and Histological Evaluation of Wild Boars’ Tissues Positive for Zearalenone Contamination in the Campania Region, Southern Italy. Antioxidants 2023, 12, 1748. [Google Scholar] [CrossRef]
- Dimatteo, M.; Di Napoli, E.; Paciello, O.; d’Aquino, I.; Iaccarino, D.; D’amore, M.; Guida, M.; Cozzolino, L.; Serpe, F.P.; Fusco, G.; et al. Pathological Changes and CYP1A1 Expression as Biomarkers of Pollution in Sarpa Salpa and Diplodus Sargus. Animals 2024, 14, 3160. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Damiano, S.; Andretta, E.; Longobardi, C.; Prisco, F.; Paciello, O.; Squillacioti, C.; Mirabella, N.; Florio, S.; Ciarcia, R. Effects of curcumin on the renal toxicity induced by ochratoxin A in rats. Antioxidants 2020, 9, 332. [Google Scholar] [CrossRef] [PubMed]
- Damiano, S.; Longobardi, C.; Andretta, E.; Prisco, F.; Piegari, G.; Squillacioti, C.; Montagnaro, S.; Pagnini, F.; Badino, P.; Florio, S.; et al. Antioxidative Effects of Curcumin on the Hepatotoxicity Induced by Ochratoxin A in Rats. Antioxidants 2021, 10, 125. [Google Scholar] [CrossRef] [PubMed]
- Melini, S.; Pirozzi, C.; Lama, A.; Comella, F.; Opallo, N.; Del Piano, F.; Di Napoli, E.; Mollica, M.P.; Paciello, O.; Ferrante, M.C.; et al. Co-Micronized Palmitoylethanolamide and Rutin Associated with Hydroxytyrosol Recover Diabesity-Induced Hepatic Dysfunction in Mice: In Vitro Insights into the Synergistic Effect. Phytother. Res. 2024, 38, 6035–6047. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

| KIDNEY | Score 0 | Score 1 | Score 2 | Score 3 | ||||
|---|---|---|---|---|---|---|---|---|
| OTA+ | OTA− | OTA+ | OTA− | OTA+ | OTA− | OTA+ | OTA− | |
| Glomerular atrophy | 1 | 7 | 6 | 3 | 3 | 0 | 0 | 0 |
| Inflammation | 0 | 5 | 6 | 5 | 4 | 0 | 0 | 0 |
| Proteinaceous material within tubular lumina | 0 | 7 | 7 | 3 | 3 | 0 | 0 | 0 |
| LIVER | Score 0 | Score 1 | Score 2 | Score 3 | ||||
|---|---|---|---|---|---|---|---|---|
| OTA+ | OTA− | OTA+ | OTA− | OTA+ | OTA− | OTA+ | OTA− | |
| Inflammation | 2 | 7 | 5 | 3 | 3 | 0 | 0 | 0 |
| Steatosis | 0 | 7 | 4 | 3 | 6 | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Damiano, S.; Longobardi, C.; Di Napoli, E.; Russo, V.; Piegari, G.; Raffaele, A.; Ferrucci, F.; Rubino, A.; Ciarcia, R. Histopathological Assessment and Oxidative Biomarker Analysis of Wild Boar Tissues Affected by Ochratoxin A Contamination in the Campania Region, Southern Italy. Toxins 2025, 17, 428. https://doi.org/10.3390/toxins17090428
Damiano S, Longobardi C, Di Napoli E, Russo V, Piegari G, Raffaele A, Ferrucci F, Rubino A, Ciarcia R. Histopathological Assessment and Oxidative Biomarker Analysis of Wild Boar Tissues Affected by Ochratoxin A Contamination in the Campania Region, Southern Italy. Toxins. 2025; 17(9):428. https://doi.org/10.3390/toxins17090428
Chicago/Turabian StyleDamiano, Sara, Consiglia Longobardi, Evaristo Di Napoli, Valeria Russo, Giuseppe Piegari, Antonio Raffaele, Francesco Ferrucci, Antonio Rubino, and Roberto Ciarcia. 2025. "Histopathological Assessment and Oxidative Biomarker Analysis of Wild Boar Tissues Affected by Ochratoxin A Contamination in the Campania Region, Southern Italy" Toxins 17, no. 9: 428. https://doi.org/10.3390/toxins17090428
APA StyleDamiano, S., Longobardi, C., Di Napoli, E., Russo, V., Piegari, G., Raffaele, A., Ferrucci, F., Rubino, A., & Ciarcia, R. (2025). Histopathological Assessment and Oxidative Biomarker Analysis of Wild Boar Tissues Affected by Ochratoxin A Contamination in the Campania Region, Southern Italy. Toxins, 17(9), 428. https://doi.org/10.3390/toxins17090428







