X Marks the Clot: Evolutionary and Clinical Implications of Divergences in Procoagulant Australian Elapid Snake Venoms
Abstract
1. Introduction
2. Results
2.1. Animal Plasma Assays
2.2. Human Plasma Assays
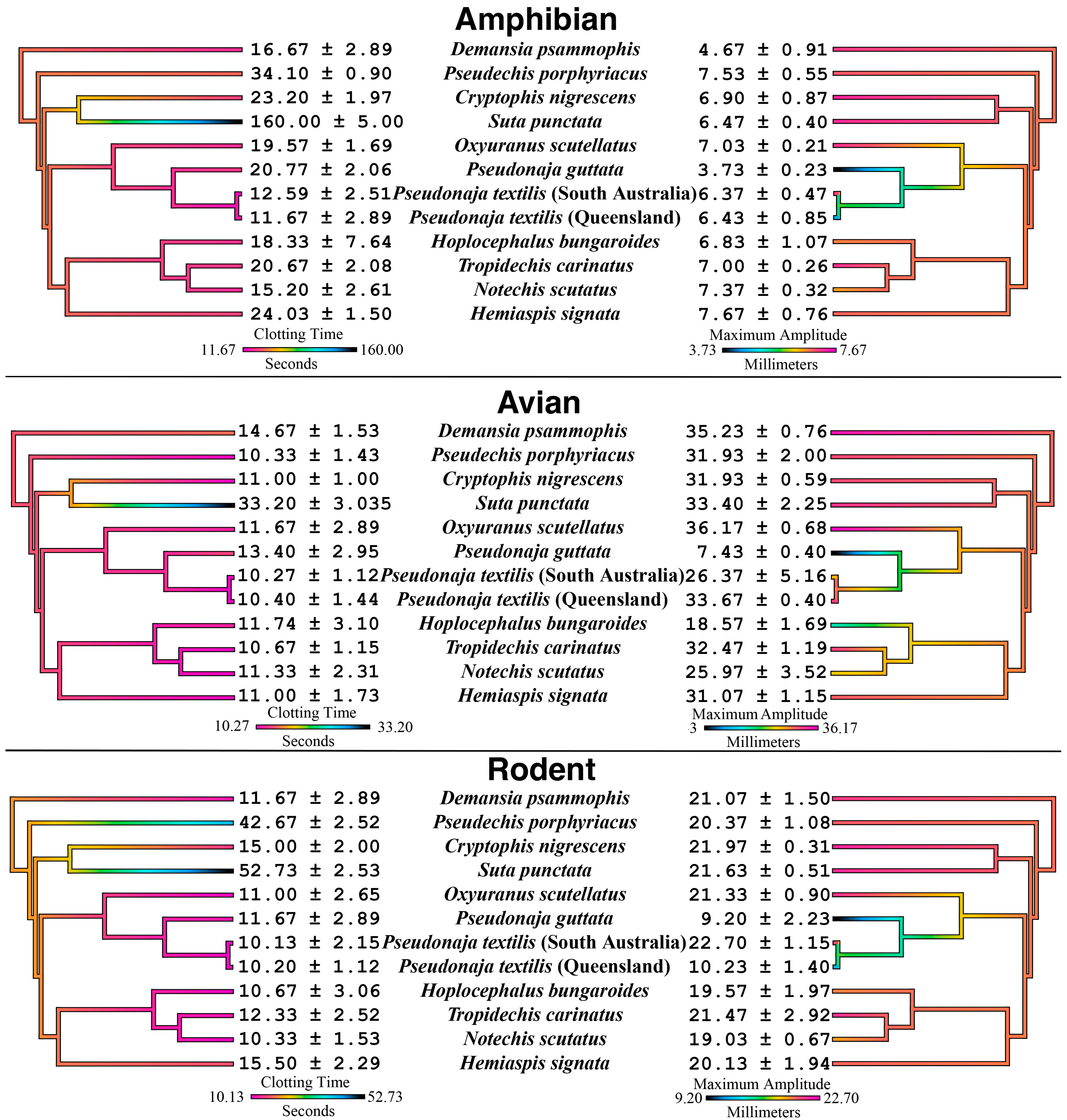
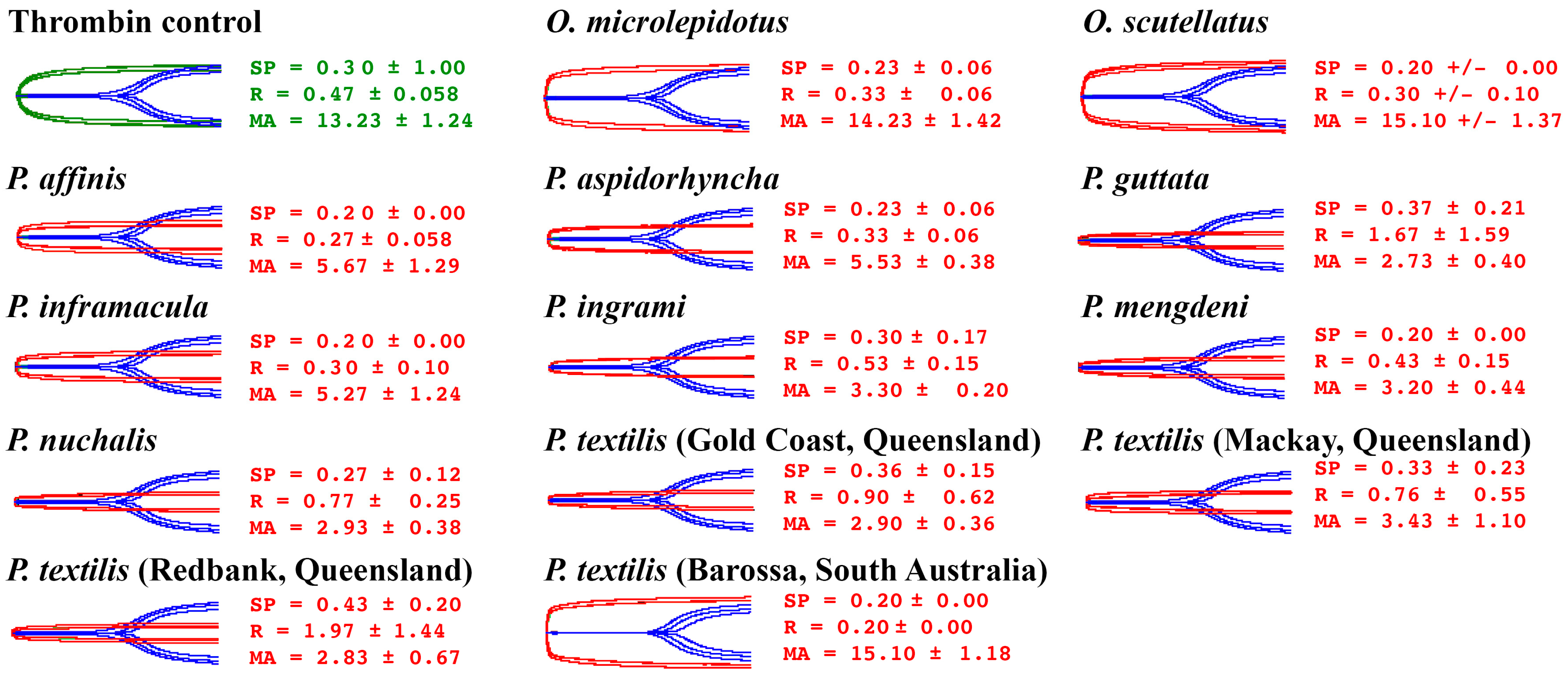
3. Discussion
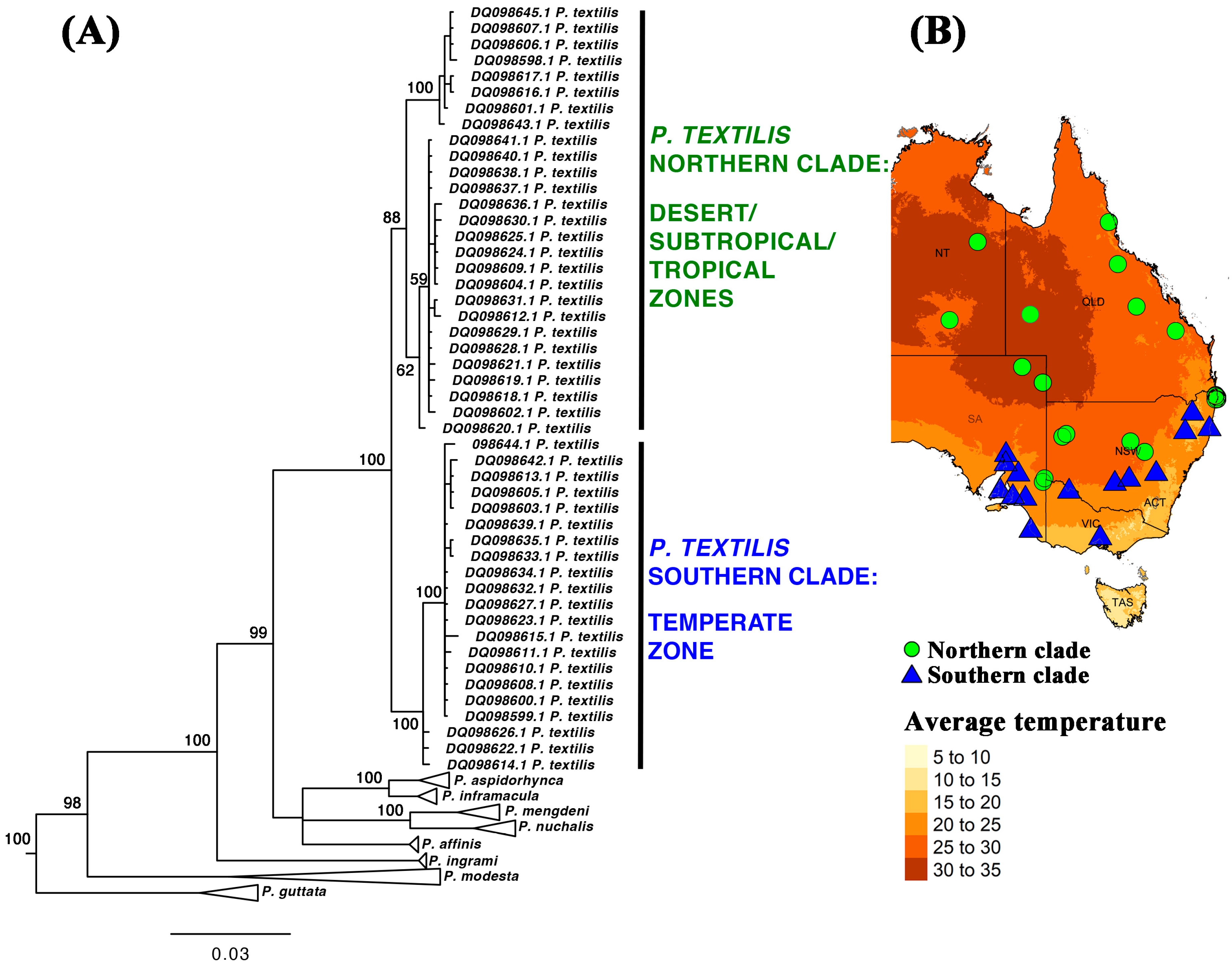
3.1. Prey-Driven Venom Specialization: Evolutionary Implications
3.2. Variations in Procoagulant Activation Mechanisms
3.3. Clinical Implications of Coagulotoxic Variation
4. Conclusions
5. Materials and Methods
5.1. Venom Stock Preparation
5.2. Plasma Sample Preparation
5.3. Thromboelastography
5.4. Statistical Analysis of Thromboelastography Results
5.5. Pseudonaja Textilis Organismal Genetics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Allen, G.E.; Brown, S.G.; Buckley, N.A.; O’Leary, M.A.; Page, C.B.; Currie, B.J.; White, J.; Isbister, G.K.; Investigators, A.S.P. Clinical effects and antivenom dosing in brown snake (Pseudonaja spp.) envenoming—Australian snakebite project (ASP-14). PLoS ONE 2012, 7, e53188. [Google Scholar] [CrossRef] [PubMed]
- Arthur, C.K.; McCallum, D.; Loveday, D.J.; Collins, A.; Isbister, J.P.; Fisher, M.M. Effects of taipan (Oxyuranus scutellatus) venom on erythrocyte morphology and blood viscosity in a human victim in vivo and in vitro. Trans. R. Soc. Trop. Med. Hyg. 1991, 85, 401–403. [Google Scholar] [CrossRef]
- Berling, I.; Brown, S.G.; Miteff, F.; Levi, C.; Isbister, G.K. Intracranial haemorrhages associated with venom induced consumption coagulopathy in Australian snakebites (ASP-21). Toxicon 2015, 102, 8–13. [Google Scholar] [CrossRef]
- Berling, I.; Isbister, G.K. Hematologic effects and complications of snake envenoming. Transfus. Med. Rev. 2015, 29, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Broad, A.J.; Sutherland, S.K.; Coulter, A.R. Lethality in mice of dangerous Australian and other snake venom. Toxicon 1979, 17, 661–664. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.G.; Caruso, N.; Borland, M.L.; McCoubrie, D.L.; Celenza, A.; Isbister, G.K. Clotting factor replacement and recovery from snake venom-induced consumptive coagulopathy. Intensive Care Med. 2009, 35, 1532–1538. [Google Scholar] [CrossRef]
- Casamento, A.J.; Isbister, G.K. Thrombotic microangiopathy in two tiger snake envenomations. Anaesth. Intensive Care 2011, 39, 1124–1127. [Google Scholar] [CrossRef]
- Chaisakul, J.; Isbister, G.K.; Konstantakopoulos, N.; Tare, M.; Parkington, H.C.; Hodgson, W.C. In vivo and in vitro cardiovascular effects of Papuan taipan (Oxyuranus scutellatus) venom: Exploring “sudden collapse”. Toxicol. Lett. 2012, 213, 243–248. [Google Scholar] [CrossRef]
- Chaisakul, J.; Isbister, G.K.; Kuruppu, S.; Konstantakopoulos, N.; Hodgson, W.C. An examination of cardiovascular collapse induced by eastern brown snake (Pseudonaja textilis) venom. Toxicol. Lett. 2013, 221, 205–211. [Google Scholar] [CrossRef]
- Chaisakul, J.; Isbister, G.K.; O’Leary, M.A.; Parkington, H.C.; Smith, A.I.; Hodgson, W.C.; Kuruppu, S. Prothrombin activator-like toxin appears to mediate cardiovascular collapse following envenoming by Pseudonaja textilis. Toxicon 2015, 102, 48–54. [Google Scholar] [CrossRef]
- Gan, M.; O’Leary, M.A.; Brown, S.G.; Jacoby, T.; Spain, D.; Tankel, A.; Gavaghan, C.; Garrett, P.; Isbister, G.K. Envenoming by the rough-scaled snake (Tropidechis carinatus): A series of confirmed cases. Med. J. Aust. 2009, 191, 183–186. [Google Scholar] [CrossRef]
- Gulati, A.; Isbister, G.K.; Duffull, S.B. Effect of Australian elapid venoms on blood coagulation: Australian Snakebite Project (ASP-17). Toxicon 2013, 61, 94–104. [Google Scholar] [CrossRef]
- Isbister, G.K. Procoagulant snake toxins: Laboratory studies, diagnosis, and understanding snakebite coagulopathy. Semin. Thromb. Hemost. 2009, 35, 93–103. [Google Scholar] [CrossRef]
- Isbister, G.K. Snakebite doesn’t cause disseminated intravascular coagulation: Coagulopathy and thrombotic microangiopathy in snake envenoming. Semin. Thromb. Hemost. 2010, 36, 444–451. [Google Scholar] [CrossRef]
- Isbister, G.K.; Buckley, N.A.; Page, C.B.; Scorgie, F.E.; Lincz, L.F.; Seldon, M.; Brown, S.G.; Investigators, A.S.P. A randomized controlled trial of fresh frozen plasma for treating venom-induced consumption coagulopathy in cases of Australian snakebite (ASP-18). J. Thromb. Haemost. 2013, 11, 1310–1318. [Google Scholar] [CrossRef] [PubMed]
- Isbister, G.K.; Currie, B.J.; Little, M.; Daly, F.F.; Isbister, J.P. Coagulopathy from tiger snake envenoming and its treatment. Pathology 2002, 34, 588–590. [Google Scholar] [CrossRef] [PubMed]
- Isbister, G.K.; Dawson, A.H.; Whyte, I.M. Two cases of bites by the black-bellied swamp snake (Hemiaspis signata). Toxicon 2002, 40, 317–319. [Google Scholar] [CrossRef]
- Isbister, G.K.; Duffull, S.B.; Brown, S.G. Failure of antivenom to improve recovery in Australian snakebite coagulopathy. QJM 2009, 102, 563–568. [Google Scholar] [CrossRef]
- Isbister, G.K.; Little, M.; Cull, G.; McCoubrie, D.; Lawton, P.; Szabo, F.; Kennedy, J.; Trethewy, C.; Luxton, G.; Brown, S.G.; et al. Thrombotic microangiopathy from Australian brown snake (Pseudonaja) envenoming. Intern. Med. J. 2007, 37, 523–528. [Google Scholar] [CrossRef]
- Isbister, G.K.; O’Leary, M.A.; Elliott, M.; Brown, S.G. Tiger snake (Notechis spp.) envenoming: Australian Snakebite Project (ASP-13). Med. J. Aust. 2012, 197, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Isbister, G.K.; O’Leary, M.A.; Schneider, J.J.; Brown, S.G.; Currie, B.J.; Investigators, A.S.P. Efficacy of antivenom against the procoagulant effect of Australian brown snake (Pseudonaja sp.) venom: In vivo and in vitro studies. Toxicon 2007, 49, 57–67. [Google Scholar] [CrossRef]
- Isbister, G.K.; Scorgie, F.E.; O’Leary, M.A.; Seldon, M.; Brown, G.A.; Lincz, L.F.; for the ASP Investigators. Factor deficiencies in venom-induced consumption coagulopathy resulting from Australian elapid envenomation: Australian Snakebite Project (ASP-10). J. Thromb. Haemost. 2010, 8, 2504–2513. [Google Scholar] [CrossRef]
- Isbister, G.K.; Scorgie, F.E.; Seldon, M.; Lincz, L.F. Clinical relevance of brown snake (Pseudonaja spp.) factor V escaping hemostatic regulation. Blood 2009, 114, 2563. [Google Scholar] [CrossRef] [PubMed]
- Isbister, G.K.; Woods, D.; Alley, S.; O’Leary, M.A.; Seldon, M.; Lincz, L.F. Endogenous thrombin potential as a novel method for the characterization of procoagulant snake venoms and the efficacy of antivenom. Toxicon 2010, 56, 75–85. [Google Scholar] [CrossRef]
- Johnston, C.I.; Ryan, N.M.; O’Leary, M.A.; Brown, S.G.; Isbister, G.K. Australian taipan (Oxyuranus spp.) envenoming: Clinical effects and potential benefits of early antivenom therapy—Australian Snakebite Project (ASP-25). Clin. Toxicol. 2017, 55, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Johnston, C.I.; Ryan, N.M.; Page, C.B.; Buckley, N.A.; Brown, S.G.; O’Leary, M.A.; Isbister, G.K. The Australian Snakebite Project, 2005–2015 (ASP-20). Med. J. Aust. 2017, 207, 119–125. [Google Scholar] [CrossRef]
- Lane, J.; O’Leary, M.A.; Isbister, G.K. Coagulant effects of black snake (Pseudechis spp.) venoms and in vitro efficacy of commercial antivenom. Toxicon 2011, 58, 239–246. [Google Scholar] [CrossRef]
- Lim, A.Y.; Singh, P.N.; Isbister, G.K. Severe rhabdomyolysis from red-bellied black snake (Pseudechis porphyriacus) envenoming despite antivenom. Toxicon 2016, 117, 46–48. [Google Scholar] [CrossRef]
- Maduwage, K.; Buckley, N.A.; de Silva, H.J.; Lalloo, D.G.; Isbister, G.K. Snake antivenom for snake venom induced consumption coagulopathy. Cochrane Database Syst. Rev. 2015, 2015, CD011428. [Google Scholar] [CrossRef] [PubMed]
- Maduwage, K.; Isbister, G.K. Current treatment for venom-induced consumption coagulopathy resulting from snakebite. PLoS Negl. Trop. Dis. 2014, 8, e3220. [Google Scholar] [CrossRef]
- Maduwage, K.P.; O’Leary, M.A.; Silva, A.; Isbister, G.K. Detection of snake venom in post-antivenom samples by dissociation treatment followed by enzyme immunoassay. Toxins 2016, 8, 130. [Google Scholar] [CrossRef]
- Maduwage, K.P.; Scorgie, F.E.; Lincz, L.F.; O’Leary, M.A.; Isbister, G.K. Procoagulant snake venoms have differential effects in animal plasmas: Implications for antivenom testing in animal models. Thromb. Res. 2016, 137, 174–177. [Google Scholar] [CrossRef]
- Noutsos, T.; Currie, B.J.; Isoardi, K.Z.; Brown, S.G.A.; Isbister, G.K. Snakebite-associated thrombotic microangiopathy: An Australian prospective cohort study [ASP30]. Clin. Toxicol. 2022, 60, 205–213. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, M.A.; Isbister, G.K.; Schneider, J.J.; Brown, S.G.; Currie, B.J. Enzyme immunoassays in brown snake (Pseudonaja spp.) envenoming: Detecting venom, antivenom and venom-antivenom complexes. Toxicon 2006, 48, 4–11. [Google Scholar] [CrossRef]
- O’Leary, M.A.; Maduwage, K.; Isbister, G.K. Use of immunoturbidimetry to detect venom-antivenom binding using snake venoms. J. Pharmacol. Toxicol. Methods 2013, 67, 177–181. [Google Scholar] [CrossRef]
- O’Leary, M.A.; Schneider, J.J.; Krishnan, B.P.; Lavis, C.; McKendry, A.; Ong, L.K.; Isbister, G.K. Cross-neutralisation of Australian brown and tiger snake venoms with commercial antivenoms: Cross-reactivity or antivenom mixtures? Toxicon 2007, 50, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, S.K.; Leonard, R.L. Snakebite deaths in Australia 1992–1994 and a management update. Med. J. Aust. 1995, 163, 616–618. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, S.K.; Lovering, K.E. Antivenoms: Use and adverse reactions over a 12-month period in Australia and Papua New Guinea. Med. J. Aust. 1979, 2, 671–674. [Google Scholar] [CrossRef]
- Sutherland, S.K.; Tibbals, J. Australian Animal Toxins: The Creatures, Their Toxins and Care of the Poisoned Patient, 2nd ed.; Oxford University Press: Oxford, UK, 2001. [Google Scholar]
- Tibballs, J.; Sutherland, S.K.; Kerr, S. Studies on Australian snake venoms, Part II: The haematological effects of brown snake (Pseudonaja) species in the dog. Anaesth. Intensive Care 1991, 19, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Tanos, P.P.; Isbister, G.K.; Lalloo, D.G.; Kirkpatrick, C.M.; Duffull, S.B. A model for venom-induced consumptive coagulopathy in snake bite. Toxicon 2008, 52, 769–780. [Google Scholar] [CrossRef]
- Tibballs, J.; Sutherland, S.K.; Rivera, R.A.; Masci, P.P. The cardiovascular and haematological effects of purified prothrombin activator from the common brown snake (Pseudonaja textilis) and their antagonism with heparin. Anaesth. Intensive Care 1992, 20, 28–32. [Google Scholar] [CrossRef]
- Fry, B.G. From genome to “venome”: Molecular origin and evolution of the snake venom proteome inferred from phylogenetic analysis of toxin sequences and related body proteins. Genome Res. 2005, 15, 403–420. [Google Scholar] [CrossRef] [PubMed]
- Earl, S.; Sunagar, K.; Jackson, T.N.W.; Reeks, T.; Fry, B.G. Factor Va Proteins. In Venomous Reptiles and Their Toxins: Evolution, Pathophysiology and Biodiscovery; Fry, B.G., Ed.; Oxford University Press: New York, NY, USA, 2015; pp. 255–260. [Google Scholar]
- Zdenek, C.N.; den Brouw, B.O.; Dashevsky, D.; Gloria, A.; Youngman, N.; Watson, E.; Green, P.; Hay, C.; Dunstan, N.; Allen, L.; et al. Clinical implications of convergent procoagulant toxicity and differential antivenom efficacy in Australian elapid snake venoms. Toxicol. Lett. 2019, 316, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Zdenek, C.N.; Hay, C.; Arbuckle, K.; Jackson, T.N.W.; Bos, M.H.A.; Op den Brouw, B.; Debono, J.; Allen, L.; Dunstan, N.; Morley, T.; et al. Coagulotoxic effects by brown snake (Pseudonaja) and taipan (Oxyuranus) venoms, and the efficacy of a new antivenom. Toxicol. Vitr. 2019, 58, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekara, U.; Chowdhury, A.; Seneci, L.; Zdenek, C.N.; Dunstan, N.; Fry, B.G. From venom to vein: Factor VII activation as a major pathophysiological target for procoagulant Australian elapid snake venoms. Toxins 2024, 16, 430. [Google Scholar] [CrossRef]
- Buckley, N.; Dawson, A.H. Unusual results of brown snake envenomation. Med. J. Aust. 1986, 158, 866. [Google Scholar] [CrossRef]
- Eramanis, L.M.; Woodward, A.; Courtman, N.; Hughes, D.; Padula, A.; Winkel, K.D.; Boller, M. Coagulation factor activity patterns of venom-induced consumption coagulopathy in naturally occurring tiger snake (Notechis scutatus) envenomed dogs treated with antivenom. Toxicon 2020, 181, 36–44. [Google Scholar] [CrossRef]
- Lalloo, D.G.; Trevett, A.J.; Owens, D.; Minei, J.; Naraqi, S.; Saweri, A.; Hutton, R.A.; Theakston, R.D.; Warrell, D.A. Coagulopathy following bites by the Papuan taipan (Oxyuranus scutellatus canni). Blood Coagul. Fibrinolysis 1995, 6, 65–72. [Google Scholar] [CrossRef]
- White, J.; Fassett, R. Acute renal failure and coagulopathy after snakebite. Med. J. Aust. 1983, 2, 142–143. [Google Scholar] [CrossRef]
- Barlow, A.; Pook, C.E.; Harrison, R.A.; Wuster, W. Coevolution of diet and prey-specific venom activity supports the role of selection in snake venom evolution. Proc. Biol. Sci. R. Soc. 2009, 276, 2443–2449. [Google Scholar] [CrossRef]
- Bernardoni, J.L.; Sousa, L.F.; Wermelinger, L.S.; Lopes, A.S.; Prezoto, B.C.; Serrano, S.M.; Zingali, R.B.; Moura-da-Silva, A.M. Functional variability of snake venom metalloproteinases: Adaptive advantages in targeting different prey and implications for human envenomation. PLoS ONE 2014, 9, e109651. [Google Scholar] [CrossRef]
- Cipriani, V.; Debono, J.; Goldenberg, J.; Jackson, T.N.W.; Arbuckle, K.; Dobson, J.; Koludarov, I.; Li, B.; Hay, C.; Dunstan, N.; et al. Correlation between ontogenetic dietary shifts and venom variation in Australian brown snakes (Pseudonaja). Comp. Biochem. Physiol. Toxicol. Pharmacol. CBP 2017, 197, 53–60. [Google Scholar] [CrossRef]
- Daltry, J.C.; Wuster, W.; Thorpe, R.S. Diet and snake venom evolution. Nature 1996, 379, 537–540. [Google Scholar] [CrossRef]
- Gibbs, H.L.; Mackessy, S.P. Functional basis of a molecular adaptation: Prey-specific toxic effects of venom from Sistrurus rattlesnakes. Toxicon 2009, 53, 672–679. [Google Scholar] [CrossRef]
- Harris, R.J.; Youngman, N.J.; Zdenek, C.N.; Huynh, T.M.; Nouwens, A.; Hodgson, W.C.; Harrich, D.; Dunstan, N.; Portes-Junior, J.A.; Fry, B.G. Assessing the binding of venoms from aquatic elapids to the nicotinic acetylcholine receptor orthosteric site of different prey models. Int. J. Mol. Sci. 2020, 21, 7377. [Google Scholar] [CrossRef]
- Healy, K.; Carbone, C.; Jackson, A.L. Snake venom potency and yield are associated with prey-evolution, predator metabolism and habitat structure. Ecol. Lett. 2019, 22, 527–537. [Google Scholar] [CrossRef]
- Hogan, M.P.; Holding, M.L.; Nystrom, G.S.; Colston, T.J.; Bartlett, D.A.; Mason, A.J.; Ellsworth, S.A.; Rautsaw, R.M.; Lawrence, K.C.; Strickland, J.L.; et al. The genetic regulatory architecture and epigenomic basis for age-related changes in rattlesnake venom. Proc. Natl. Acad. Sci. USA 2024, 121, e2313440121. [Google Scholar] [CrossRef]
- Holding, M.L.; Strickland, J.L.; Rautsaw, R.M.; Hofmann, E.P.; Mason, A.J.; Hogan, M.P.; Nystrom, G.S.; Ellsworth, S.A.; Colston, T.J.; Borja, M.; et al. Phylogenetically diverse diets favor more complex venoms in North American pitvipers. Proc. Natl. Acad. Sci. USA 2021, 118, e2015579118. [Google Scholar] [CrossRef] [PubMed]
- Jackson, T.N.; Koludarov, I.; Ali, S.A.; Dobson, J.; Zdenek, C.N.; Dashevsky, D.; Op den Brouw, B.; Masci, P.P.; Nouwens, A.; Josh, P.; et al. Rapid radiations and the race to redundancy: An investigation of the evolution of Australian elapid snake venoms. Toxins 2016, 8, 309. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Fry, B.G.; Kini, R.M. Eggs-only diet: Its implications for the toxin profile changes and ecology of the Marbled Sea Snake (Aipysurus eydouxii). J. Mol. Evol. 2005, 60, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Lyons, K.; Dugon, M.M.; Healy, K. Diet breadth mediates the prey specificity of venom potency in snakes. Toxins 2020, 12, 74. [Google Scholar] [CrossRef]
- Mason, A.J.; Holding, M.L.; Rautsaw, R.M.; Rokyta, D.R.; Parkinson, C.L.; Gibbs, H.L. Venom gene sequence diversity and expression jointly shape diet adaptation in pitvipers. Mol. Biol. Evol. 2022, 39, msac082. [Google Scholar] [CrossRef] [PubMed]
- Modahl, C.M.; Mrinalini; Frietze, S.; Mackessy, S.P. Adaptive evolution of distinct prey-specific toxin genes in rear-fanged snake venom. Proceedings. Biol. Sci. R. Soc. 2018, 285, 20181003. [Google Scholar] [CrossRef]
- Nachtigall, P.G.; Freitas-de-Sousa, L.A.; Mason, A.J.; Moura-da-Silva, A.M.; Grazziotin, F.G.; Junqueira-de-Azevedo, I.L.M. Differences in PLA2 constitution distinguish the venom of two endemic Brazilian mountain lanceheads, Bothrops cotiara and Bothrops fonsecai. Toxins 2022, 14, 237. [Google Scholar] [CrossRef]
- Naik, H.; Kgaditse, M.M.; Alexander, G.J. Ancestral Reconstruction of diet and fang condition in the Lamprophiidae: Implications for the evolution of venom systems in snakes. J. Herpetol. 2021, 55, 1–10. [Google Scholar] [CrossRef]
- Richards, D.P.; Barlow, A.; Wuster, W. Venom lethality and diet: Differential responses of natural prey and model organisms to the venom of the saw-scaled vipers (Echis). Toxicon 2012, 59, 110–116. [Google Scholar] [CrossRef]
- Schaeffer, R.; Pascolutti, V.J.; Jackson, T.N.W.; Arbuckle, K. Diversity begets diversity: When diet drives snake venom evolution, but evenness rather than richness is what counts. Toxins 2023, 15, 251. [Google Scholar] [CrossRef]
- Sousa, L.F.; Zdenek, C.N.; Dobson, J.S.; Op den Brouw, B.; Coimbra, F.; Gillett, A.; Del-Rei, T.H.M.; Chalkidis, H.M.; Sant’Anna, S.; Teixeira-da-Rocha, M.M.; et al. Coagulotoxicity of Bothrops (Lancehead Pit-Vipers) venoms from Brazil: Differential biochemistry and antivenom efficacy resulting from prey-driven venom variation. Toxins 2018, 10, 411. [Google Scholar] [CrossRef] [PubMed]
- Tioyama, E.C.; Bayona-Serrano, J.D.; Portes-Junior, J.A.; Nachtigall, P.G.; de Souza, V.C.; Beraldo-Neto, E.; Grazziotin, F.G.; Junqueira-de-Azevedo, I.L.M.; Moura-da-Silva, A.M.; Freitas-de-Sousa, L.A. The Venom Composition of the snake tribe philodryadini: ‘Omic’ techniques reveal intergeneric variability among South American racers. Toxins 2023, 15, 415. [Google Scholar] [CrossRef] [PubMed]
- Youngman, N.J.; Zdenek, C.N.; Dobson, J.S.; Bittenbinder, M.A.; Gillett, A.; Hamilton, B.; Dunstan, N.; Allen, L.; Veary, A.; Veary, E.; et al. Mud in the blood: Novel potent anticoagulant coagulotoxicity in the venoms of the Australian elapid snake genus Denisonia (mud adders) and relative antivenom efficacy. Toxicol. Lett. 2019, 302, 1–6. [Google Scholar] [CrossRef]
- Youngman, N.J.; Chowdhury, A.; Zdenek, C.N.; Coster, K.; Sundman, E.; Braun, R.; Fry, B.G. Utilising venom activity to infer dietary composition of the Kenyan horned viper (Bitis worthingtoni). Comp. Biochem. Physiol. Toxicol. Pharmacol. CBP 2021, 240, 108921. [Google Scholar] [CrossRef]
- Zancolli, G.; Calvete, J.J.; Cardwell, M.D.; Greene, H.W.; Hayes, W.K.; Hegarty, M.J.; Herrmann, H.W.; Holycross, A.T.; Lannutti, D.I.; Mulley, J.F.; et al. When one phenotype is not enough: Divergent evolutionary trajectories govern venom variation in a widespread rattlesnake species. Proc. R. Soc. B Biol. Sci. 2019, 286, 2735. [Google Scholar] [CrossRef] [PubMed]
- Zdenek, C.N.; Harris, R.J.; Kuruppu, S.; Youngman, N.J.; Dobson, J.S.; Debono, J.; Khan, M.; Smith, I.; Yarski, M.; Harrich, D.; et al. A taxon-specific and high-throughput method for measuring ligand binding to nicotinic acetylcholine receptors. Toxins 2019, 11, 600. [Google Scholar] [CrossRef] [PubMed]
- Casewell, N.R.; Wuster, W.; Vonk, F.J.; Harrison, R.A.; Fry, B.G. Complex cocktails: The evolutionary novelty of venoms. Trends Ecol. Evol. 2013, 28, 219–229. [Google Scholar] [CrossRef]
- Chandrasekara, U.; Harris, R.J.; Fry, B.G. The target selects the toxin: Specific amino acids in snake-prey nicotinic acetylcholine receptors that are selectively bound by king cobra venoms. Toxins 2022, 14, 528. [Google Scholar] [CrossRef]
- Chandrasekara, U.; Mancuso, M.; Seneci, L.; Bourke, L.; Trembath, D.F.; Sumner, J.; Zdenek, C.N.; Fry, B.G. A Russian doll of resistance: Nested gains and losses of venom immunity in varanid lizards. Int. J. Mol. Sci. 2024, 25, 2628. [Google Scholar] [CrossRef] [PubMed]
- Dashevsky, D.; Harris, R.J.; Zdenek, C.N.; Benard-Valle, M.; Alagon, A.; Portes-Junior, J.A.; Tanaka-Azevedo, A.M.; Grego, K.F.; Sant’Anna, S.S.; Frank, N.; et al. Red-on-yellow queen: Bio-layer interferometry reveals functional diversity within Micrurus venoms and toxin resistance in prey species. J. Mol. Evol. 2024, 92, 317–328. [Google Scholar] [CrossRef]
- de Oliveira, L.; Jared, C.; da Costa Prudente, A.L.; Zaher, H.; Antoniazzi, M.M. Oral glands in dipsadine “goo-eater” snakes: Morphology and histochemistry of the infralabial glands in Atractus reticulatus, Dipsas indica, and Sibynomorphus mikanii. Toxicon 2008, 51, 898–913. [Google Scholar] [CrossRef]
- Gibbs, H.L.; Sanz, L.; Chiucchi, J.E.; Farrell, T.M.; Calvete, J.J. Proteomic analysis of ontogenetic and diet-related changes in venom composition of juvenile and adult Dusky Pigmy rattlesnakes (Sistrurus miliarius barbouri). J. Proteom. 2011, 74, 2169–2179. [Google Scholar] [CrossRef]
- Hague, M.T.J.; Stokes, A.N.; Feldman, C.R.; Brodie, E.D., Jr.; Brodie, E.D., 3rd. The geographic mosaic of arms race coevolution is closely matched to prey population structure. Evol. Lett. 2020, 4, 317–332. [Google Scholar] [CrossRef]
- Holding, M.L.; Biardi, J.E.; Gibbs, H.L. Coevolution of venom function and venom resistance in a rattlesnake predator and its squirrel prey. Proceedings. Biol. Sci. R. Soc. 2016, 283, 20152841. [Google Scholar] [CrossRef]
- Mackessy, S.P.; Sixberry, N.A.; Heyborne, W.H.; Fritts, T. Venom of the Brown Treesnake, Boiga irregularis: Ontogenetic shifts and taxa-specific toxicity. Toxicon 2006, 47, 537–548. [Google Scholar] [CrossRef]
- Pawlak, J.; Mackessy, S.P.; Fry, B.G.; Bhatia, M.; Mourier, G.; Fruchart-Gaillard, C.; Servent, D.; Menez, R.; Stura, E.; Menez, A.; et al. Denmotoxin, a three-finger toxin from the colubrid snake Boiga dendrophila (Mangrove Catsnake) with bird-specific activity. J. Biol. Chem. 2006, 281, 29030–29041. [Google Scholar] [CrossRef] [PubMed]
- Schield, D.R.; Perry, B.W.; Adams, R.H.; Holding, M.L.; Nikolakis, Z.L.; Gopalan, S.S.; Smith, C.F.; Parker, J.M.; Meik, J.M.; DeGiorgio, M.; et al. The roles of balancing selection and recombination in the evolution of rattlesnake venom. Nat. Ecol. Evol. 2022, 6, 1367–1380. [Google Scholar] [CrossRef] [PubMed]
- Smiley-Walters, S.A.; Farrell, T.M.; Gibbs, H.L. The importance of species: Pygmy rattlesnake venom toxicity differs between native prey and related non-native species. Toxicon 2018, 144, 42–47. [Google Scholar] [CrossRef]
- Youngman, N.J.; Llinas, J.; Haworth, M.; Gillett, A.; Jones, L.; Walker, A.A.; Fry, B.G. Untangling interactions between Bitis vipers and their prey using coagulotoxicity against diverse vertebrate plasmas. Toxicon 2022, 216, 37–44. [Google Scholar] [CrossRef]
- Zdenek, C.N.; Chowdhury, A.; Haw, G.Y.H.; Violette, A.; Fourmy, R.; Christ, T.; Vonk, F.J.; Fry, B.G. Taxon-selective venom variation in adult and neonate Daboia russelii (Russell’s Viper), and antivenom efficacy. Toxicon 2022, 205, 11–19. [Google Scholar] [CrossRef]
- Bos, M.H.; Boltz, M.; St Pierre, L.; Masci, P.P.; de Jersey, J.; Lavin, M.F.; Camire, R.M. Venom factor V from the common brown snake escapes hemostatic regulation through procoagulant adaptations. Blood 2009, 114, 686–692. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, A.; Youngman, N.J.; Liu, J.; Lewin, M.R.; Carter, R.W.; Fry, B.G. The relative efficacy of chemically diverse small-molecule enzyme-inhibitors against anticoagulant activities of Black Snake (Pseudechis spp.) venoms. Toxicol. Lett. 2022, 366, 26–32. [Google Scholar] [CrossRef]
- Masci, P.P.; Mirtschin, P.J.; Nias, T.N.; Turnbull, R.K.; Kuchel, T.R.; Whitaker, A.N. Brown snakes (Pseudonaja genus): Venom yields, prothrombin activator neutralization and implications affecting antivenom usage. Anaesth. Intensive Care 1998, 26, 276–281. [Google Scholar] [CrossRef]
- Jones, L.; Neri-Castro, E.; Youngman, N.J.; Llinas, J.; Haworth, M.; Gillett, A.; Fry, B.G. Tailored toxins: Coagulotoxic variations in Atropoides, Cerrophidion, Metlapilcoatlus, and Porthidium viperid venoms against diverse vertebrate plasmas. Toxicon 2025, 264, 108453. [Google Scholar] [CrossRef] [PubMed]
- Boyer, L.; Alagón, A.; Fry, B.G.; Jackson, T.N.W.; Sunagar, K.; Chippaux, J.P. Signs, symptoms and treatment of envenomation. In Venomous Reptiles and Their Toxins: Evolution, Pathophysiology and Biodiscovery; Fry, B.G., Ed.; Oxford University Press: New York, NY, USA, 2015; pp. 32–60. [Google Scholar]
- Slagboom, J.; Kool, J.; Harrison, R.A.; Casewell, N.R. Haemotoxic snake venoms: Their functional activity, impact on snakebite victims and pharmaceutical promise. Br. J. Haematol. 2017, 177, 947–959. [Google Scholar] [CrossRef] [PubMed]
- Shine, R. Australian snakes: A natural history; Cornell University Press: New York, NY, USA, 1995. [Google Scholar]
- Skinner, A.; Donnellan, S.C.; Hutchinson, M.N.; Hutchinson, R.G. A phylogenetic analysis of Pseudonaja (Hydrophiinae, Elapidae, Serpentes) based on mitochondrial DNA sequences. Mol. Phylogenet Evol. 2005, 37, 558–571. [Google Scholar] [CrossRef]
- Williams, D.J.; O’Shea, M.; Daguerre, R.L.; Pook, C.E.; Wüster, W.; Hayden, C.J.; McVay, J.D.; Paiva, O.; Matainaho, T.; Winkel, K.D.; et al. Origin of the eastern brownsnake, Pseudonaja textilis (Dumeril, Bibron and Dumeril) (Serpentes: Elapidae: Hydrophiinae) in New Guinea: Evidence of multiple dispersals from Australia, and comments on the status of Pseudonaja textilis pughi Hoser 2003. Zootaxa 2008, 1703, 47–61. [Google Scholar] [CrossRef]
- Lee, M.S.; Sanders, K.L.; King, B.; Palci, A. Diversification rates and phenotypic evolution in venomous snakes (Elapidae). R. Soc. Open Sci. 2016, 3, 150277. [Google Scholar] [CrossRef] [PubMed]
- Sanders, K.L.; Lee, M.S.; Leys, R.; Foster, R.; Keogh, J.S. Molecular phylogeny and divergence dates for Australasian elapids and sea snakes (hydrophiinae): Evidence from seven genes for rapid evolutionary radiations. J. Evol. Biol. 2008, 21, 682–695. [Google Scholar] [CrossRef]
- Sousa, L.F.; Bernardoni, J.L.; Zdenek, C.N.; Dobson, J.; Coimbra, F.; Gillett, A.; Lopes-Ferreira, M.; Moura-da-Silva, A.M.; Fry, B.G. Differential coagulotoxicity of metalloprotease isoforms from Bothrops neuwiedi snake venom and consequent variations in antivenom efficacy. Toxicol. Lett. 2020, 333, 211–221. [Google Scholar] [CrossRef]
- Shine, R. Constraints, allometry, and adaptation: Food habits and reproductive biology of Australian Brownsnakes (Pseudonaja: Elapidae). Herpetologica 1989, 45, 195–207. [Google Scholar]
- Skejic, J.; Hodgson, W.C. Population divergence in venom bioactivities of elapid snake Pseudonaja textilis: Role of procoagulant proteins in rapid rodent prey incapacitation. PLoS ONE 2013, 8, e63988. [Google Scholar] [CrossRef]
- Trabi, M.; Sunagar, K.; Jackson, T.N.W.; Fry, B.G. Factor Xa Enzymes. In Venomous Reptiles and Their Toxins: Evolution, Pathophysiology and Biodiscovery; Fry, B.G., Ed.; Oxford University Press: New York, NY, USA, 2015; pp. 261–266. [Google Scholar]
- Birrell, G.W.; Earl, S.; Masci, P.P.; de Jersey, J.; Wallis, T.P.; Gorman, J.J.; Lavin, M.F. Molecular diversity in venom from the Australian Brown snake, Pseudonaja textilis. Mol. Cell. Proteom. 2006, 5, 379–389. [Google Scholar] [CrossRef]
- Birrell, G.W.; Earl, S.T.; Wallis, T.P.; Masci, P.P.; de Jersey, J.; Gorman, J.J.; Lavin, M.F. The diversity of bioactive proteins in Australian snake venoms. Mol. Cell. Proteom. 2007, 7, 973–986. [Google Scholar] [CrossRef] [PubMed]
- Filippovich, I.; Sorokina, N.; St Pierre, L.; Flight, S.; de Jersey, J.; Perry, N.; Masci, P.P.; Lavin, M.F. Cloning and functional expression of venom prothrombin activator protease from Pseudonaja textilis with whole blood procoagulant activity. Br. J. Haematol. 2005, 131, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Lavin, M.F.; Masci, P.P. Prothrombinase complexes with different physiological roles. Thromb. Haemost. 2009, 102, 421–423. [Google Scholar] [CrossRef] [PubMed]
- Masci, P.P.; Whitaker, A.N.; de Jersey, J. Purification and characterization of a prothrombin activator from the venom of the Australian brown snake, Pseudonaja textilis textilis. Biochem. Int. 1988, 17, 825–835. [Google Scholar]
- Morrison, J.J.; Tesseraux, I.; Pearn, J.H.; Harris, J.; Masci, P.P. Venom of the Australian rough-scaled snake, Tropidechis carinatus: Lethal potency and electrophysiological actions. Toxicon 1984, 22, 759–765. [Google Scholar] [CrossRef]
- St Pierre, L.; Birrell, G.W.; Earl, S.T.; Wallis, T.P.; Gorman, J.J.; de Jersey, J.; Masci, P.P.; Lavin, M.F. Diversity of toxic components from the venom of the evolutionarily distinct black whip snake, Demansia vestigiata. J. Proteome Res. 2007, 6, 3093–3107. [Google Scholar] [CrossRef]
- St Pierre, L.; Masci, P.P.; Filipovich, I.; Sorokina, N.; Marsh, N.; Miller, D.J.; Lavin, M.F. Comparative analysis of prothrombin activators from the venom of Australian elapids. Mol. Biol. Evol. 2005, 22, 1853–1864. [Google Scholar] [CrossRef]
- Doley, R.; Kini, R.M. Protein complexes in snake venom. Cell. Mol. Life Sci. 2009, 66, 2851–2871. [Google Scholar] [CrossRef]
- Han, S.X.; Kwong, S.; Ge, R.; Kolatkar, P.R.; Woods, A.E.; Blanchet, G.; Kini, R.M. Regulation of expression of venom toxins: Silencing of prothrombin activator trocarin D by AG-rich motifs. FASEB J. 2016, 30, 2411–2425. [Google Scholar] [CrossRef]
- Joseph, J.S.; Chung, M.C.; Mirtschin, P.J.; Kini, R.M. Effect of snake venom procoagulants on snake plasma: Implications for the coagulation cascade of snakes. Toxicon 2002, 40, 175–183. [Google Scholar] [CrossRef]
- Joseph, J.S.; Kini, R.M. Snake venom prothrombin activators homologous to blood coagulation factor Xa. Haemostasis 2001, 31, 234–240. [Google Scholar] [CrossRef]
- Joseph, J.S.; Kini, R.M. Snake venom prothrombin activators similar to blood coagulation factor Xa. Curr. Drug Targets Cardiovasc. Haematol. Disord. 2004, 4, 397–416. [Google Scholar] [CrossRef] [PubMed]
- Joseph, J.S.; Thirumangalathu, S.; Tsang, F.; Wong, F.W.; Kini, R.M. Trocarin, a blood coagulation factor Xa homologue from snake venom, causes inflammation and mitogenesis. Toxicon 2003, 42, 769–776. [Google Scholar] [CrossRef]
- Kini, R.M. The intriguing world of prothrombin activators from snake venom. Toxicon 2005, 45, 1133–1145. [Google Scholar] [CrossRef]
- Kini, R.M.; Rao, V.S.; Joseph, J.S. Procoagulant proteins from snake venoms. Haemostasis 2001, 31, 218–224. [Google Scholar] [CrossRef]
- Kwong, S.; Woods, A.E.; Mirtschin, P.J.; Ge, R.; Kini, R.M. The recruitment of blood coagulation factor X into snake venom gland as a toxin: The role of promoter cis-elements in its expression. Thromb. Haemost. 2009, 102, 469–478. [Google Scholar] [CrossRef]
- Latinović, Z.; Leonardi, A.; Koh, C.Y.; Kini, R.M.; Trampuš Bakija, A.; Pungerčar, J.; Križaj, I. The procoagulant snake venom serine protease potentially having a dual, blood coagulation factor V and X-Activating activity. Toxins 2020, 12, 358. [Google Scholar] [CrossRef] [PubMed]
- Le, T.N.M.; Abu Reza, M.; Swarup, S.; Kini, R.M. Gene duplication of coagulation factor V and origin of venom prothrombin activator in Pseudonaja textilis snake. Thromb. Haemost. 2005, 93, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.S.; Joseph, J.S.; Kini, R.M. Group D prothrombin activators from snake venom are structural homologues of mammalian blood coagulation factor Xa. Biochem. J. 2003, 369, 635–642. [Google Scholar] [CrossRef]
- Rao, V.S.; Kini, R.M. Pseutarin C, a prothrombin activator from Pseudonaja textilis venom: Its structural and functional similarity to mammalian coagulation factor Xa-Va complex. Thromb. Haemost. 2002, 88, 611–619. [Google Scholar] [CrossRef]
- Rao, V.S.; Swarup, S.; Kini, R.M. The nonenzymatic subunit of pseutarin C, a prothrombin activator from Eastern Brown snake (Pseudonaja textilis) venom, shows structural similarity to mammalian coagulation factor V. Blood 2003, 102, 1347–1354. [Google Scholar] [CrossRef]
- Rao, V.S.; Swarup, S.; Kini, R.M. The catalytic subunit of pseutarin C, a group C prothrombin activator from the venom of Pseudonaja textilis, is structurally similar to mammalian blood coagulation factor Xa. Thromb. Haemost. 2004, 92, 509–521. [Google Scholar] [CrossRef] [PubMed]
- Reza, A.; Swarup, S.; Manjunatha Kini, R. Two parallel prothrombin activator systems in Australian rough-scaled snake, Tropidechis carinatus. Structural comparison of venom prothrombin activator with blood coagulation factor X. Thromb. Haemost. 2005, 93, 40–47. [Google Scholar] [CrossRef]
- Reza, M.A.; Minh Le, T.N.; Swarup, S.; Kini, R.M. Molecular evolution caught in action: Gene duplication and evolution of molecular isoforms of prothrombin activators in Pseudonaja textilis (brown snake). J. Thromb. Haemost. 2006, 4, 1346–1353. [Google Scholar] [CrossRef] [PubMed]
- Reza, M.A.; Swarup, S.; Kini, R.M. Gene structures of trocarin D and coagulation factor X, two functionally diverse prothrombin activators from Australian rough scaled snake. Pathophysiol. Haemost. Thromb. 2005, 34, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Reza, M.A.; Swarup, S.; Kini, R.M. Structure of two genes encoding parallel prothrombin activators in Tropidechis carinatus snake: Gene duplication and recruitment of factor X gene to the venom gland. J. Thromb. Haemost. 2006, 5, 117–126. [Google Scholar] [CrossRef]
- Chester, A.; Crawford, G.P. In vitro coagulant properties of venoms from Australian snakes. Toxicon 1982, 20, 501–504. [Google Scholar] [CrossRef] [PubMed]
- Herrera, M.; Fernandez, J.; Vargas, M.; Villalta, M.; Segura, A.; Leon, G.; Angulo, Y.; Paiva, O.; Matainaho, T.; Jensen, S.D.; et al. Comparative proteomic analysis of the venom of the taipan snake, Oxyuranus scutellatus, from Papua New Guinea and Australia: Role of neurotoxic and procoagulant effects in venom toxicity. J. Proteom. 2012, 75, 2128–2140. [Google Scholar] [CrossRef]
- Herrera, M.; Paiva, O.K.; Pagotto, A.H.; Segura, A.; Serrano, S.M.; Vargas, M.; Villalta, M.; Jensen, S.D.; Leon, G.; Williams, D.J.; et al. Antivenomic characterization of two antivenoms against the venom of the taipan, Oxyuranus scutellatus, from Papua New Guinea and Australia. Am. J. Trop. Med. Hyg. 2014, 91, 887–894. [Google Scholar] [CrossRef]
- Judge, P.R. Coastal taipan (Oxyuranus scutellatus) envenomation of a dog. Aust. Vet. J. 2015, 93, 412–416. [Google Scholar] [CrossRef]
- Debono, J.; Bos, M.H.A.; Frank, N.; Fry, B. Clinical implications of differential antivenom efficacy in neutralising coagulotoxicity produced by venoms from species within the arboreal viperid snake genus Trimeresurus. Toxicol. Lett. 2019, 316, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Hohna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing, R-4.3.3; R Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- Wickham, H. dplyr: A Grammar of Data Manipulation, R Package Version 1.1.4; Posit PBC: Boston, MA, USA, 2023; Available online: https://cran.r-project.org/web/packages/dplyr/index.html (accessed on 1 July 2025).
- Hijmans, R.J. geodata: Download Geographic Data, R Package Version 0.6-2; GeoAI Data Society: Seoul, Republic of Korea, 2024; Available online: https://cran.r-project.org/web/packages/geodata/index.html (accessed on 1 July 2025).
- Pebesma, E. sf: Simple Features for R, R Package Version 1.0-16; 2024. Available online: https://cran.r-project.org/web/packages/sf/index.html (accessed on 1 July 2025).
- Pebesma, E.; Bivand, R. Spatial Data Science: With Applications in R; Chapman and Hall/CRC: London, UK, 2023. [Google Scholar]
- Tennekes, M. tmap: Thematic Maps, R Package Version 3.3-4; 2023. Available online: https://cran.r-project.org/web/packages/tmap/index.html (accessed on 1 July 2025).
- Hijmans, R.J. raster: Geographic Data Analysis and Modeling, R Package Version 3.6-32; 2024. Available online: https://cran.r-project.org/web/packages/raster/index.html (accessed on 1 July 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morecroft, H.; Zdenek, C.N.; Chowdhury, A.; Dunstan, N.; Hay, C.; Fry, B.G. X Marks the Clot: Evolutionary and Clinical Implications of Divergences in Procoagulant Australian Elapid Snake Venoms. Toxins 2025, 17, 417. https://doi.org/10.3390/toxins17080417
Morecroft H, Zdenek CN, Chowdhury A, Dunstan N, Hay C, Fry BG. X Marks the Clot: Evolutionary and Clinical Implications of Divergences in Procoagulant Australian Elapid Snake Venoms. Toxins. 2025; 17(8):417. https://doi.org/10.3390/toxins17080417
Chicago/Turabian StyleMorecroft, Holly, Christina N. Zdenek, Abhinandan Chowdhury, Nathan Dunstan, Chris Hay, and Bryan G. Fry. 2025. "X Marks the Clot: Evolutionary and Clinical Implications of Divergences in Procoagulant Australian Elapid Snake Venoms" Toxins 17, no. 8: 417. https://doi.org/10.3390/toxins17080417
APA StyleMorecroft, H., Zdenek, C. N., Chowdhury, A., Dunstan, N., Hay, C., & Fry, B. G. (2025). X Marks the Clot: Evolutionary and Clinical Implications of Divergences in Procoagulant Australian Elapid Snake Venoms. Toxins, 17(8), 417. https://doi.org/10.3390/toxins17080417






