Heating up the Blunts: Prothrombin Activation, with Factor Va as an Obligate Cofactor, Is the Dominant Procoagulant Mechanism of Blunt-Nosed Viper Venoms (Macrovipera Species)
Abstract
1. Introduction
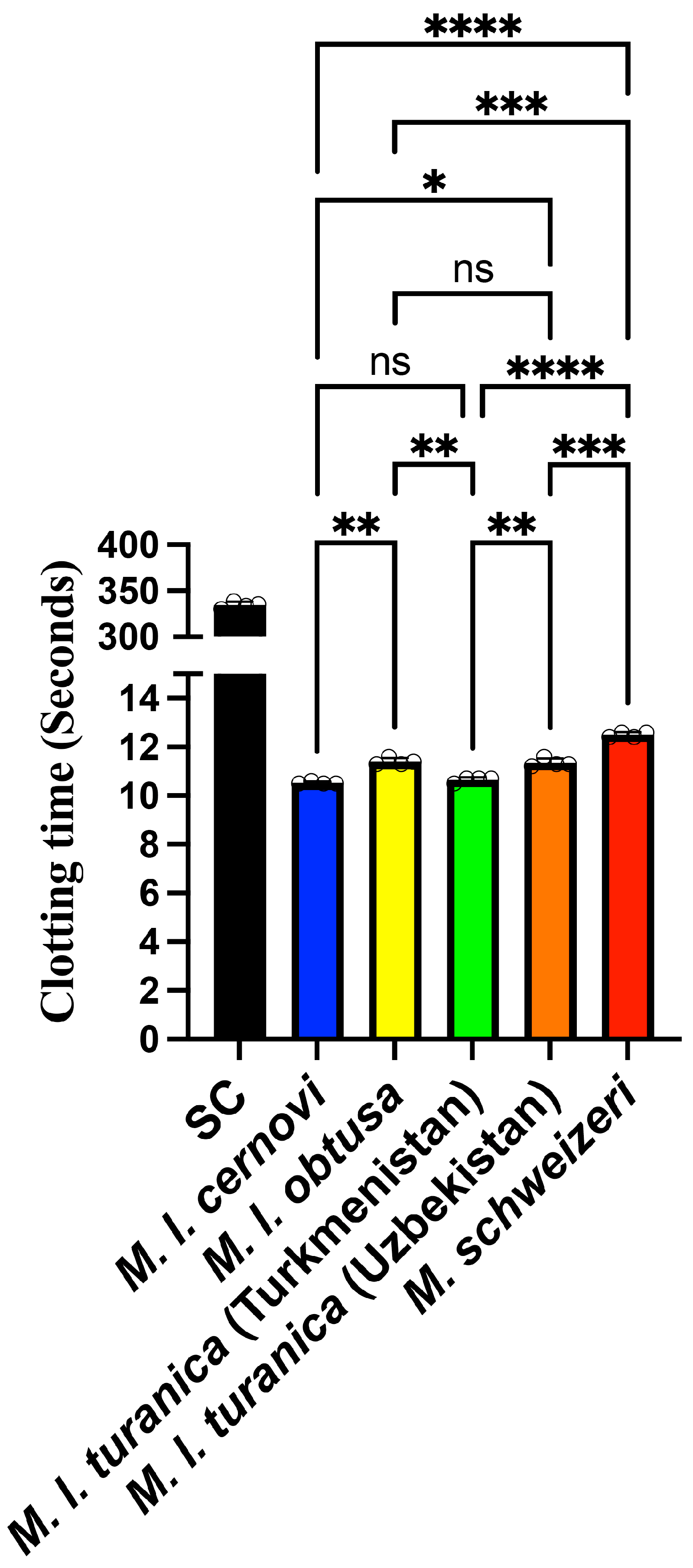
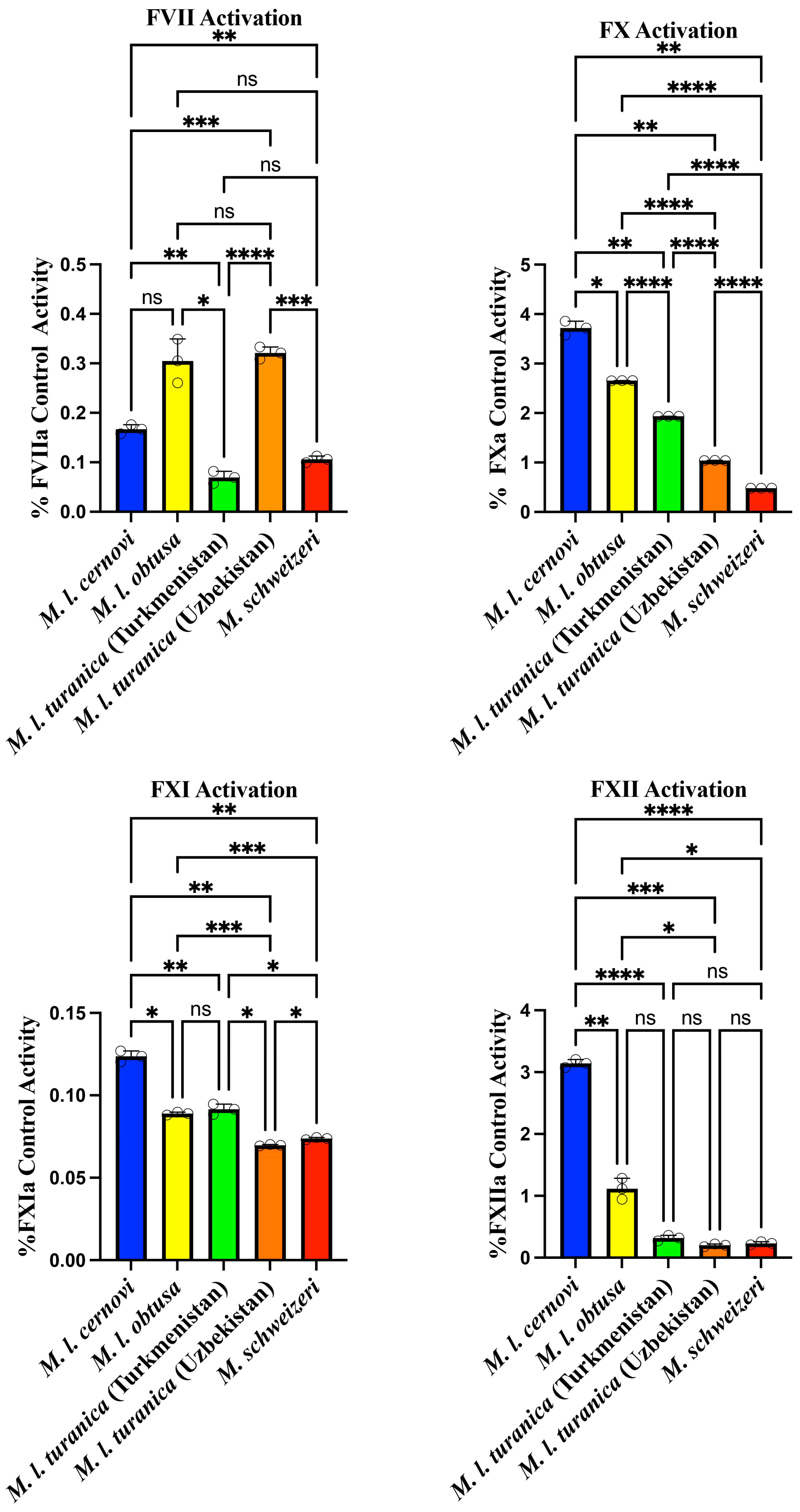
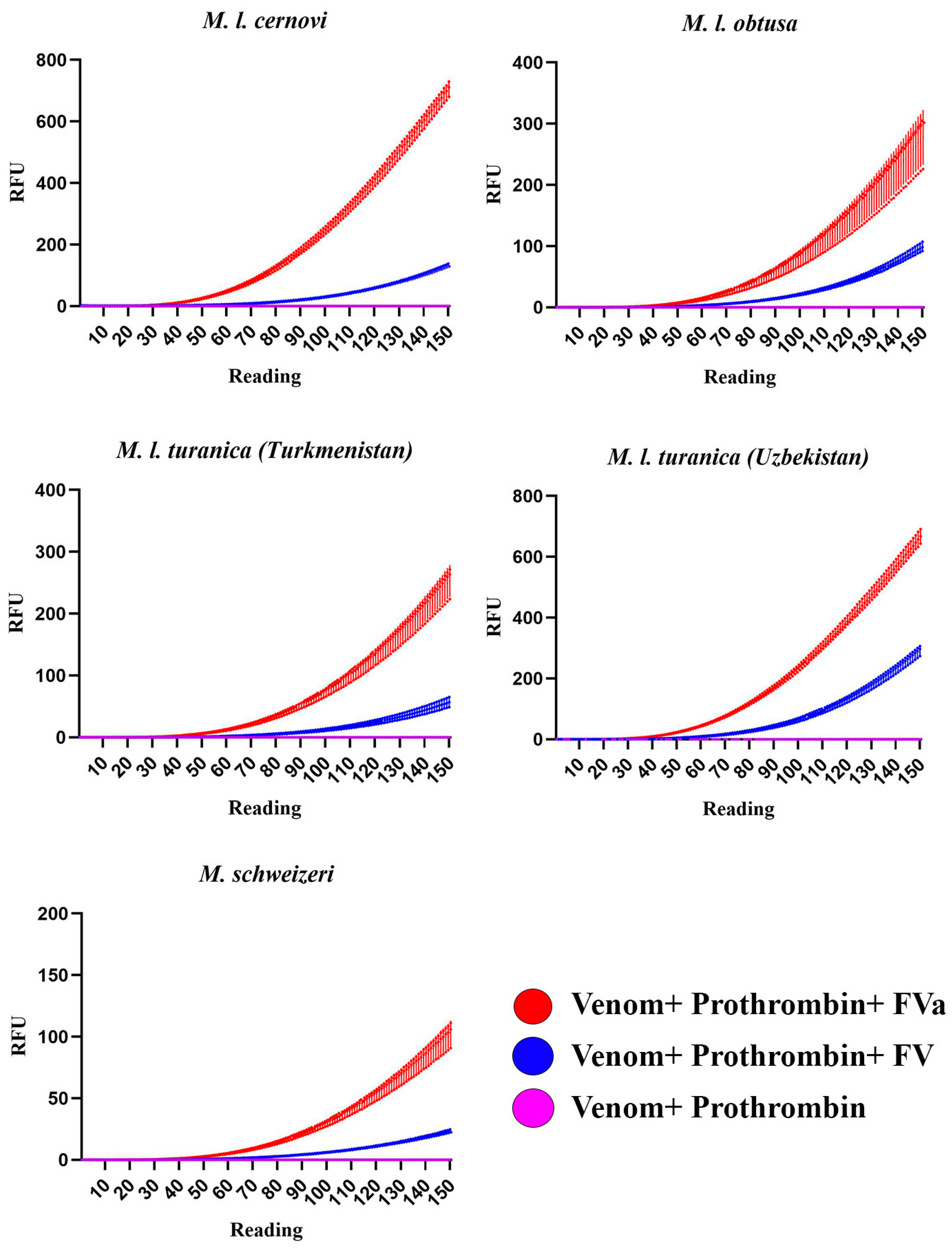
2. Results and Discussion
3. Materials and Methods
3.1. Venom Sources and Preparation
3.2. Stago STA-R Max Coagulation Assays
3.3. Fluoroskan Assays for FVII, FX, FXI, FXII, and Prothrombin Activation
3.3.1. Activation of FVII, FX, FXI, or FXII Plate Setup [15]
Blank Control Wells
- 10 μL phospholipid (Stago #00597)
- 20 μL of OK buffer
Zymogen Control Wells
- 10 μL phospholipid (Stago #00597)
- 10 μL of 10 μg/mL of either FVII (Prolytix #HCVII-0030), FX (Prolytix #HCX-0050), FXI (Prolytix #HCXI-0150), FXII (Prolytix #HCXII-0155), or prothrombin (Prolytix # HCP-0010)
- 10 μL of OK buffer
Activated Enzyme Control Wells
- 10 μL phospholipid (Stago #00597)
- 10 μL of 10 μg/mL of either FVIIa (HCVIIA-0031), FXa (Prolytix # HCBXA-0061), FXIa (Prolytix # HCXIA-0160), FXIIa (Prolytix #HCXII-0155 activated with kaolin), or thrombin (Prolytix # HCT-0020)
- 10 μL of OK buffer
Venom Control Wells
- 10 μL phospholipid (Stago #00597)
- 10 μL of 1 μg/mL venom
- 10 μL of OK buffer
Experimental Wells
- 10 μL phospholipid (Stago #00597)
- 10 μL of 10 μg/mL of either FVII (Prolytix #HCVII-0030), FX (Prolytix #HCX-0050), FXI (Prolytix #HCXI-0150), FXII (Prolytix #HCXII-0155), or prothrombin (Prolytix # HCP-0010)
- 10 μL of 1 μg/mL venom
3.3.2. Prothrombin Activation Plate Setup [29]
Blank Control Wells
- 10 μL phospholipid (Stago #00597)
- 20 μL OK buffer
Zymogen Control Wells
- 10 μL phospholipid (Stago #00597)
- 10 μL 1 μg/mL prothrombin (Prolytix HCP-0010)
- 10 μL OK buffer
Activated Enzyme Control Wells
- 10 μL phospholipid (Stago #00597)
- 10 μL 1 μg/mL thrombin (Prolytix HCT-0020)
- 10 μL OK buffer
Venom Control Wells
- 10 μL phospholipid (Stago #00597)
- 10 μL 0.1 μg/mL venom
- 10 μL OK buffer
Experimental Wells
- 10 μL phospholipid (Stago #00597)
- Prothrombin tested under three separate conditions:
- ○
- 10 μL 1 μg/mL prothrombin (Prolytix HCP-0010)
- ○
- 10 μL of a combined solution consisting of 1 μg/mL prothrombin (Prolytix HCP-0010) + 7 µg/mL Factor V (Prolytix #HCV-0100).
- ○
- 10 μL of a combined solution consisting of 1 μg/mL prothrombin (Prolytix HCP-0010) + 4 µg/mL Factor Va (Prolytix #HCVA-0110).
- 10 μL 0.1 μg/mL venom
3.4. One-Dimensional Polyacrylamide Gel Electrophoresis (SDS-PAGE)
3.5. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Casewell, N.R.; Wuster, W.; Vonk, F.J.; Harrison, R.A.; Fry, B.G. Complex cocktails: The evolutionary novelty of venoms. Trends Ecol. Evol. 2013, 28, 219–229. [Google Scholar] [CrossRef]
- Fry, B.G.; Roelants, K.; Champagne, D.E.; Scheib, H.; Tyndall, J.D.; King, G.F.; Nevalainen, T.J.; Norman, J.A.; Lewis, R.J.; Norton, R.S.; et al. The toxicogenomic multiverse: Convergent recruitment of proteins into animal venoms. Annu. Rev. Genom. Hum. Genet. 2009, 10, 483–511. [Google Scholar] [CrossRef]
- Jenner, R.A.; Casewell, N.R.; Undheim, E.A.B. What is animal venom? Rethinking a manipulative weapon. Trends Ecol. Evol. 2025. online ahead of print. [Google Scholar] [CrossRef]
- Schendel, V.; Rash, L.D.; Jenner, R.A.; Undheim, E.A.B. The diversity of venom: The Importance of behavior and venom system morphology in understanding its ecology and evolution. Toxins 2019, 11, 666. [Google Scholar] [CrossRef]
- Boyer, L.; Alagón, A.; Fry, B.G.; Jackson, T.N.W.; Sunagar, K.; Chippaux, J.P. Signs, symptoms and treatment of envenomation. In Venomous Reptiles and Their Toxins: Evolution, Pathophysiology and Biodiscovery; Fry, B.G., Ed.; Oxford University Press: New York, NY, USA, 2015; pp. 32–60. [Google Scholar]
- Gopalakrishnakone, P.; Inagaki, H.; Mukherjee, A.K.; Rahmy, T.R.; Vogel, C.W. Snake Venoms; Springer: Berlin, Germany, 2017. [Google Scholar]
- Lu, Q.; Clemetson, J.M.; Clemetson, K.J. Snake venoms and hemostasis. J. Thromb. Haemost. 2005, 3, 1791–1799. [Google Scholar] [CrossRef]
- Avella, I.; Damm, M.; Di Nicola, M.R.; Dresler, J.; Igci, N.; Karis, M.; Kazemi, S.M.; Kreuels, B.; Paolino, G.; Sarigiannis, Y.; et al. The biology and toxinology of blunt-nosed vipers. NPJ Biodivers 2025, 4, 21. [Google Scholar] [CrossRef] [PubMed]
- Phelps, T.; Warrell, D.A. Old World Vipers; Chimaira: Frankfurt, Germany, 2010. [Google Scholar]
- Di Nicola, M.R.; Pozzi, A.; Avella, I.; Mezzadri, S.; Paolino, G. Taxonomy, biology and natural history of the Milos viper Macrovipera schweizeri (Werner, 1935): Literature review and observations on autumnal activity and importance of residual pools. Spixiana 2022, 45, 111–130. [Google Scholar]
- Frétey, T. Capitalised Epithets in the Works of Linnaeus (1758–1767): Findings and Consequences in Herpetology. Bionomina 2019, 16, 22–45. [Google Scholar] [CrossRef]
- Op den Brouw, B.; Coimbra, F.C.P.; Bourke, L.A.; Huynh, T.M.; Vlecken, D.H.W.; Ghezellou, P.; Visser, J.C.; Dobson, J.S.; Fernandez-Rojo, M.A.; Ikonomopoulou, M.P.; et al. Extensive Variation in the Activities of Pseudocerastes and Eristicophis Viper Venoms Suggests Divergent Envenoming Strategies Are Used for Prey Capture. Toxins 2021, 13, 112. [Google Scholar] [CrossRef] [PubMed]
- Di Nicola, M.R.; Pontara, A.; Kass, G.E.; Kramer, N.I.; Avella, I.; Pampena, R.; Mercuri, S.R.; Dorne, J.L.C.; Paolino, G. Vipers of Major clinical relevance in Europe: Taxonomy, venom composition, toxicology and clinical management of human bites. Toxicology 2021, 453, 152724. [Google Scholar] [CrossRef]
- Chowdhury, A.; Zdenek, C.N.; Dobson, J.S.; Bourke, L.A.; Soria, R.; Fry, B.G. Clinical implications of differential procoagulant toxicity of the palearctic viperid genus Macrovipera, and the relative neutralization efficacy of antivenoms and enzyme inhibitors. Toxicol. Lett. 2021, 340, 77–88. [Google Scholar] [CrossRef]
- Kempson, K.; Chowdhury, A.; Violette, A.; Fourmy, R.; Soria, R.; Fry, B.G. Age Is Just a Number: Ontogenetic Conservation in activation of blood clotting Factors VII, X, and XII by Caucasus Blunt-Nosed Viper (Macrovipera lebetina obtusa) Venoms. Toxins 2024, 16, 520. [Google Scholar] [CrossRef]
- Siigur, E.; Tonismagi, K.; Trummal, K.; Samel, M.; Vija, H.; Subbi, J.; Siigur, J. Factor X activator from Vipera lebetina snake venom, molecular characterization and substrate specificity. Biochim. Biophys. Acta 2001, 1568, 90–98. [Google Scholar] [CrossRef]
- Siigur, J.; Siigur, E. Activation of Factor X by Snake Venom Proteases. In Toxins and Hemostasis; Kini, R.M., Clemetson, K.J., Markland, F.S., McLane, M.A., Morita, T., Eds.; Bench to Bedside; Springer: Berlin/Heidelberg, Germany, 2010; p. 447. [Google Scholar]
- Siigur, J.; Aaspollu, A.; Siigur, E. Biochemistry and pharmacology of proteins and peptides purified from the venoms of the snakes Macrovipera lebetina subspecies. Toxicon 2019, 158, 16–32. [Google Scholar] [CrossRef]
- Tans, G.; Rosing, J. Snake venom activators of factor X: An overview. Haemostasis 2001, 31, 225–233. [Google Scholar] [CrossRef]
- Yamada, D.; Sekiya, F.; Morita, T. Prothrombin and factor X activator activities in the venoms of Viperidae snakes. Toxicon 1997, 35, 1581–1589. [Google Scholar] [CrossRef] [PubMed]
- Siigur, E.; Aaspõllu, A.; Trummal, K.; Tõnismägi, K.; Tammiste, I.; Kalkkinen, N.; Siigur, J. Factor X activator from Vipera lebetina venom is synthesized from different genes. Biochim. Biophys. Acta 2004, 1702, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Bourke, L.A.; Salazar-Valenzuela, D.; Mancuso, M.; Quirola, D.R.; Loaiza-Lange, A.; Zdenek, C.N.; Lewin, M.R.; Arbeláez-Ortiz, E.; Fry, B.G. Venom versatility: Dynamic anticoagulant and procoagulant variations between and within Bothrocophias (toad-head) and basal Bothrops (lance-head) pit vipers. Biochimie 2025, in press. [Google Scholar] [CrossRef] [PubMed]
- Prentice, C.R.; Ratnoff, O.D. The action of Russell’s viper venom on factor V and the prothrombin-converting principle. Br. J. Haematol. 1969, 16, 291–302. [Google Scholar] [CrossRef]
- Rosing, J.; Govers-Riemslag, J.W.; Yukelson, L.; Tans, G. Factor V activation and inactivation by venom proteases. Haemostasis 2001, 31, 241–246. [Google Scholar] [CrossRef]
- Siigur, E.; Samel, M.; Tonismagi, K.; Subbi, J.; Reintamm, T.; Siigur, J. Isolation, properties and N-terminal amino acid sequence of a factor V activator from Vipera lebetina (Levantine viper) snake venom. Biochim. Biophys. Acta 1998, 1429, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.J.; Rolfs, M.R.; Day, W.C. Observations on the Factor V activator present in Russell’s viper venom and its action on Factor, V. Biochim. Biophys. Acta 1972, 286, 205–211. [Google Scholar] [CrossRef]
- Latinović, Z.; Leonardi, A.; Koh, C.Y.; Kini, R.M.; Trampuš Bakija, A.; Pungerčar, J.; Križaj, I. The procoagulant snake venom serine protease potentially having a dual, blood coagulation factor V and X-Activating activity. Toxins 2020, 12, 358. [Google Scholar] [CrossRef]
- Rawala, R.; Saraswati, S.; Niewiarowski, S.; Colman, R.W. Molecular changes during the activation of bovine factor V by snake venom proteases. Circulation 1978, 58, 209. [Google Scholar]
- Chandrasekara, U.; Chowdhury, A.; Seneci, L.; Zdenek, C.N.; Dunstan, N.; Fry, B.G. From venom to vein: Factor VII activation as a major pathophysiological target for procoagulant Australian elapid snake venoms. Toxins 2024, 16, 430. [Google Scholar] [CrossRef]
- Joseph, J.S.; Chung, M.C.; Jeyaseelan, K.; Kini, R.M. Amino acid sequence of trocarin, a prothrombin activator from Tropidechis carinatus venom: Its structural similarity to coagulation factor Xa. Blood 1999, 94, 621–631. [Google Scholar] [CrossRef]
- Joseph, J.S.; Kini, R.M. Snake venom prothrombin activators homologous to blood coagulation factor Xa. Haemostasis 2001, 31, 234–240. [Google Scholar] [CrossRef]
- Joseph, J.S.; Kini, R.M. Snake venom prothrombin activators similar to blood coagulation factor Xa. Curr. Drug Targets Cardiovasc. Haematol. Disord. 2004, 4, 397–416. [Google Scholar] [CrossRef]
- Kini, R.M. The intriguing world of prothrombin activators from snake venom. Toxicon 2005, 45, 1133–1145. [Google Scholar] [CrossRef] [PubMed]
- Kwong, S.; Woods, A.E.; Mirtschin, P.J.; Ge, R.; Kini, R.M. The recruitment of blood coagulation factor X into snake venom gland as a toxin: The role of promoter cis-elements in its expression. Thromb. Haemost. 2009, 102, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Le, T.N.M.; Reza, M.A.; Swarup, S.; Kini, R.M. Gene duplication of coagulation factor V and origin of venom prothrombin activator in Pseudonaja textilis snake. Thromb. Haemost. 2005, 93, 420–429. [Google Scholar] [CrossRef]
- McCleary, R.J.; Kini, R.M. Snake bites and hemostasis/thrombosis. Thromb. Res. 2013, 132, 642–646. [Google Scholar] [CrossRef]
- Earl, S.; Sunagar, K.; Jackson, T.N.W.; Reeks, T.; Fry, B.G. Factor Va Proteins. In Venomous Reptiles and Their Toxins: Evolution, Pathophysiology and Biodiscovery; Fry, B.G., Ed.; Oxford University Press: New York, NY, USA, 2015; pp. 255–260. [Google Scholar]
- Rao, V.S.; Joseph, J.S.; Kini, R.M. Group D prothrombin activators from snake venom are structural homologues of mammalian blood coagulation factor Xa. Biochem. J. 2003, 369, 635–642. [Google Scholar] [CrossRef]
- Rao, V.S.; Swarup, S.; Kini, R.M. The nonenzymatic subunit of pseutarin C, a prothrombin activator from Eastern Brown snake (Pseudonaja textilis) venom, shows structural similarity to mammalian coagulation factor V. Blood 2003, 102, 1347–1354. [Google Scholar] [CrossRef]
- Rao, V.S.; Swarup, S.; Kini, R.M. The catalytic subunit of pseutarin C, a group C prothrombin activator from the venom of Pseudonaja textilis, is structurally similar to mammalian blood coagulation factor Xa. Thromb. Haemost. 2004, 92, 509–521. [Google Scholar] [CrossRef] [PubMed]
- Reza, A.; Swarup, S.; Manjunatha Kini, R. Two parallel prothrombin activator systems in Australian rough-scaled snake, Tropidechis carinatus. Structural comparison of venom prothrombin activator with blood coagulation factor X. Thromb. Haemost. 2005, 93, 40–47. [Google Scholar] [CrossRef]
- Reza, M.A.; Minh Le, T.N.; Swarup, S.; Kini, R.M. Molecular evolution caught in action: Gene duplication and evolution of molecular isoforms of prothrombin activators in Pseudonaja textilis (brown snake). J. Thromb. Haemost. 2006, 4, 1346–1353. [Google Scholar] [CrossRef] [PubMed]
- Trabi, M.; Sunagar, K.; Jackson, T.N.W.; Fry, B.G. Factor Xa Enzymes. In Venomous Reptiles and Their Toxins: Evolution, Pathophysiology and Biodiscovery; Fry, B.G., Ed.; Oxford University Press: New York, NY, USA, 2015; pp. 261–266. [Google Scholar]
- Reza, M.A.; Swarup, S.; Kini, R.M. Gene structures of trocarin D and coagulation factor X, two functionally diverse prothrombin activators from Australian rough scaled snake. Pathophysiol. Haemost. Thromb. 2005, 34, 205–208. [Google Scholar] [CrossRef]
- Reza, M.A.; Swarup, S.; Kini, R.M. Structure of two genes encoding parallel prothrombin activators in Tropidechis carinatus snake: Gene duplication and recruitment of factor X gene to the venom gland. J. Thromb. Haemost. 2006, 5, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Zdenek, C.N.; den Brouw, B.O.; Dashevsky, D.; Gloria, A.; Youngman, N.; Watson, E.; Green, P.; Hay, C.; Dunstan, N.; Allen, L.; et al. Clinical implications of convergent procoagulant toxicity and differential antivenom efficacy in Australian elapid snake venoms. Toxicol. Lett. 2019, 316, 171–182. [Google Scholar] [CrossRef]
- Zdenek, C.N.; Hay, C.; Arbuckle, K.; Jackson, T.N.W.; Bos, M.H.A.; Op den Brouw, B.; Debono, J.; Allen, L.; Dunstan, N.; Morley, T.; et al. Coagulotoxic effects by brown snake (Pseudonaja) and taipan (Oxyuranus) venoms, and the efficacy of a new antivenom. Toxicol. Vitr. 2019, 58, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Fry, B.G. From genome to “venome”: Molecular origin and evolution of the snake venom proteome inferred from phylogenetic analysis of toxin sequences and related body proteins. Genome Res. 2005, 15, 403–420. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Champagne, P.S.; Seneci, L.; Fry, B.G. Heating up the Blunts: Prothrombin Activation, with Factor Va as an Obligate Cofactor, Is the Dominant Procoagulant Mechanism of Blunt-Nosed Viper Venoms (Macrovipera Species). Toxins 2025, 17, 398. https://doi.org/10.3390/toxins17080398
Champagne PS, Seneci L, Fry BG. Heating up the Blunts: Prothrombin Activation, with Factor Va as an Obligate Cofactor, Is the Dominant Procoagulant Mechanism of Blunt-Nosed Viper Venoms (Macrovipera Species). Toxins. 2025; 17(8):398. https://doi.org/10.3390/toxins17080398
Chicago/Turabian StyleChampagne, Patrick S., Lorenzo Seneci, and Bryan G. Fry. 2025. "Heating up the Blunts: Prothrombin Activation, with Factor Va as an Obligate Cofactor, Is the Dominant Procoagulant Mechanism of Blunt-Nosed Viper Venoms (Macrovipera Species)" Toxins 17, no. 8: 398. https://doi.org/10.3390/toxins17080398
APA StyleChampagne, P. S., Seneci, L., & Fry, B. G. (2025). Heating up the Blunts: Prothrombin Activation, with Factor Va as an Obligate Cofactor, Is the Dominant Procoagulant Mechanism of Blunt-Nosed Viper Venoms (Macrovipera Species). Toxins, 17(8), 398. https://doi.org/10.3390/toxins17080398






