Profiling the Paralytic Effects and Lethality of Cone Snail Venom Toxins Using Nanofractionation Analytics with In Vivo Zebrafish Larvae Assays
Abstract
1. Introduction
2. Results and Discussion
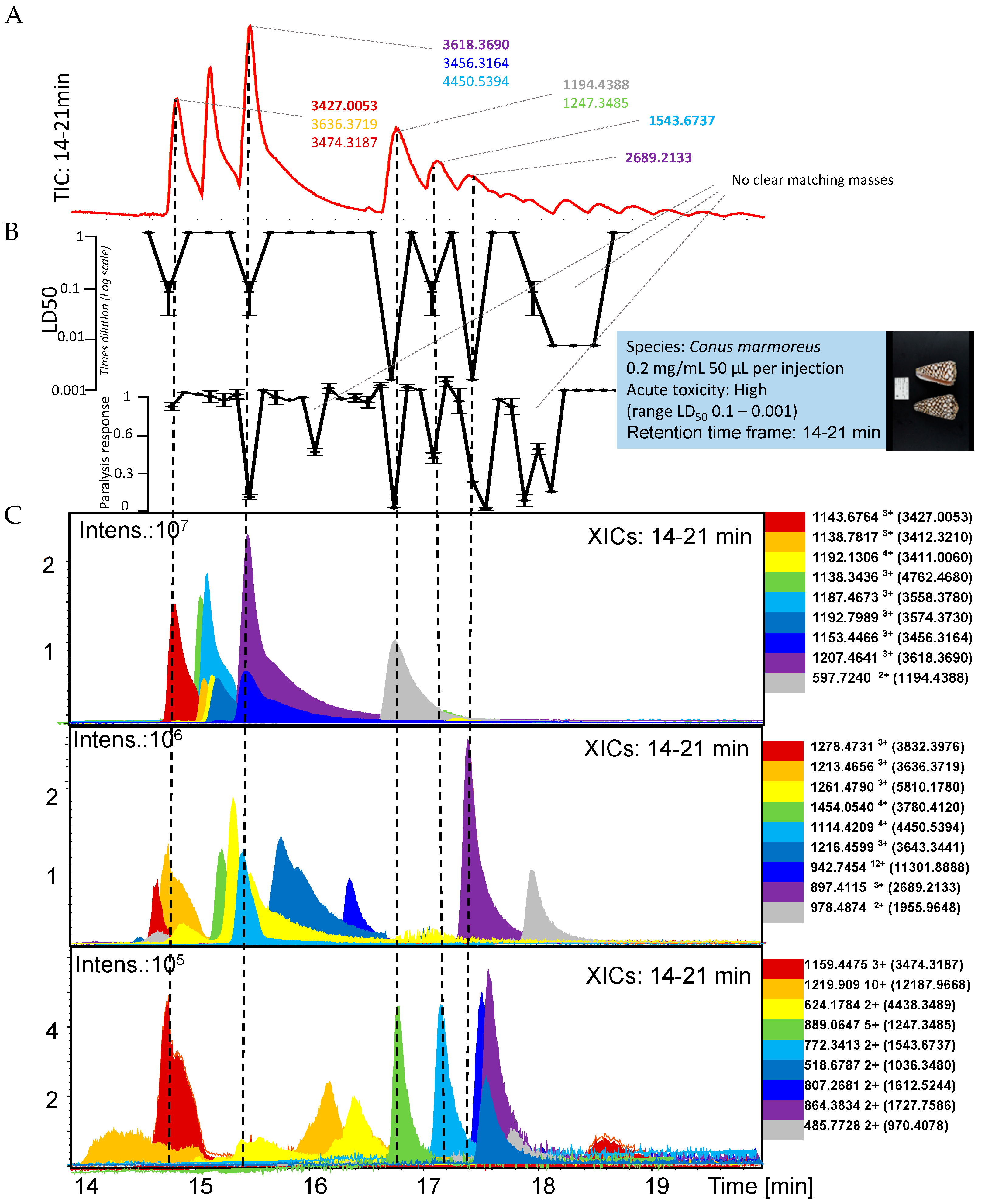
2.1. Conus Marmoreus
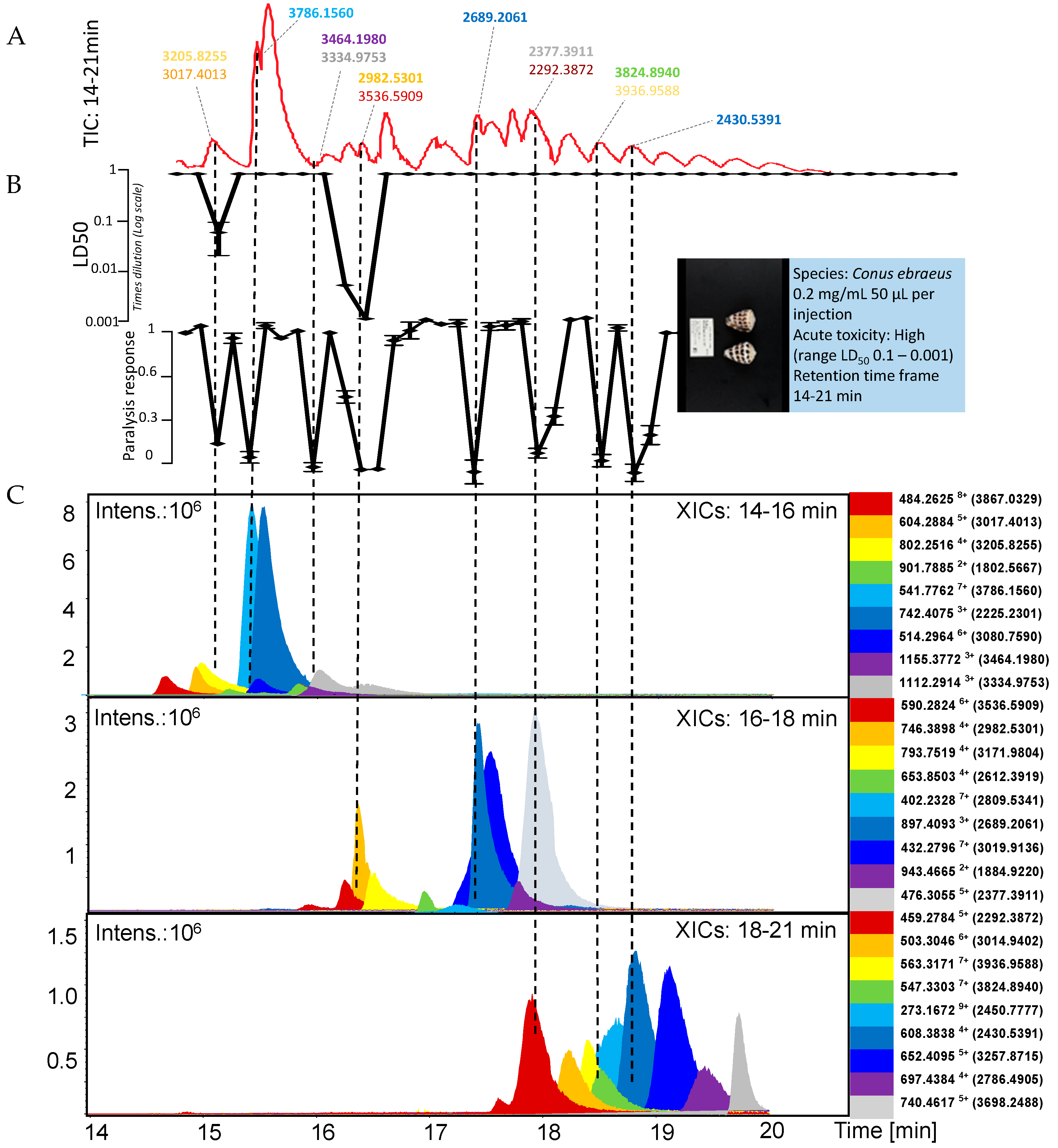
2.2. Conus Ebraeus
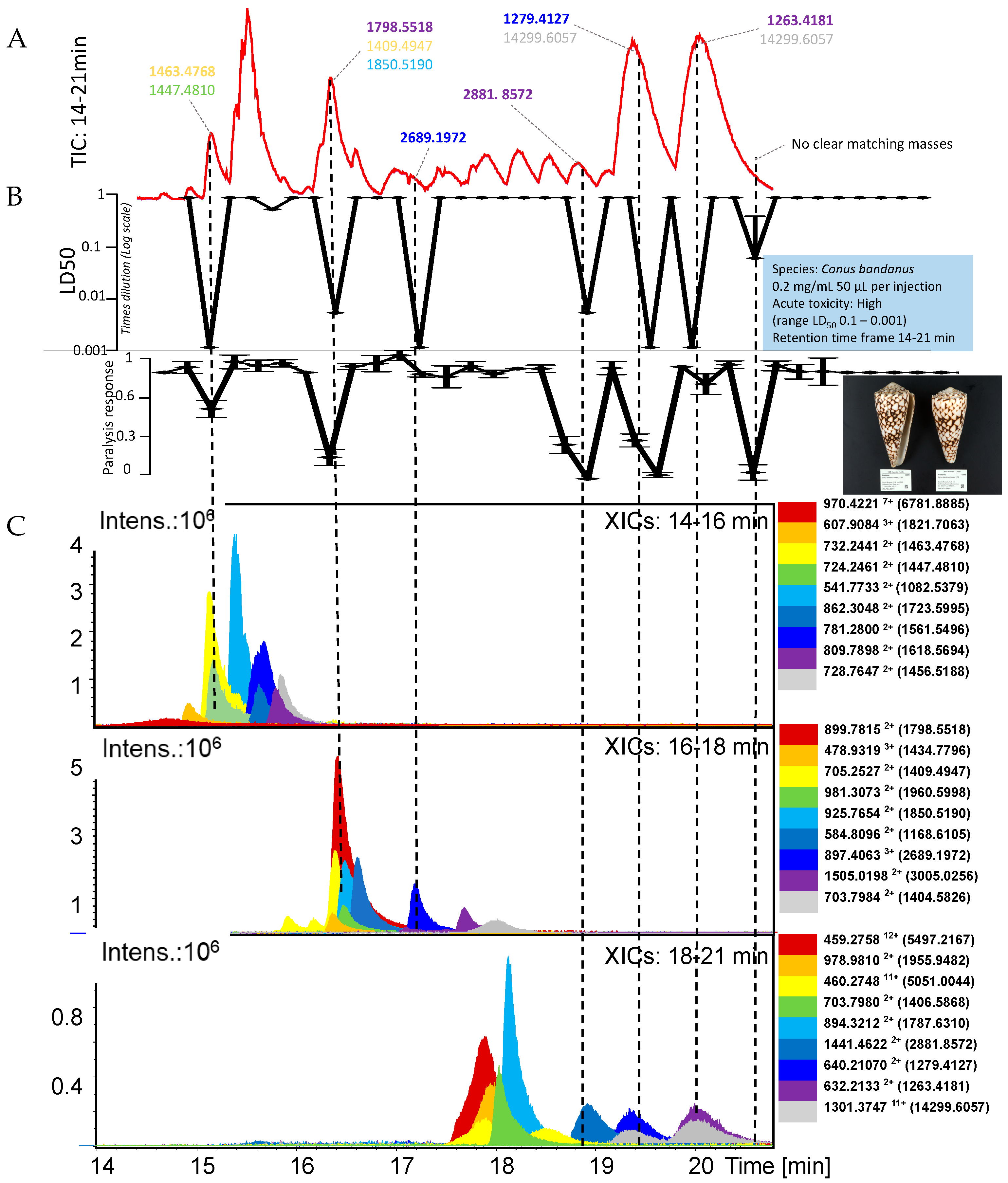
2.3. Conus Bandanus
2.4. Concluding Remarks
3. Materials and Methods
3.1. Chemical, Biological Reagents and Venoms
3.2. Cone Snail Venom Fractionation and Mass Spectrometry
3.3. Zebrafish Animal Care and Handling
3.4. Zebrafish Lethality Toxicity Assay
3.5. Zebrafish Paralysis Assay
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ratibou, Z.; Inguimbert, N.; Dutertre, S. Predatory and Defensive Strategies in Cone Snails. Toxins 2024, 16, 94. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Peng, C.; Yang, J.; Yi, Y.; Zhang, J.; Shi, Q. Cone Snails: A Big Store of Conotoxins for Novel Drug Discovery. Toxins 2017, 9, 397. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F.C.; Lewis, R.J. Sodium Channels and Pain: From Toxins to Therapies. Br. J. Pharmacol. 2018, 175, 2138–2157. [Google Scholar] [CrossRef]
- Hasan, M.M.; Starobova, H.; Mueller, A.; Vetter, I.; Lewis, R.J. Subcutaneous ω-Conotoxins Alleviate Mechanical Pain in Rodent Models of Acute Peripheral Neuropathy. Mar. Drugs 2021, 19, 106. [Google Scholar] [CrossRef]
- Wallace, M.S.; Rauck, R.; Fisher, R.; Charapata, S.G.; Ellis, D.; Dissanayake, S. Intrathecal Ziconotide for Severe Chronic Pain: Safety and Tolerability Results of an Open-Label, Long-Term Trial. Anesth. Analg. 2008, 106, 628–637. [Google Scholar] [CrossRef] [PubMed]
- Pei, S.; Wang, N.; Mei, Z.; Zhangsun, D.; Craik, D.J.; McIntosh, J.M.; Zhu, X.; Luo, S. Conotoxins Targeting Voltage-Gated Sodium Ion Channels. Pharmacol. Rev. 2024, 76, 828–845. [Google Scholar] [CrossRef]
- Martínez-Hernández, L.; López-Vera, E.; Aguilar, M.B. Peptide Toxins from Marine Conus Snails with Activity on Potassium Channels and/or Currents. Toxins 2024, 16, 504. [Google Scholar] [CrossRef]
- Ho, T.N.; Tran, T.H.; Le, H.S.; Lewis, R.J. Advances in the Synthesis and Engineering of Conotoxins. Eur. J. Med. Chem. 2025, 282, 117038. [Google Scholar] [CrossRef]
- Freuville, L.; Matthys, C.; Quinton, L.; Gillet, J.-P. Venom-Derived Peptides for Breaking through the Glass Ceiling of Drug Development. Front. Chem. 2024, 12, 1465459. [Google Scholar] [CrossRef]
- Ben-Yakar, A. High-Content and High-Throughput In Vivo Drug Screening Platforms Using Microfluidics. ASSAY Drug Dev. Technol. 2019, 17, 8–13. [Google Scholar] [CrossRef]
- Miscevic, F.; Rotstein, O.; Wen, X.-Y. Advances in Zebrafish High Content and High Throughput Technologies. Comb. Chem. High Throughput Screen. 2012, 15, 515–521. [Google Scholar] [CrossRef]
- Arrahman, A.; Xu, H.; Khan, M.A.; Bos, T.S.; Slagboom, J.; Van Der Velden, G.C.; Nehrdich, U.; Casewell, N.R.; Richardson, M.K.; Tudorache, C.; et al. Parallel in vitro Ion Channel and in vivo Zebrafish Assaying of Elapid Snake Venoms Following Chromatographic Separation of Toxin Components. SLAS Discov. 2025, 34, 100239. [Google Scholar] [CrossRef]
- Von Reumont, B.M.; Anderluh, G.; Antunes, A.; Ayvazyan, N.; Beis, D.; Caliskan, F.; Crnković, A.; Damm, M.; Dutertre, S.; Ellgaard, L.; et al. Modern Venomics—Current Insights, Novel Methods, and Future Perspectives in Biological and Applied Animal Venom Research. GigaScience 2022, 11, giac048. [Google Scholar] [CrossRef]
- Himaya, S.; Lewis, R. Venomics-Accelerated Cone Snail Venom Peptide Discovery. Int. J. Mol. Sci. 2018, 19, 788. [Google Scholar] [CrossRef] [PubMed]
- Conticello, S.G.; Gilad, Y.; Avidan, N.; Ben-Asher, E.; Levy, Z.; Fainzilber, M. Mechanisms for evolving hypervariability: The case of conopeptides. Mol. Biol. Evol. 2001, 18, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Yao, G.E.; Peng, C.; Zhu, Y.; Fan, C.; Jiang, H.; Chen, J.; Cao, Y.; Shi, Q. High-Throughput Identification and Analysis of Novel Conotoxins from Three Vermivorous Cone Snails by Transcriptome Sequencing. Mar. Drugs 2019, 17, 193. [Google Scholar] [CrossRef]
- Luo, S.; Zhangsun, D.; Feng, J.; Wu, Y.; Zhu, X.; Hu, Y. Diversity of the O-superfamily conotoxins from Conus miles. J. Pept. Sci. 2007, 13, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Vijayasarathy, M.; Balaram, P. Cone snail prolyl-4-hydroxylase α-subunit sequences derived from transcriptomic data and mass spectrometric analysis of variable proline hydroxylation in C. amadis venom. J. Proteom. 2018, 194, 37–48. [Google Scholar] [CrossRef]
- Li, X.; Chen, W.; Zhangsun, D.; Luo, S. Diversity of Conopeptides and Their Precursor Genes of Conus Litteratus. Mar. Drugs 2020, 18, 464. [Google Scholar] [CrossRef]
- Abalde, S.; Tenorio, M.J.; Afonso, C.M.L.; Zardoya, R. Conotoxin diversity in Chelyconus ermineus (Born, 1778) and the convergent origin of piscivory in the Atlantic and Indo-Pacific cones. Genome Biol. Evol. 2018, 10, 2643–2662. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, N.; Hu, J.; Zhao, C.; Yu, Z.; Dai, Q. Identification of novel I-superfamily conopeptides from several clades of Conus species found in the South China Sea. Peptides 2009, 30, 1782–1787. [Google Scholar] [CrossRef]
- Hu, H.; Bandyopadhyay, P.K.; Olivera, B.M.; Yandell, M. Characterization of the Conus bullatus genome and its venom-duct transcriptome. BMC Genom. 2011, 12, 60. [Google Scholar] [CrossRef]
- Dutertre, S.; Jin, A.-H.; Kaas, Q.; Jones, A.; Alewood, P.F.; Lewis, R.J. Deep venomics reveals the mechanism for expanded peptide diversity in cone snail venom. Mol. Cell. Proteom. 2013, 12, 312–329. [Google Scholar] [CrossRef]
- Dudajr, T.; Bolin, M.B.; Meyer, C.P.; Kohn, A.J. Hidden diversity in a hyperdiverse gastropod genus: Discovery of previously unidentified members of a Conus species complex. Mol. Phylogenetics Evol. 2008, 49, 867–876. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, H.; Liu, N.A.; Wu, C.; Jiang, J.; Yue, J.; Jing, Y.; Dai, Q. Diversity and evolution of conotoxins in Conus virgo, Conus eburneus, Conus imperialis and Conus marmoreus from the South China Sea. Toxicon 2012, 60, 982–989. [Google Scholar] [CrossRef] [PubMed]
- Jin, A.H.; Dutertre, S.; Dutt, M.; Lavergne, V.; Jones, A.; Lewis, R.J.; Alewood, P.F. Transcriptomic-Proteomic Correlation in the Predation-Evoked Venom of the Cone Snail, Conus imperialis. Mar. Drugs 2019, 17, 177. [Google Scholar]
- Chang, D.; Duda, T.F. Extensive and continuous duplication facilitates rapid evolution and diversification of gene families. Mol. Biol. Evol. 2012, 29, 2019–2029. [Google Scholar] [CrossRef] [PubMed]
- Duda, T.F.; Lee, T. Ecological release and venom evolution of a predatory marine snail at Easter Island. PLoS ONE 2009, 4, e005558. [Google Scholar] [CrossRef]
- Lluisma, A.O.; Milash, B.A.; Moore, B.; Olivera, B.M.; Bandyopadhyay, P.K. Novel venom peptides from the cone snail Conus pulicarius discovered through next-generation sequencing of its venom duct transcriptome. Mar. Genom. 2012, 5, 43–51. [Google Scholar] [CrossRef]
- Zhou, M.; Wang, L.; Wu, Y.; Zhu, X.; Feng, Y.; Chen, Z.; Li, Y.; Sun, D.; Ren, Z.; Xu, A. Characterizing the evolution and functions of the M-superfamily conotoxins. Toxicon 2013, 76, 150–159. [Google Scholar] [CrossRef]
- Kauferstein, S.; Melaun, C.; Mebs, D. Direct cDNA cloning of novel conopeptide precursors of the O-superfamily. Peptides 2005, 26, 361–367. [Google Scholar] [CrossRef]
- Elliger, C.A.; Richmond, T.A.; Lebaric, Z.N.; Pierce, N.T.; Sweedler, J.V.; Gilly, W.F. Diversity of conotoxin types from Conus californicus reflects a diversity of prey types and a novel evolutionary history. Toxicon 2011, 57, 311–322. [Google Scholar] [CrossRef]
- Möller, C.; Dovell, S.; Melaun, C.; Marí, F. Definition of the R-superfamily of conotoxins: Structural convergence of helix-loop-helix peptidic scaffolds. Peptides 2018, 107, 75–82. [Google Scholar] [CrossRef]
- Bernáldez-Sarabia, J.; Figueroa-Montiel, A.; Dueñas, S.; Cervantes-Luévano, K.; Beltrán, J.A.; Ortiz, E.; Jiménez, S.; Possani, L.D.; Paniagua-Solís, J.F.; Gonzalez-Canudas, J.; et al. The Diversified O-Superfamily in Californiconus californicus Presents a Conotoxin with Antimycobacterial Activity. Toxins 2019, 11, 128. [Google Scholar] [CrossRef] [PubMed]
- Puillandre, N.; Watkins, M.; Olivera, B.M. Evolution of Conus Peptide Genes: Duplication and Positive Selection in the A-Superfamily. J. Mol. Evol. 2010, 70, 190–202. [Google Scholar] [CrossRef]
- Nielsen, L.D.; Foged, M.M.; Albert, A.; Bertelsen, A.B.; Søltoft, C.L.; Robinson, S.D.; Petersen, S.V.; Purcell, A.W.; Olivera, B.M.; Norton, R.S.; et al. The three-dimensional structure of an H-superfamily conotoxin reveals a granulin fold arising from a common ICK cysteine. J. Biol. Chem. 2019, 294, 8745–8759. [Google Scholar] [CrossRef] [PubMed]
- Franco, A.; Dovell, S.; Möller, C.; Grandal, M.; Clark, E.; Marí, F. Structural plasticity of mini-M conotoxins—Expression of all mini-M subtypes by Conus regius. FEBS J. 2018, 285, 887–902. [Google Scholar] [CrossRef]
- Gupta, K.; Kumar, M.; Balaram, P. Disulfide bond assignments by mass spectrometry of native natural peptides: Cysteine pairing in disulfide bonded conotoxins. Anal. Chem. 2010, 82, 8313–8319. [Google Scholar] [CrossRef] [PubMed]
- Pi, C.; Liu, J.; Peng, C.; Liu, Y.; Jiang, X.; Zhao, Y.U.; Tang, S.; Wang, L.; Dong, M.; Chen, S.; et al. Diversity and evolution of conotoxins based on gene expression profiling of Conus litteratus. Genomics 2006, 88, 809–819. [Google Scholar] [CrossRef]
- Han, Y.; Wang, Q.I.; Jiang, H.; Liu, L.I.; Xiao, C.; Yuan, D.; Shao, X.; Dai, Q.; Cheng, J.-F.; Chi, C. Characterization of novel M-superfamily conotoxins with new disulfide linkage. FEBS J. 2006, 273, 4972–4982. [Google Scholar] [CrossRef]
- Dobson, R.; Collodoro, M.; Gilles, N.; Turtoi, A.; De Pauw, E.; Quinton, L. Secretion and maturation of conotoxins in the venom ducts of Conus textile. Toxicon 2012, 60, 1370–1379. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.; Kil, Y.J.; Ueberheide, B.; Chait, B.T.; Tayo, L.; Cruz, L.; Lu, B.; Yates, J.R.; Bern, M. Constrained de novo sequencing of conotoxins. J. Proteome Res. 2012, 11, 4191–4200. [Google Scholar] [CrossRef]
- Safavi-Hemami, H.; Siero, W.A.; Kuang, Z.; Williamson, N.A.; Karas, J.A.; Page, L.R.; MacMillan, D.; Callaghan, B.; Kompella, S.N.; Adams, D.J.; et al. Embryonic toxin expression in the cone snail Conus victoriae: Primed to kill or divergent function? J. Biol. Chem. 2011, 286, 22546–22557. [Google Scholar] [CrossRef]
- Myers, R.A.; Zafaralla, G.; Gray, W.R.; Abbott, J.A.; Cruz, L.J.; Olivera, B.M. Alpha-Conotoxins, small peptide probes of nicotinic acetylcholine receptors. Biochemistry 1991, 30, 9370–9377. [Google Scholar] [CrossRef]
- Han, Y.-H.; Wang, Q.I.; Jiang, H.; Miao, X.-W.; Chen, J.-S.; Chi, C.-W. Sequence diversity of T-superfamily conotoxins from Conus marmoreus. Toxicon 2005, 45, 481–487. [Google Scholar] [CrossRef]
- Robinson, S.D.; Safavi-Hemami, H.; McIntosh, L.D.; Purcell, A.W.; Norton, R.S.; Papenfuss, A.T. Diversity of Conotoxin Gene Superfamilies in the Venomous Snail, Conus victoriae. PLoS ONE 2014, 9, e087648. [Google Scholar] [CrossRef] [PubMed]
- Lavergne, V.; Dutertre, S.; Jin, A.-H.; Lewis, R.J.; Taft, R.J.; Alewood, P.F. Systematic interrogation of the Conus marmoreus venom duct transcriptome with ConoSorter reveals 158 novel conotoxins and 13 new gene superfamilies. BMC Genom. 2013, 14, 708. [Google Scholar] [CrossRef]
- Jin, A.; Dekan, Z.; Smout, M.J.; Wilson, D.; Dutertre, S.; Vetter, I.; Lewis, R.J.; Loukas, A.; Daly, N.L.; Alewood, P.F. Conotoxin Φ-MiXXVIIA from the Superfamily G2 Employs a Novel Cysteine Framework That Mimics Granulin and Displays Anti-Apoptotic Activity. Angew. Chem. Int. Ed. 2017, 56, 14973–14976. [Google Scholar] [CrossRef]
- Jin, A.-H.; Israel, M.R.; Inserra, M.C.; Smith, J.J.; Lewis, R.J.; Alewood, P.F.; Vetter, I.; Dutertre, S. δ-Conotoxin SuVIA Suggests an Evolutionary Link between Ancestral Predator Defence and the Origin of Fish-Hunting Behaviour in Carnivorous Cone Snails. Proc. R. Soc. B 2015, 282, 20150817. [Google Scholar] [CrossRef]
- Yanagawa, Y.; Abe, T.; Satake, M.; Odani, S.; Suzuki, J.; Ishikawa, K. A Novel Sodium Channel Inhibitor from Conus Geographus: Purification, Structure, and Pharmacological Properties. Biochemistry 1988, 27, 6256–6262. [Google Scholar] [CrossRef] [PubMed]
- Kauferstein, S.; Huys, I.; Kuch, U.; Melaun, C.; Tytgat, J.; Mebs, D. Novel Conopeptides of the I-Superfamily Occur in Several Clades of Cone Snails. Toxicon 2004, 44, 539–548. [Google Scholar] [CrossRef]
- Espino, S.S.; Dilanyan, T.; Imperial, J.S.; Aguilar, M.B.; Teichert, R.W.; Bandyopadhyay, P.; Olivera, B.M. Glycine-Rich Conotoxins from the Virgiconus Clade. Toxicon 2016, 113, 11–17. [Google Scholar] [CrossRef]
- Himaya, S.W.A.; Jin, A.-H.; Dutertre, S.; Giacomotto, J.; Mohialdeen, H.; Vetter, I.; Alewood, P.F.; Lewis, R.J. Comparative Venomics Reveals the Complex Prey Capture Strategy of the Piscivorous Cone Snail Conus Catus. J. Proteome Res. 2015, 14, 4372–4381. [Google Scholar] [CrossRef] [PubMed]
- Gowd, K.H.; Dewan, K.K.; Iengar, P.; Krishnan, K.S.; Balaram, P. Probing Peptide Libraries from Conus Achatinus Using Mass Spectrometry and cDNA Sequencing: Identification of δ and Ω-conotoxins. J. Mass Spectrom. 2008, 43, 791–805. [Google Scholar] [PubMed]
- Tran, H.N.T.; McMahon, K.L.; Deuis, J.R.; Vetter, I.; Schroeder, C.I. Structural and Functional Insights into the Inhibition of Human Voltage-Gated Sodium Channels by μ-Conotoxin KIIIA Disulfide Isomers. J. Biol. Chem. 2022, 298, 101728. [Google Scholar] [PubMed]
- Giribaldi, J.; Wilson, D.; Nicke, A.; El Hamdaoui, Y.; Laconde, G.; Faucherre, A.; Moha Ou Maati, H.; Daly, N.L.; Enjalbal, C.; Dutertre, S. Synthesis, Structure and Biological Activity of CIA and CIB, Two α-Conotoxins from the Predation-Evoked Venom of Conus catus. Toxins 2018, 10, 222. [Google Scholar] [CrossRef]
- Aguilar, M.B.; Lezama-Monfil, L.; Maillo, M.; Pedraza-Lara, H.; López-Vera, E.; Heimer De La Cotera, E.P. A Biologically Active Hydrophobic T-1-Conotoxin from the Venom of Conus spurius. Peptides 2006, 27, 500–505. [Google Scholar] [CrossRef]
- Dutt, M.; Dutertre, S.; Jin, A.; Lavergne, V.; Alewood, P.F.; Lewis, R.J. Venomics Reveals Venom Complexity of the Piscivorous Cone Snail, Conus tulipa. Mar. Drugs 2019, 17, 71. [Google Scholar] [CrossRef]
- Ziegman, R.; Brust, A.; Jha, P.; Cardoso, F.C.; Lewis, R.J.; Alewood, P.F. ‘Messy’ Processing of χ-Conotoxin MrIA Generates Homologues with Reduced hNET Potency. Mar. Drugs 2019, 17, 165. [Google Scholar] [CrossRef]
- Loughnan, M.L.; Nicke, A.; Jones, A.; Adams, D.J.; Alewood, P.F.; Lewis, R.J. Chemical and Functional Identification and Characterization of Novel Sulfated α-Conotoxins from the Cone Snail Conus a Nemone. J. Med. Chem. 2004, 47, 1234–1241. [Google Scholar] [CrossRef]
- Bao, N.; Lecaer, J.-P.; Nghia, N.D.; Vinh, P.T.K. Isolation and Structural Identification of a New T1-Conotoxin with Unique Disulfide Connectivities Derived from Conus bandanus. J. Venom. Anim. Toxins Incl. Trop. Dis. 2020, 26, e20190095. [Google Scholar] [CrossRef]
- Nguyen, B.; Le Caer, J.-P.; Aráoz, R.; Thai, R.; Lamthanh, H.; Benoit, E.; Molgó, J. Isolation, Purification and Functional Characterization of Alpha-BnIA from Conus bandanus Venom. Toxicon 2014, 91, 155–163. [Google Scholar] [CrossRef]
- Nguyen, B.; Caer, J.-P.; Mourier, G.; Thai, R.; Lamthanh, H.; Servent, D.; Benoit, E.; Molgó, J. Characterization of a Novel Conus bandanus Conopeptide Belonging to the M-Superfamily Containing Bromotryptophan. Mar. Drugs 2014, 12, 3449–3465. [Google Scholar] [CrossRef]
- Sharpe, I.A.; Palant, E.; Schroeder, C.I.; Kaye, D.M.; Adams, D.J.; Alewood, P.F.; Lewis, R.J. Inhibition of the Norepinephrine Transporter by the Venom Peptide χ-MrIA. J. Biol. Chem. 2003, 278, 40317–40323. [Google Scholar] [CrossRef]
- Hansson, K.; Ma, X.; Eliasson, L.; Czerwiec, E.; Furie, B.; Furie, B.C.; Rorsman, P.; Stenflo, J. The First γ-Carboxyglutamic Acid-Containing Contryphan. J. Biol. Chem. 2004, 279, 32453–32463. [Google Scholar] [CrossRef] [PubMed]
- Pardos-Blas, J.R.; Tenorio, M.J.; Galindo, J.C.G.; Zardoya, R. Comparative Venomics of the Cryptic Cone Snail Species Virroconus ebraeus and Virroconus judaeus. Mar. Drugs 2022, 20, 149. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kool, J.; Arrahman, A.; Xu, H.; Liu, J.; Lewis, R.J.; Tudorache, C.; Cardoso, F.C. Profiling the Paralytic Effects and Lethality of Cone Snail Venom Toxins Using Nanofractionation Analytics with In Vivo Zebrafish Larvae Assays. Toxins 2025, 17, 504. https://doi.org/10.3390/toxins17100504
Kool J, Arrahman A, Xu H, Liu J, Lewis RJ, Tudorache C, Cardoso FC. Profiling the Paralytic Effects and Lethality of Cone Snail Venom Toxins Using Nanofractionation Analytics with In Vivo Zebrafish Larvae Assays. Toxins. 2025; 17(10):504. https://doi.org/10.3390/toxins17100504
Chicago/Turabian StyleKool, Jeroen, Arif Arrahman, Haifeng Xu, Jiaxing Liu, Richard J. Lewis, Christian Tudorache, and Fernanda C. Cardoso. 2025. "Profiling the Paralytic Effects and Lethality of Cone Snail Venom Toxins Using Nanofractionation Analytics with In Vivo Zebrafish Larvae Assays" Toxins 17, no. 10: 504. https://doi.org/10.3390/toxins17100504
APA StyleKool, J., Arrahman, A., Xu, H., Liu, J., Lewis, R. J., Tudorache, C., & Cardoso, F. C. (2025). Profiling the Paralytic Effects and Lethality of Cone Snail Venom Toxins Using Nanofractionation Analytics with In Vivo Zebrafish Larvae Assays. Toxins, 17(10), 504. https://doi.org/10.3390/toxins17100504







