Role of the Gene tri14 in Biosynthesis of the Trichothecene Toxin Harzianum A in Trichoderma arundinaceum
Abstract
1. Introduction
2. Results
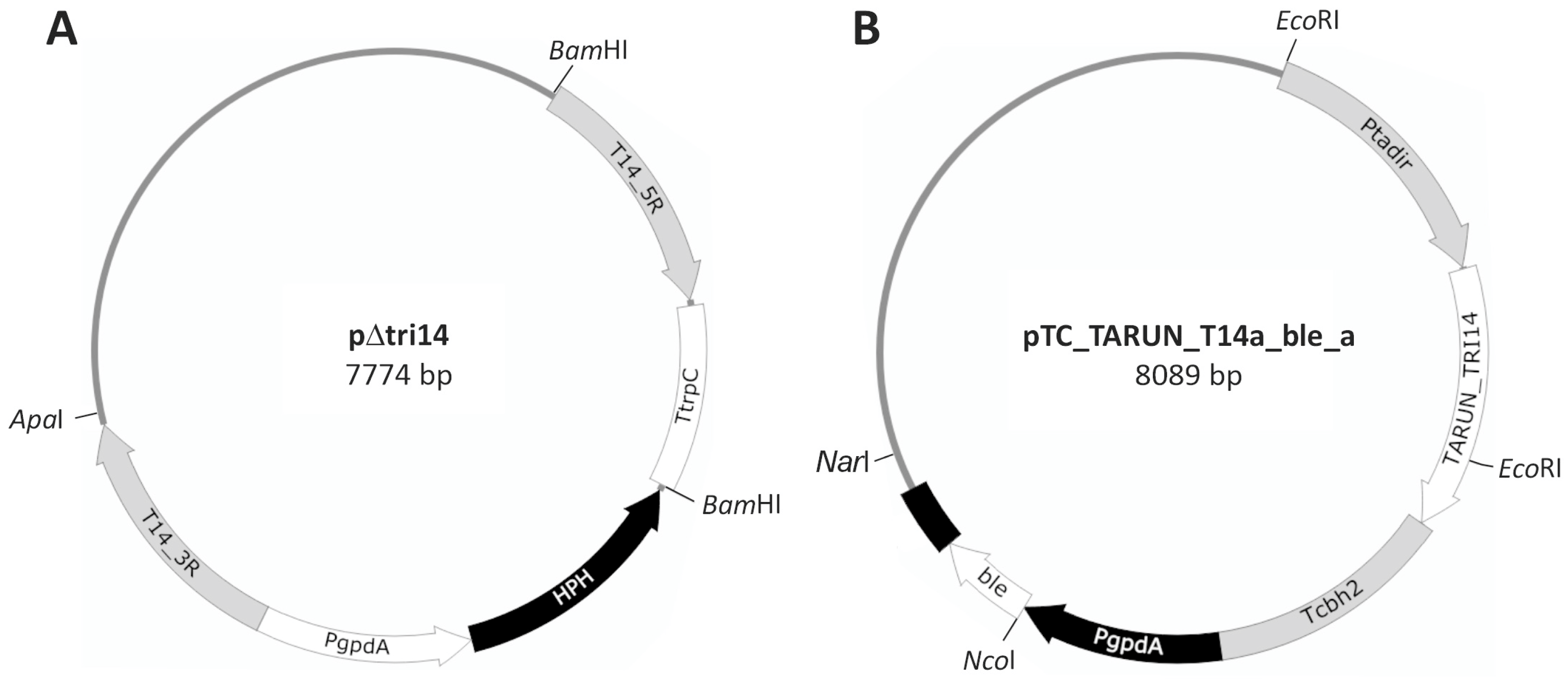
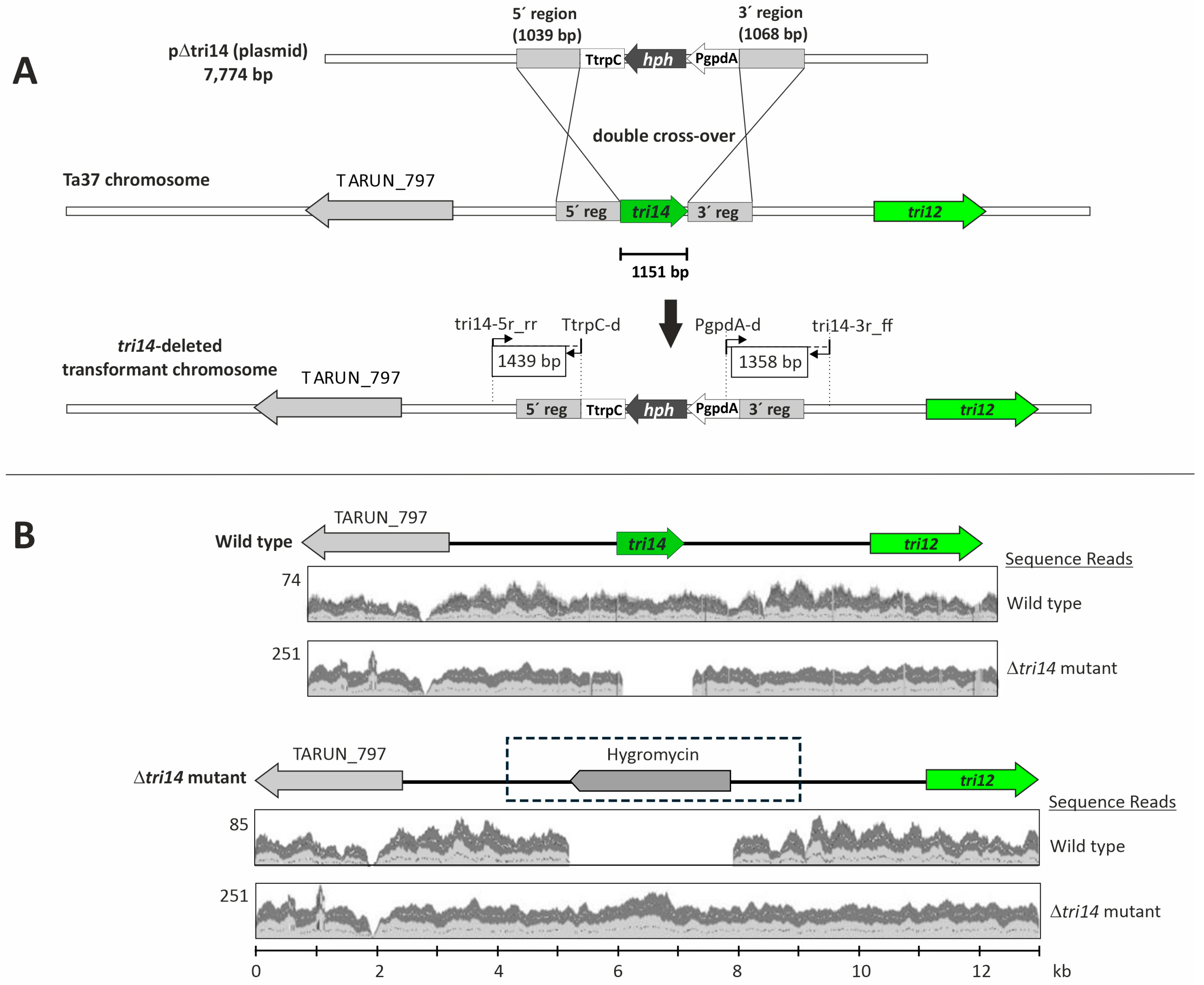
2.1. T. arundinaceum tri14 Deletion
2.2. Effect of tri14 Deletion on HA Production
2.3. Isolation of Δtri14 Add-Back and Ta37 Transformants Overexpressing T. arundinaceum tri14 Gene
2.4. HA Production in Strains Overexpressing tri14 Gene
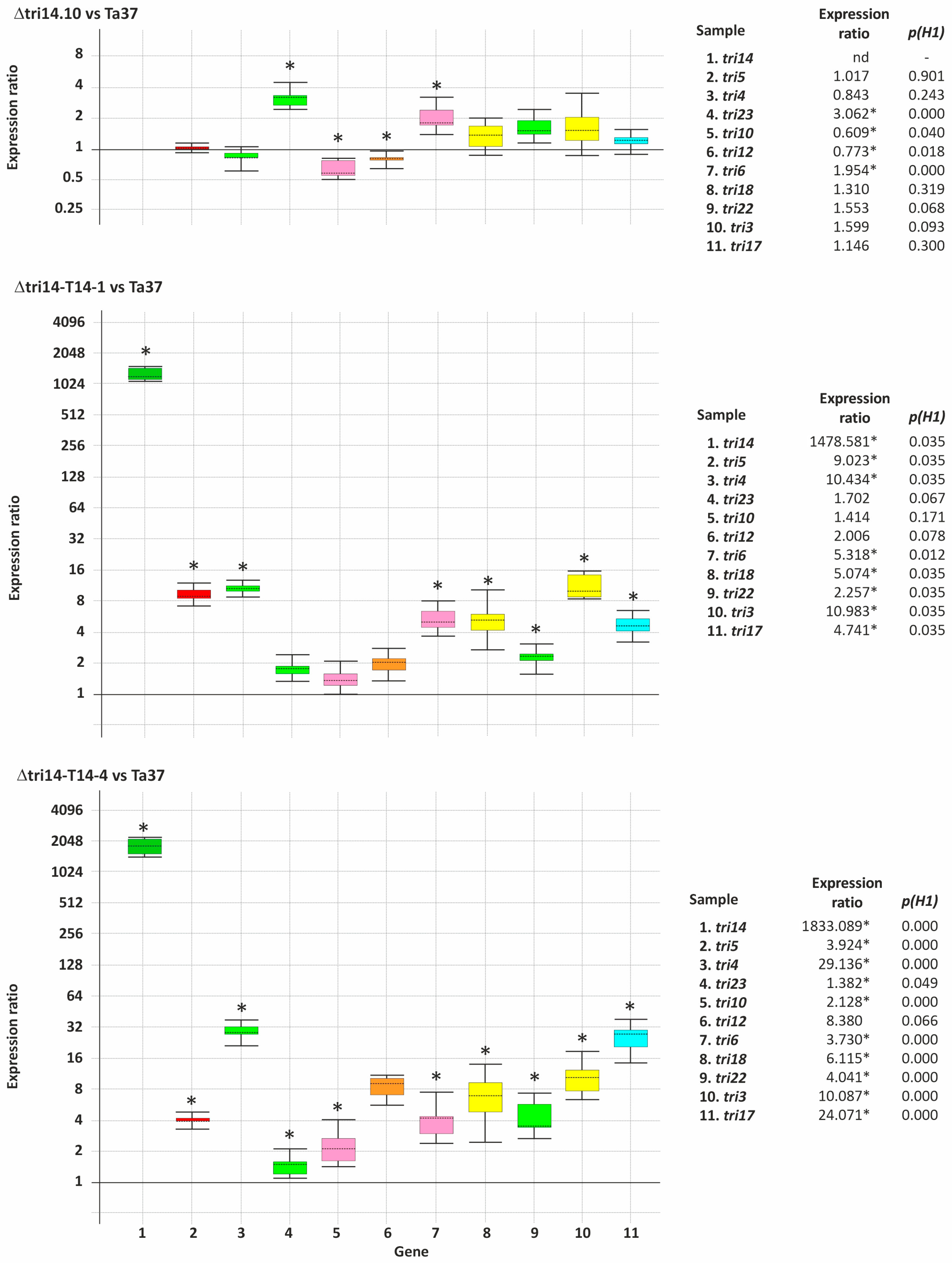
2.5. Effect of Deletion and Overexpression of tri14 on Expression of Other Tri Genes
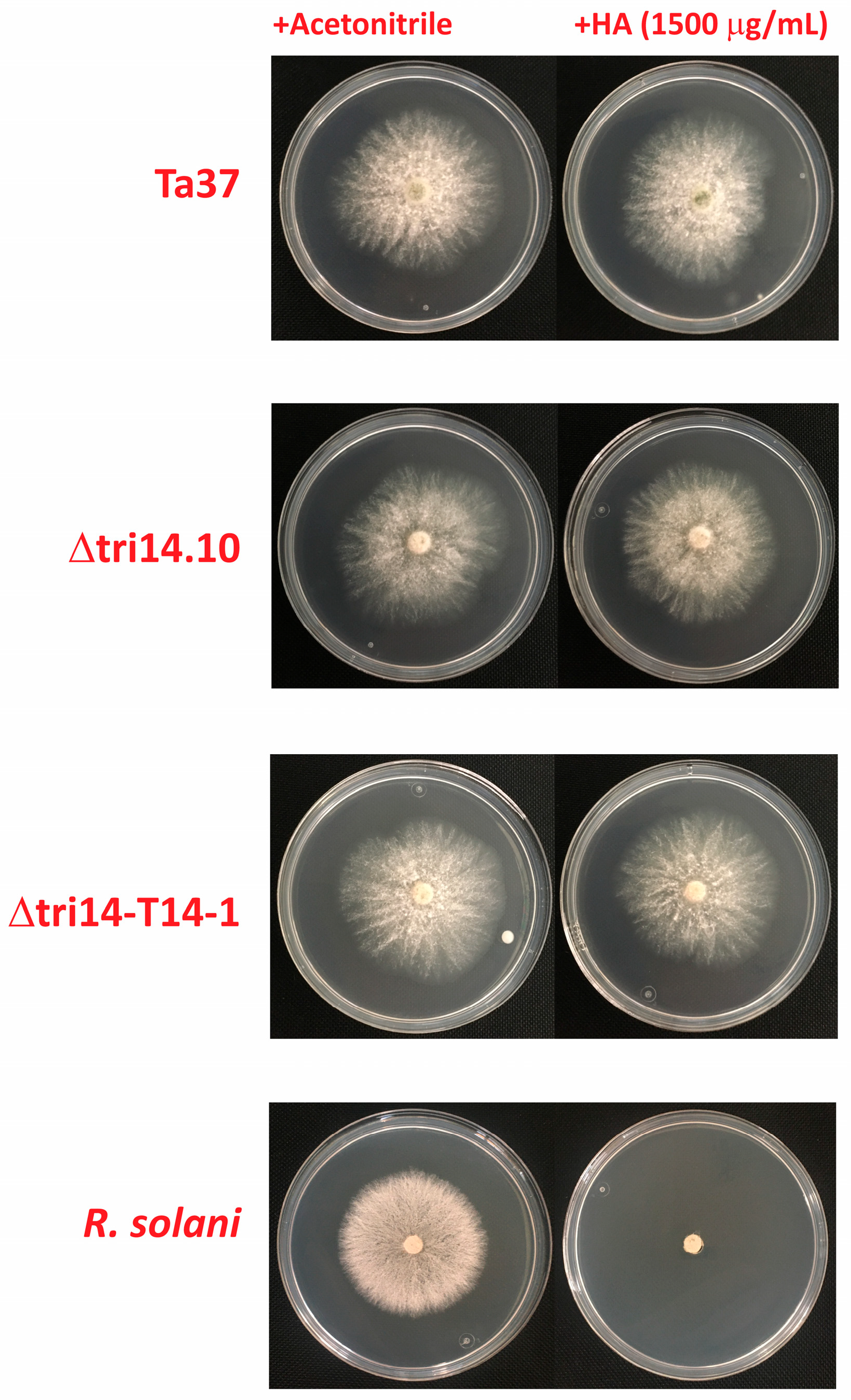
2.6. Antifungal Activity
2.7. In Silico Analysis of Tri14
Effect of tri14 Deletion on Resistance to Farnesol
2.8. tri14 and HA Resistance
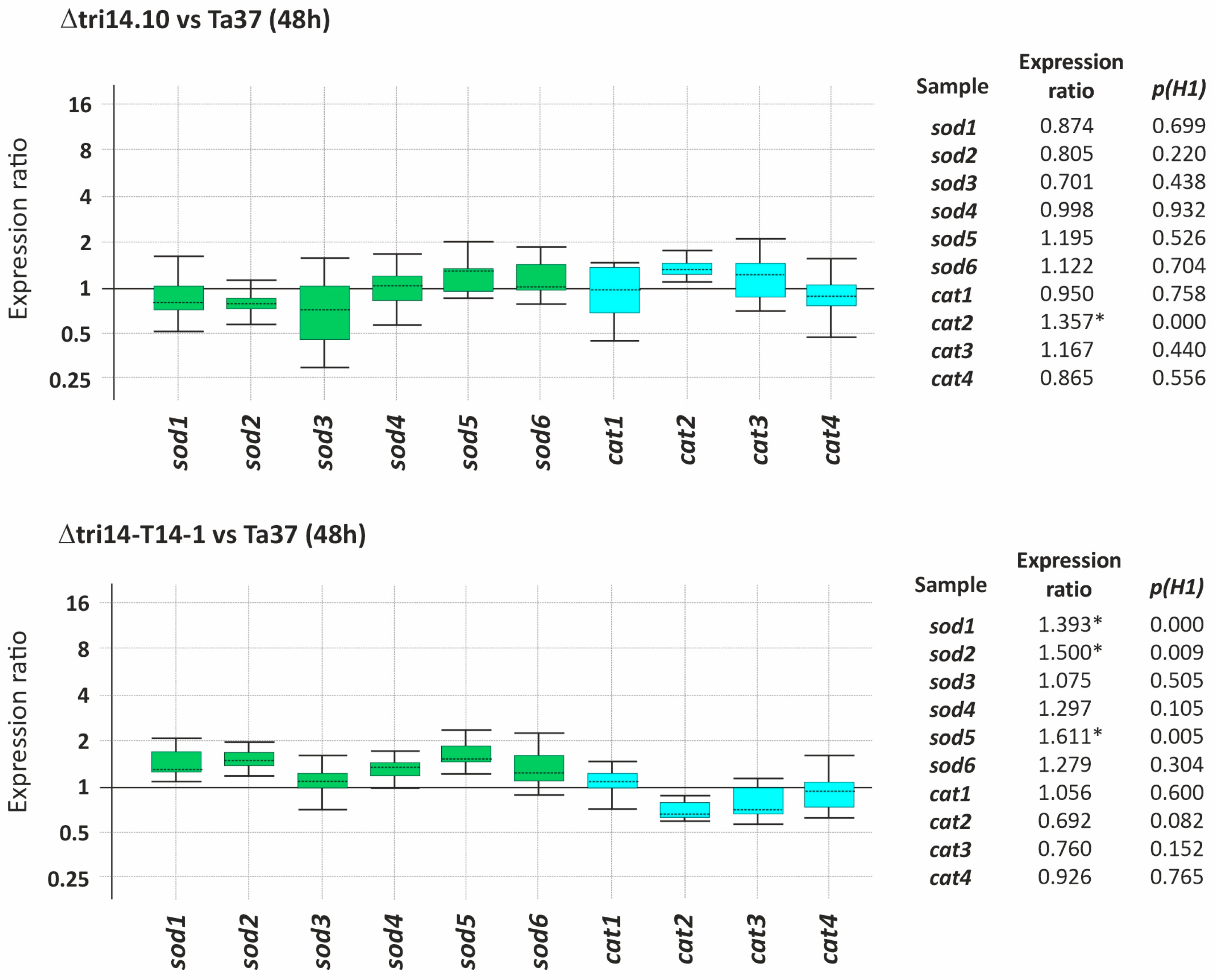
2.9. tri14 and Expression of ROS Detoxification Genes
2.10. tri14 and Resistance to H2O2
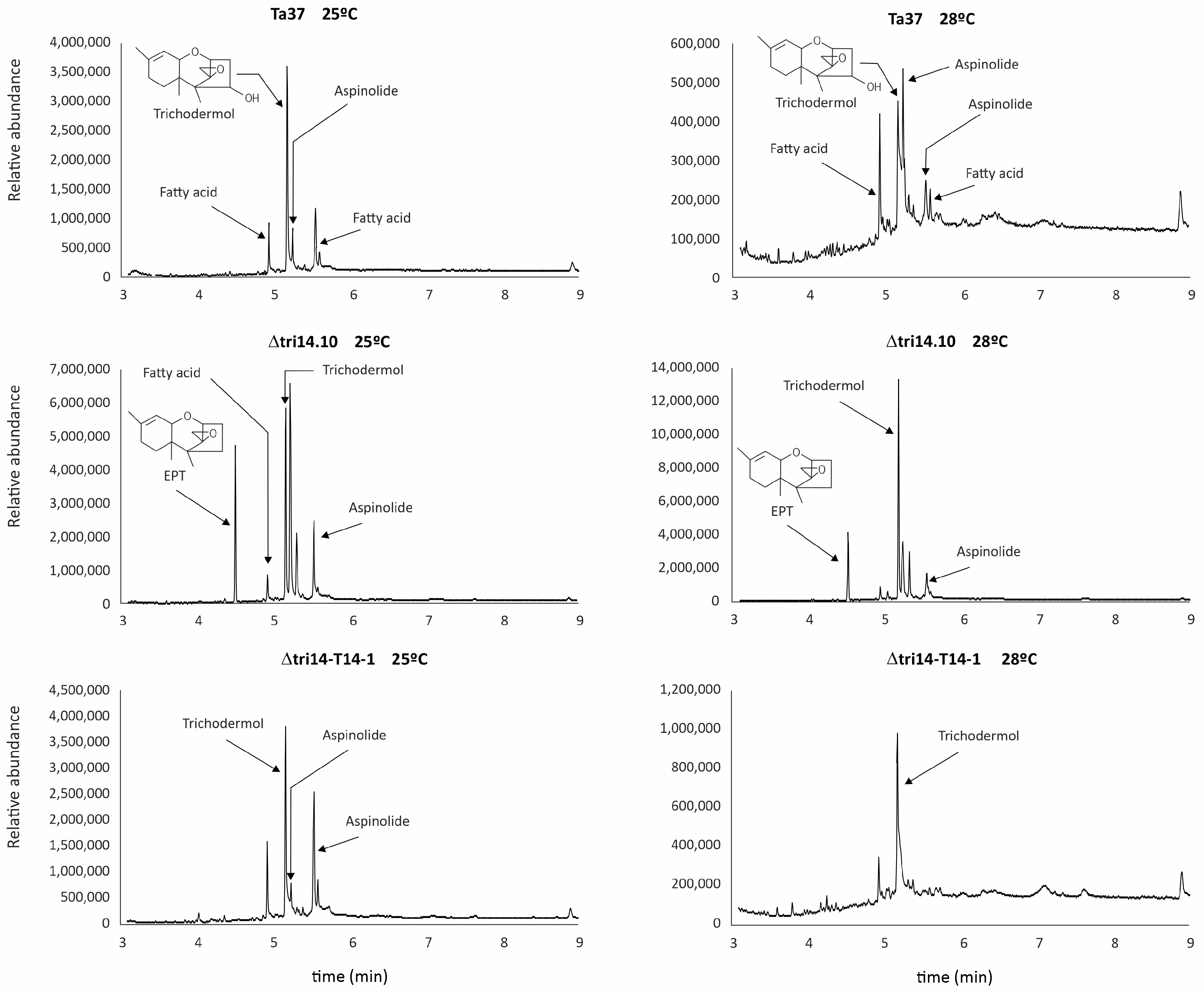
2.11. tri14 Deletion and Metabolite Profile
3. Discussion
4. Materials and Methods
4.1. Strains Used and Culture Conditions
4.2. Plasmid Construction
4.2.1. Construction of Plasmid pΔtri14 for T. arundinaceum tri14 Deletion
4.2.2. Construction of T. arundinaceum tri14 Overexpression Plasmid
4.3. Transformation Procedures
4.4. Metabolomics Characterization
4.4.1. HA Purification and Quantification
4.4.2. Gas Chromatography–Mass Spectrometry (GC-MS) Analysis
4.5. Antifungal Assays
4.5.1. HA Self-Inhibition Activity
4.5.2. Farnesol Resistance
4.6. Quantitative PCR (qPCR)
4.7. Genome Sequencing and Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Ta37 | Trichoderma arundinaceum IBT 40837 |
| EPT | 12,13-epoxytrichothec-9-ene |
| DON | Deoxynivalenol |
| ROS | Reactive oxygen species |
| HA | Harzianum A |
| HPLC | High-performance liquid chromatography |
| TLC | Thin-layer chromatography |
| GC-MS | Gas chromatography–mass spectrometry |
| RT-PCR | Reverse transcription polymerase chain reaction |
References
- Desjardins, A.E. From yellow rain to green wheat: 25 years of trichothecene biosynthesis research. J. Agric. Food Chem. 2009, 57, 4478–4484. [Google Scholar] [CrossRef]
- Proctor, R.H.; McCormick, S.P.; Gutiérrez, S. Genetic bases for variation in structure and biological activity of trichothecene toxins produced by diverse fungi. Appl. Microbiol. Biotechnol. 2020, 104, 5185–5199. [Google Scholar] [CrossRef]
- Ye, W.; Liu, T.; Zhu, M.; Zhang, W.; Li, H.; Huang, Z.; Li, S. De novo transcriptome analysis of plant pathogenic fungus Myrothecium roridum and identification of genes associated with trichothecene mycotoxin biosynthesis. Int. J. Mol. Sci. 2017, 18, 497. [Google Scholar] [CrossRef]
- Gutiérrez, S.; McCormick, S.P.; Cardoza, R.E.; Lindo, L.; Alexander, N.J.; Proctor, R.H. Trichoderma trichothecenes: Beyond their toxic effect. In New and Future Developments in Microbial Biotechnology and Bioengineering; Gupta, V.K., Zeilinger, S., Singh, H.B., Druzhinina, I., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 281–301. [Google Scholar]
- Kimura, M.; Tokai, T.; Takahashi-Ando, N.; Ohsato, S.; Fujimura, M. Molecular and genetic studies of Fusarium trichothecene biosynthesis: Pathways, genes, and evolution. Biosci. Biotechnol. Biochem. 2007, 71, 2105–2123. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, M.; Yang, J.; Yang, X.; Zhang, J.; Zhao, Z. Type A trichothecene metabolic profile differentiation, mechanisms, biosynthetic pathways and evolution in Fusarium species-A mini review. Toxins 2023, 15, 446. [Google Scholar] [CrossRef] [PubMed]
- Koster, B.; Wong, B.; Straus, N.; Malloch, D. A multi-gene phylogeny for Stachybotrys evidences lack of trichodiene synthase (tri5) gene for isolates of one of three intrageneric lineages. Mycol. Res. 2009, 113 Pt 8, 877–886. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Li, E.; Liu, L.; Liao, M.; Zhu, Z.; Zhuang, W.; Bao, L.; Liu, H. Botryane sesquiterpenoids, cyclopentadepsipeptides, xanthones, and trichothecenes from Trichoderma oligosporum. Planta Med. 2018, 84, 1055–1063. [Google Scholar] [CrossRef]
- Ryu, S.M.; Lee, H.M.; Song, E.G.; Seo, Y.H.; Lee, J.; Guo, Y.; Kim, B.S.; Kim, J.J.; Hong, J.S. Antiviral activities of trichothecenes isolated from Trichoderma albolutescens against pepper mottle virus. J. Agric. Food Chem. 2017, 65, 4273–4279. [Google Scholar] [CrossRef]
- Vicente, I.; Baroncelli, R.; Morán-Diez, M.E.; Bernardi, R.; Puntoni, G.; Hermosa, R.; Monte, E.; Vannacci, G.; Sarrocco, S. Combined comparative genomics and gene expression analyses provide insights into the terpene synthases inventory in Trichoderma. Microorganisms 2020, 8, 1603. [Google Scholar] [CrossRef] [PubMed]
- Ding, G.; Chen, A.J.; Lan, J.; Zhang, H.; Chen, X.; Liu, X.; Zou, Z. Sesquiterpenes and cyclopeptides from the endophytic fungus Trichoderma asperellum. Chem. Biodivers. 2012, 9, 1205–1212. [Google Scholar] [CrossRef] [PubMed]
- Klaiklay, S.; Rukachaisirikul, V.; Saithong, S.; Phongpaichit, S.; Sakayaroj, J. Trichothecenes from a soil-derived Trichoderma brevicompactum. J. Nat. Prod. 2019, 82, 687–693. [Google Scholar] [CrossRef]
- Nielsen, K.F.; Gräfenhan, T.; Zafari, D.; Thrane, U. Trichothecene production by Trichoderma brevicompactum. J. Agric. Food Chem. 2005, 53, 8190–8196. [Google Scholar] [CrossRef]
- Shentu, X.; Yao, J.; Yuan, X.; He, L.; Sun, F.; Ochi, K.; Yu, X. tri11, tri3, and tri4 genes are required for trichodermin biosynthesis of Trichoderma brevicompactum. AMB Express 2018, 8, 58. [Google Scholar] [CrossRef]
- Hao, G.; Proctor, R.H.; Brown, D.W.; Rhoades, N.A.; Naumann, T.A.; Kim, H.; Gutiérrez, S.; McCormick, S.P. TRI14 Is critical for Fusarium graminearum infection and spread in wheat. Appl. Microbiol. 2024, 4, 839–855. [Google Scholar] [CrossRef]
- Hohn, T.M.; Beremand, P. Isolation and nucleotide sequence of a sesquiterpene cyclase gene from the trichothecene-producing fungus Fusarium sporotrichioides. Gene 1989, 79, 131–138. [Google Scholar] [CrossRef]
- Gao, J.; Liu, D.; Nguyen, C.; McCormick, S.P.; Proctor, R.H.; Luo, S.; Zou, Y.; Hai, Y. Biosynthesis of the central tricyclic skeleton of trichothecene mycotoxins. J. Am. Chem. Soc. 2025, 147, 10331–10338. [Google Scholar] [CrossRef] [PubMed]
- Lindo, L.; McCormick, S.P.; Cardoza, R.E.; Busman, M.; Alexander, N.J.; Proctor, R.H.; Gutiérrez, S. Requirement of two acyltransferases for 4-O-acylation during biosynthesis of harzianum A, an antifungal trichothecene produced by Trichoderma arundinaceum. J. Agric. Food Chem. 2019, 67, 723–734. [Google Scholar] [CrossRef]
- Kasahara, E.; Kitamura, Y.; Katada, M.; Mizuki, M.; Okumura, N.; Sano, T.; Koizumi, Y.; Maeda, K.; Takahashi-Ando, N.; Kimura, M.; et al. Attempting to create a pathway to 15-deacetylcalonectrin with limited accumulation in cultures of Fusarium Tri3 mutants: Insight into trichothecene biosynthesis machinery. Int. J. Mol. Sci. 2024, 25, 6414. [Google Scholar] [CrossRef]
- Chen, H.; Mao, L.; Zhao, N.; Xia, C.; Liu, J.; Kubicek, C.P.; Wu, W.; Xu, S.; Zhang, C. Verification of TRI3 acetylation of trichodermol to trichodermin in the plant endophyte Trichoderma taxi. Front. Microbiol. 2021, 12, 731425. [Google Scholar] [CrossRef] [PubMed]
- Tokai, T.; Takahashi-Ando, N.; Izawa, M.; Kamakura, T.; Yoshida, M.; Fujimura, M.; Kimura, M. 4-O-acetylation and 3-O-acetylation of trichothecenes by trichothecene 15-O-acetyltransferase encoded by Fusarium Tri3. Biosci. Biotechnol. Biochem. 2008, 72, 2485–2489. [Google Scholar] [CrossRef] [PubMed]
- Dyer, R.B.; Plattner, R.D.; Kendra, D.F.; Brown, D.W. Fusarium graminearum TRI14 is required for high virulence and DON production on wheat but not for DON synthesis in vitro. J. Agric. Food Chem. 2005, 53, 9281–9287. [Google Scholar] [CrossRef]
- Shentu, X.P.; Yuan, X.F.; Liu, W.P.; Xu, J.F.; Yu, X.P. Cloning and functional analysis of tri14 in Trichoderma brevicompactum. Am. J. Biochem. Biotechnol. 2015, 11, 169–175. [Google Scholar] [CrossRef]
- Cuzick, A.; Urban, M.; Hammond-Kosack, K. Fusarium graminearum gene deletion mutants map1 and tri5 reveal similarities and differences in the pathogenicity requirements to cause disease on Arabidopsis and wheat floral tissue. New Phytol. 2008, 177, 990–1000. [Google Scholar] [CrossRef]
- Desjardins, A.E.; Plattner, R.D.; Nelsen, T.C.; Leslie, J.F. Genetic analysis of fumonisin production and virulence of Gibberella fujikuroi mating population A (Fusarium moniliforme) on maize (Zea mays) seedlings. Appl. Environ. Microbiol. 1995, 61, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Jansen, C.; von Wettstein, D.; Schafer, W.; Kogel, K.H.; Felk, A.; Maier, F.J. Infection patterns in barley and wheat spikes inoculated with wild-type and trichodiene synthase gene disrupted Fusarium graminearum. Proc. Natl. Acad. Sci. USA 2005, 102, 16892–16897. [Google Scholar] [CrossRef]
- Pfaffl, M.W.; Horgan, G.W.; Dempfle, L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002, 30, e36. [Google Scholar] [CrossRef]
- Pfaffl, M.W.; Tichopad, A.; Prgomet, C.; Neuvians, T.P. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper--Excel-based tool using pair-wise correlations. Biotechnol. Lett. 2004, 26, 509–515. [Google Scholar] [CrossRef]
- Gao, J.; Liao, J.; Yang, G.Y. CAAX-box protein, prenylation process and carcinogenesis. Am. J. Transl. Res. 2009, 1, 312–325. [Google Scholar]
- Li, W.; Wu, J.; Xiang, D.; Luo, S.; Hu, X.; Tang, T.; Sun, T.L.; Liu, X.Y. Micelles loaded with puerarin and modified with triphenylphosphonium cation possess mitochondrial targeting and demonstrate enhanced protective effect against isoprenaline-induced H9c2 cells apoptosis. Int. J. Nanomed. 2019, 14, 8345–8360. [Google Scholar] [CrossRef]
- Feng, C.; Wang, Y.; Xu, J.; Zheng, Y.; Zhou, W.; Wang, Y.; Luo, C. Precisely tailoring molecular structure of doxorubicin prodrugs to enable stable nanoassembly, rapid activation, and potent antitumor effect. Pharmaceutics 2024, 16, 1582. [Google Scholar] [CrossRef] [PubMed]
- Punt, P.J.; Oliver, R.P.; Dingemanse, M.A.; Pouwels, P.H.; van den Hondel, C.A.M.J.J. Transformation of Aspergillus based on the hygromycin B resistance marker from Escherichia coli. Gene 1987, 56, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef] [PubMed]
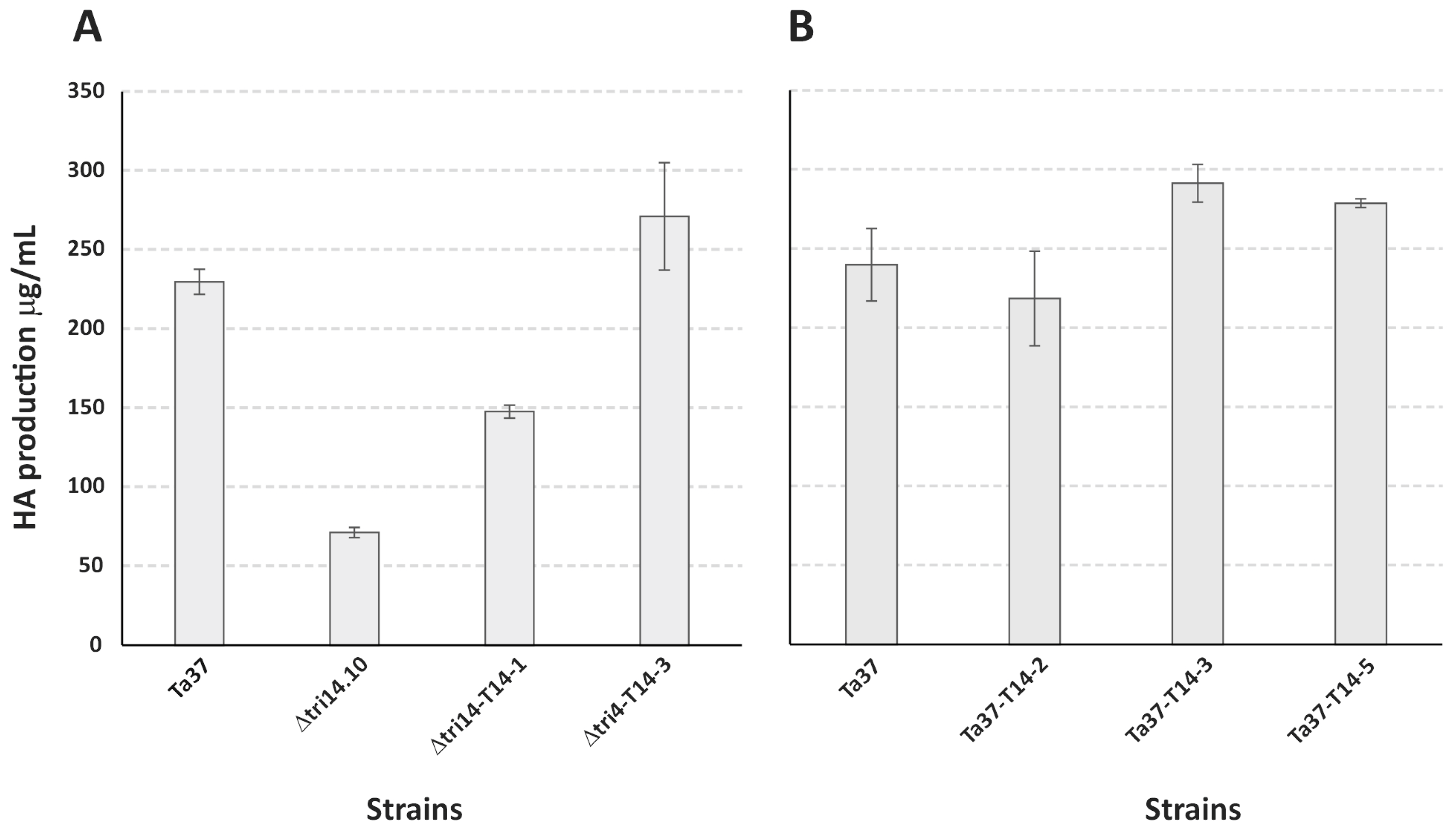

| Strain | HA Production μg/mL | % HA vs. Ta37 * |
|---|---|---|
| Ta37 | 229.67 ± 7.92 | 100 |
| Δtri14.10 | 71.16 ± 3.16 | 31 |
| Δtri14-T14-1 | 147.58 ± 4.12 | 64 |
| Δtri14-T14-3 | 270.94 ± 33.98 | 118 |
| Strain | HA Production μg/mL | % HA vs. Ta37 * |
|---|---|---|
| Ta37 | 239.75 ± 14.24 | 100 |
| Ta37-T14-2 | 218.51 ± 16.76 | 91 |
| Ta37-T14-3 | 291.25 ± 7.11 | 121 |
| Ta37-T14-5 | 278.67 ± 1.63 | 116 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Reyes, N.; Cardoza, R.E.; McCormick, S.P.; Hao, G.; Rodríguez-Fernández, J.; Proctor, R.H.; Gutiérrez, S. Role of the Gene tri14 in Biosynthesis of the Trichothecene Toxin Harzianum A in Trichoderma arundinaceum. Toxins 2025, 17, 427. https://doi.org/10.3390/toxins17090427
Martínez-Reyes N, Cardoza RE, McCormick SP, Hao G, Rodríguez-Fernández J, Proctor RH, Gutiérrez S. Role of the Gene tri14 in Biosynthesis of the Trichothecene Toxin Harzianum A in Trichoderma arundinaceum. Toxins. 2025; 17(9):427. https://doi.org/10.3390/toxins17090427
Chicago/Turabian StyleMartínez-Reyes, Natalia, Rosa E. Cardoza, Susan P. McCormick, Guixia Hao, Joaquín Rodríguez-Fernández, Robert H. Proctor, and Santiago Gutiérrez. 2025. "Role of the Gene tri14 in Biosynthesis of the Trichothecene Toxin Harzianum A in Trichoderma arundinaceum" Toxins 17, no. 9: 427. https://doi.org/10.3390/toxins17090427
APA StyleMartínez-Reyes, N., Cardoza, R. E., McCormick, S. P., Hao, G., Rodríguez-Fernández, J., Proctor, R. H., & Gutiérrez, S. (2025). Role of the Gene tri14 in Biosynthesis of the Trichothecene Toxin Harzianum A in Trichoderma arundinaceum. Toxins, 17(9), 427. https://doi.org/10.3390/toxins17090427









