Derivation of Human Toxicokinetic Parameters and Chemical-Specific Adjustment Factor of Citrinin Through a Human Intervention Trial and Hierarchical Bayesian Population Modeling
Abstract
1. Introduction
2. Results and Discussion
2.1. Sample Preparation and UHPLC-MS/MS Method
2.1.1. Method Validation
2.1.2. Matrix Effect
2.1.3. Stability
2.2. Toxicokinetic Modeling
2.2.1. TK Profiles and Deterministic Model
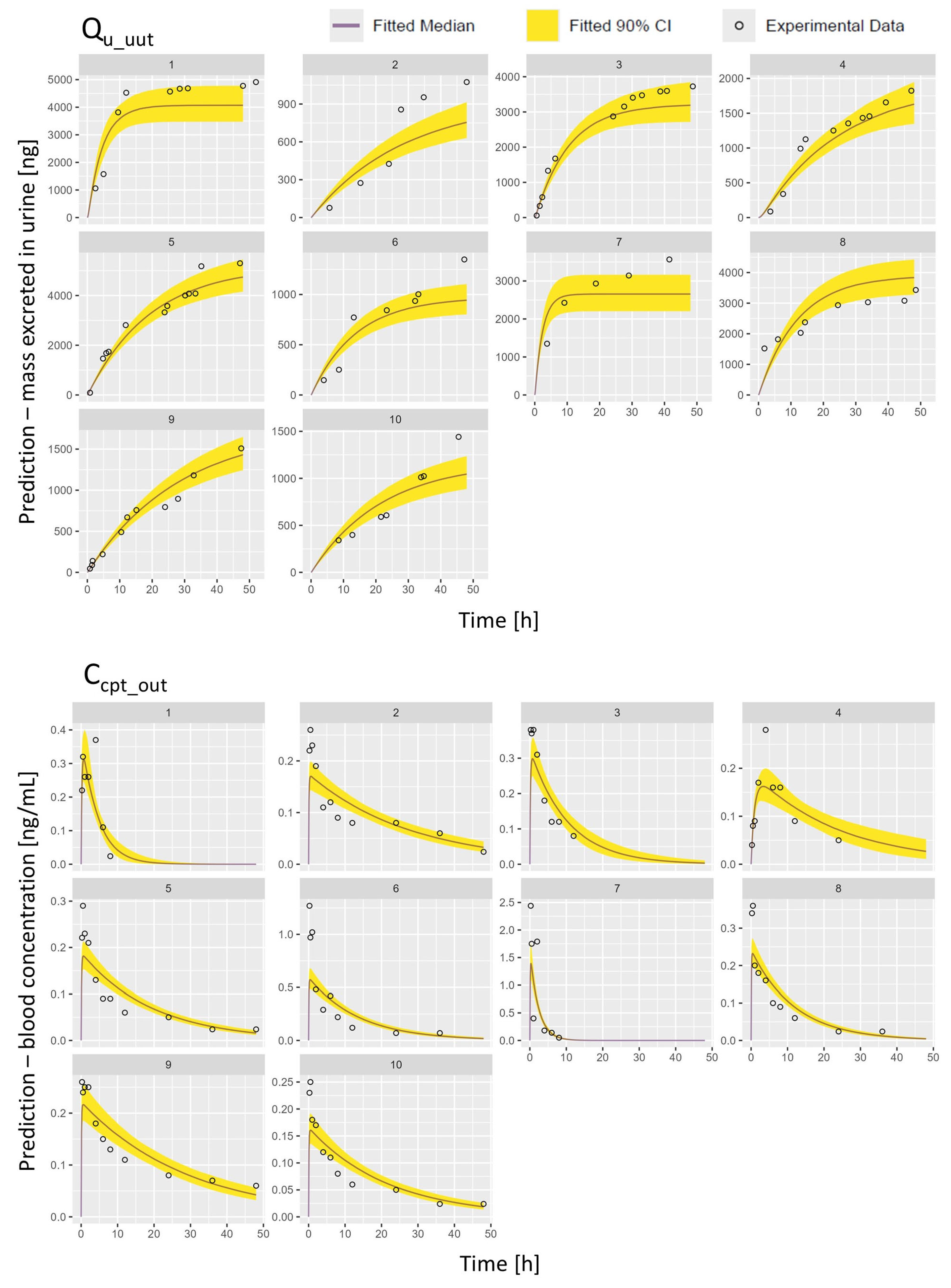
2.2.2. PopTK Model Fit and Posterior Predictions
3. Limitations, Strengths, and Conclusions
4. Materials and Methods
4.1. Materials
4.2. Human Intervention Trial
4.3. Sample Preparation
4.3.1. Whole Blood (VAMS Mitra® Tips)
4.3.2. Urine
4.3.3. Feces
4.4. UHPLC-MS/MS Analysis and Method Validation
4.5. PopTK Modeling
4.5.1. Hierarchical Bayesian Population Model
4.5.2. Model Fit and Predictions for Human Toxicokinetics
4.6. Adjustment Factor for Human Variability in Toxicokinetics (HKAF)
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 13C13-CIT | Isotopically labelled citrinin |
| ADME | Absorption, distribution, metabolization, excretion |
| AUC | Area under the blood concentration-time curve |
| bw | Body weight |
| Ccpt_out | Citrinin concentration in the central compartment (blood flow) |
| Ccpt_met_out | Metabolites concentration in the central compartment (blood flow) |
| CE | Collision energy |
| CI | Confidence interval |
| CIT | Citrinin |
| Cltot | Clearance of citrinin |
| Clmet | Clearance of citrinin’s metabolites |
| Cmax | Maximum blood concentration |
| CSAF | Chemical-specific adjustment factors |
| CV | Coefficient of variation |
| EFSA | European Food Safety Authority |
| ESI | Electrospray ionization |
| EU | European Union |
| Fgutabs | Fraction absorbed via the gastrointestinal tract |
| GI | Gastrointestinal |
| GM | Geometric mean |
| GSD | Geometric standard deviation |
| HBM | Human biomonitoring |
| HBGV | Human biomonitoring guidance values |
| HO-CIT | Dihydrocitrinone |
| HKAF | Human inter-individual toxicokinetic variability |
| IARC | International Agency for Research on Cancer |
| ICF | Informed consent form |
| ICH | International Council for Harmonisation |
| IS | Internal standard |
| kel | Total elimination rate of citrinin |
| kmet | Metabolization rate |
| kgutelim | Gut elimination rate |
| kgutabs | Gut absorption rate |
| ku | Urinary excretion rate |
| kufrac | Fraction excreted in urinec |
| kumet | Urinary excretion rate of the metabolite |
| LLOQ | Lower Limit of Quantification |
| LOD | Limit of Detection |
| MCMC | Markov chain Monte Carlo |
| ME | Matrix effect |
| mp | Mobile phase |
| MS | Mass spectrometry/spectrometer |
| MRM | Multiple reaction monitoring |
| m/z | Mass-to-charge ratio |
| NOAEL | No-observed-adverse-effect level |
| PopTK | Population toxicokinetics |
| Q | Quadrupole |
| Q_fec_out | Amount of citrinin excreted via the GI tract |
| Qu_met_out | Amount of citrinin’s metabolites excreted in urine |
| Qu_out | Amount of citrinin excreted in urine |
| Ȓ | R-hat convergence diagnostic |
| RA | Apparent recovery |
| RE | Extraction efficiency |
| ROS | Reactive oxygen species |
| RSDR | Intermediate precision |
| RSDr | Repeatability |
| Rt | Retention time |
| SALLE | Salting-out assisted liquid–liquid Extraction |
| SSE | Signal suppression enhancement |
| t1/2 | Half-life |
| TK | Toxicokinetic |
| tmax | Time to reach Cmax |
| ULOQ | Upper Limit of Quantification |
| UHPLC | Ultra-high performance liquid chromatography |
| VAMS | Volumetric absorptive microsampling |
| Vdist | Volume of distribution of citrinin |
| Vdistmet | Volume of distribution of citrinin’s metabolites |
References
- Haque, M.A.; Wang, Y.; Shen, Z.; Li, X.; Saleemi, M.K.; He, C. Mycotoxin Contamination and Control Strategy in Human, Domestic Animal and Poultry: A Review. Microb. Pathog. 2020, 142, 104095. [Google Scholar] [CrossRef]
- Flajs, D.; Peraica, M. Toxicological Properties of Citrinin. Arch. Ind. Hyg. Toxicol. 2009, 60, 457–464. [Google Scholar] [CrossRef]
- Ali, N.; Degen, G.H. Citrinin Biomarkers: A Review of Recent Data and Application to Human Exposure Assessment. Arch. Toxicol. 2019, 93, 3057–3066. [Google Scholar] [CrossRef]
- Chen, C.-C.; Chan, W.-H. Inhibition of Citrinin-Induced Apoptotic Biochemical Signaling in Human Hepatoma G2 Cells by Resveratrol. Int. J. Mol. Sci. 2009, 10, 3338–3357. [Google Scholar] [CrossRef]
- Chan, W.-H. Citrinin Induces Apoptosis via a Mitochondria-Dependent Pathway and Inhibition of Survival Signals in Embryonic Stem Cells, and Causes Developmental Injury in Blastocysts. Biochem. J. 2007, 404, 317–326. [Google Scholar] [CrossRef]
- Chan, W. Citrinin Induces Apoptosis in Mouse Embryonic Stem Cells. IUBMB Life 2008, 60, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Chagas, G.M.; Klüppel, M.L.W.; Campello, A.d.P.; Buchi, D.d.F.; de Oliveira, M.B.M. Alterations Induced by Citrinin in Cultured Kidney Cells. Cell Struct. Funct. 1994, 19, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Chagas, G.M.; Oliveira, M.B.M.; Campello, A.P.; Klüppel, M.L.W. Mechanism of Citrinin-induced Dysfunction of Mitochondria Iii. Effects on Renal Cortical and Liver Mitochondrial Swelling. J. Appl. Toxicol. 1995, 15, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Aydin, Y.; Orta Yilmaz, B.; Yildizbayrak, N.; Korkut, A.; Arabul Kursun, M.; Irez, T.; Erkan, M. Evaluation of Citrinin-Induced Toxic Effects on Mouse Sertoli Cells. Drug Chem. Toxicol. 2021, 44, 559–565. [Google Scholar] [CrossRef]
- Sun, M.-H.; Li, X.-H.; Xu, Y.; Xu, Y.; Pan, Z.-N.; Sun, S.-C. Citrinin Exposure Disrupts Organelle Distribution and Functions in Mouse Oocytes. Environ. Res. 2020, 185, 109476. [Google Scholar] [CrossRef]
- Zargar, S.; Wani, T.A. Food Toxicity of Mycotoxin Citrinin and Molecular Mechanisms of Its Potential Toxicity Effects through the Implicated Targets Predicted by Computer-Aided Multidimensional Data Analysis. Life 2023, 13, 880. [Google Scholar] [CrossRef]
- de Oliveira Filho, J.W.G.; Islam, M.T.; Ali, E.S.; Uddin, S.J.; Santos, J.V.d.O.; de Alencar, M.V.O.B.; Júnior, A.L.G.; Paz, M.F.C.J.; de Brito, M.d.R.M.; e Sousa, J.M.d.C.; et al. A Comprehensive Review on Biological Properties of Citrinin. Food Chem. Toxicol. 2017, 110, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Phillips, R.D.; Berndt, W.O.; Hayes, A.W. Distribution and Excretion of [14C]Citrinin in Rats. Toxicology 1979, 12, 285–298. [Google Scholar] [CrossRef] [PubMed]
- CONTAM Panel. Scientific Opinion on the Risks for Public and Animal Health Related to the Presence of Citrinin in Food and Feed. EFSA J. 2012, 10, 2605. [Google Scholar] [CrossRef]
- IARC Monographs. Available online: https://monographs.iarc.fr/agents-classified-by-the-iarc/ (accessed on 24 October 2024).
- Degen, G.H.; Ali, N.; Gundert-Remy, U. Preliminary Data on Citrinin Kinetics in Humans and Their Use to Estimate Citrinin Exposure Based on Biomarkers. Toxicol. Lett. 2018, 282, 43–48. [Google Scholar] [CrossRef]
- Degen, G.H.; Reinders, J.; Kraft, M.; Völkel, W.; Gerull, F.; Burghardt, R.; Sievering, S.; Engelmann, J.; Chovolou, Y.; Hengstler, J.G.; et al. Citrinin Exposure in Germany: Urine Biomarker Analysis in Children and Adults. Toxins 2023, 15, 26. [Google Scholar] [CrossRef]
- Meerpoel, C.; Vidal, A.; Andjelkovic, M.; De Boevre, M.; Tangni, E.K.; Huybrechts, B.; Devreese, M.; Croubels, S.; De Saeger, S. Dietary Exposure Assessment and Risk Characterization of Citrinin and Ochratoxin A in Belgium. Food Chem. Toxicol. 2021, 147, 111914. [Google Scholar] [CrossRef]
- Heyndrickx, E.; Sioen, I.; Huybrechts, B.; Callebaut, A.; De Henauw, S.; De Saeger, S. Human Biomonitoring of Multiple Mycotoxins in the Belgian Population: Results of the BIOMYCO Study. Environ. Int. 2015, 84, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Huybrechts, B.; Martins, J.C.; Debongnie, P.; Uhlig, S.; Callebaut, A. Fast and Sensitive LC–MS/MS Method Measuring Human Mycotoxin Exposure Using Biomarkers in Urine. Arch. Toxicol. 2015, 89, 1993–2005. [Google Scholar] [CrossRef]
- Ali, N.; Blaszkewicz, M.; Mohanto, N.C.; Rahman, M.; Alim, A.; Hossain, K.; Degen, G.H. First Results on Citrinin Biomarkers in Urines from Rural and Urban Cohorts in Bangladesh. Mycotoxin Res. 2015, 31, 9–16. [Google Scholar] [CrossRef]
- Warensjö Lemming, E.; Montano Montes, A.; Schmidt, J.; Cramer, B.; Humpf, H.-U.; Moraeus, L.; Olsen, M. Mycotoxins in Blood and Urine of Swedish Adolescents—Possible Associations to Food Intake and Other Background Characteristics. Mycotoxin Res. 2020, 36, 193–206. [Google Scholar] [CrossRef] [PubMed]
- Narváez, A.; Izzo, L.; Rodríguez-Carrasco, Y.; Ritieni, A. Citrinin Dietary Exposure Assessment Approach through Human Biomonitoring High-Resolution Mass Spectrometry-Based Data. J. Agric. Food Chem. 2021, 69, 6330–6338. [Google Scholar] [CrossRef]
- Ali, N.; Blaszkewicz, M.; Degen, G.H. Occurrence of the Mycotoxin Citrinin and Its Metabolite Dihydrocitrinone in Urines of German Adults. Arch. Toxicol. 2015, 89, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.; Blaszkewicz, M.; Alim, A.; Hossain, K.; Degen, G.H. Urinary Biomarkers of Ochratoxin A and Citrinin Exposure in Two Bangladeshi Cohorts: Follow-up Study on Regional and Seasonal Influences. Arch. Toxicol. 2016, 90, 2683–2697. [Google Scholar] [CrossRef] [PubMed]
- Njumbe Ediage, E.; Diana Di Mavungu, J.; Song, S.; Wu, A.; Van Peteghem, C.; De Saeger, S. A Direct Assessment of Mycotoxin Biomarkers in Human Urine Samples by Liquid Chromatography Tandem Mass Spectrometry. Anal. Chim. Acta 2012, 741, 58–69. [Google Scholar] [CrossRef]
- Ali, N.; Blaszkewicz, M.; Manirujjaman, M.; Degen, G.H. Biomonitoring of Concurrent Exposure to Ochratoxin A and Citrinin in Pregnant Women in Bangladesh. Mycotoxin Res. 2016, 32, 163–172. [Google Scholar] [CrossRef]
- Rezvanfar, M.A. Chemical-Specific Adjustment Factors for Interspecies Differences and Human Variability: Guidance Document for Use of Data in Dose/Concentration–Response Assessment, 3rd ed.; WHO/IPCS: Geneva, Switzerland, 2005; ISBN 9780123864543. [Google Scholar]
- Bois, F.Y.; Jamei, M.; Clewell, H.J. PBPK Modelling of Inter-Individual Variability in the Pharmacokinetics of Environmental Chemicals. Toxicology 2010, 278, 256–267. [Google Scholar] [CrossRef]
- Allen, B.C.; Hack, C.E.; Clewell, H.J. Use of Markov Chain Monte Carlo Analysis with a Physiologically-Based Pharmacokinetic Model of Methylmercury to Estimate Exposures in U.S. Women of Childbearing Age. Risk Anal. 2007, 27, 947–959. [Google Scholar] [CrossRef]
- Chiu, W.A.; Okino, M.S.; Evans, M.V. Characterizing Uncertainty and Population Variability in the Toxicokinetics of Trichloroethylene and Metabolites in Mice, Rats, and Humans Using an Updated Database, Physiologically Based Pharmacokinetic (PBPK) Model, and Bayesian Approach. Toxicol. Appl. Pharmacol. 2009, 241, 36–60. [Google Scholar] [CrossRef]
- Desharnais, B.; Camirand-Lemyre, F.; Mireault, P.; Skinner, C.D. Procedure for the Selection and Validation of a Calibration Model I-Description and Application. J. Anal. Toxicol. 2017, 41, 261–268. [Google Scholar] [CrossRef]
- The European Commission. Commission Implementing Regulation (EU) 2021/808 of 22 March 2021 on the Performance of Analytical Methods for Residues of Pharmacologically Active Substances Used in Food-Producing Animals and on the Interpretation of Results as Well as on the Methods to Be Used for Sampling and Repealing Decisions 2002/657/EC and 98/179/EC; The European Commission: Brussels, Belgium, 2021. [Google Scholar]
- European Medicines Agency. Bioanalytical Method Validation M10. Sci. Med. Health 2019, 44, 6, 7–20, 40–41, 49–57.
- Alladio, E.; Amante, E.; Bozzolino, C.; Seganti, F.; Salomone, A.; Vincenti, M.; Desharnais, B. Effective Validation of Chromatographic Analytical Methods: The Illustrative Case of Androgenic Steroids. Talanta 2020, 215, 120867. [Google Scholar] [CrossRef]
- Sulyok, M.; Berthiller, F.; Krska, R.; Schuhmacher, R. Development and Validation of a Liquid Chromatography/Tandem Mass Spectrometric Method for the Determination of 39 Mycotoxins in Wheat and Maize. Rapid Commun. Mass Spectrom. 2006, 20, 2649–2659. [Google Scholar] [CrossRef]
- Peters, F.T.; Drummer, O.H.; Musshoff, F. Validation of New Methods. Forensic Sci. Int. 2007, 165, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.J.G.; Pereira, A.M.P.T.; Pena, A.; Lino, C.M. Citrinin in Foods and Supplements: A Review of Occurrence and Analytical Methodologies. Foods 2020, 10, 14. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.S.; Magnusson, B.M.; Burczynski, F.J.; Weiss, M. Enterohepatic Circulation. Clin. Pharmacokinet. 2002, 41, 751–790. [Google Scholar] [CrossRef] [PubMed]
- Gerding, J.; Ali, N.; Schwartzbord, J.; Cramer, B.; Brown, D.L.; Degen, G.H.; Humpf, H.U. A Comparative Study of the Human Urinary Mycotoxin Excretion Patterns in Bangladesh, Germany, and Haiti Using a Rapid and Sensitive LC-MS/MS Approach. Mycotoxin Res. 2015, 31, 127–136. [Google Scholar] [CrossRef]
- WHO/IPCS. Guidance Document on Evaluating and Expressing Uncertainty in Hazard Characterization, 2nd ed.; World Health Organization International Program on Chemical Safety: Geneva, Switzerland, 2018. [Google Scholar]
- Visintin, L.; Lu, E.-H.; Lin, H.-C.; Bader, Y.; Nguyen, T.N.; Michailidis, T.M.; De Saeger, S.; Chiu, W.A.; De Boevre, M. Derivation of Human Toxicokinetic Parameters and Internal Threshold of Toxicological Concern for Tenuazonic Acid through a Human Intervention Trial and Hierarchical Bayesian Population Modeling. J. Expo. Sci. Environ. Epidemiol. 2025, 35, 632–643. [Google Scholar] [CrossRef]
- Laparre, J.; Kaabia, Z.; Mooney, M.; Buckley, T.; Sherry, M.; Le Bizec, B.; Dervilly-Pinel, G. Impact of Storage Conditions on the Urinary Metabolomics Fingerprint. Anal. Chim. Acta 2017, 951, 99–107. [Google Scholar] [CrossRef]
- Ouhibi, S.; Vidal, A.; Martins, C.; Gali, R.; Hedhili, A.; De Saeger, S.; De Boevre, M. LC-MS/MS methodology for simultaneous determination of patulin and citrinin in urine and plasma applied to a pilot study in colorectal cancer patients. Food Chem. Toxicol. 2020, 136, 110994. [Google Scholar] [CrossRef]
- Puntscher, H.; Hankele, S.; Tillmann, K.; Attakpah, E.; Braun, D.; Kütt, M.-L.; Del Favero, G.; Aichinger, G.; Pahlke, G.; Höger, H.; et al. First Insights into Alternaria Multi-Toxin in Vivo Metabolism. Toxicol. Lett. 2019, 301, 168–178. [Google Scholar] [CrossRef] [PubMed]
- European Union. European Commission Commission Implementing Regulation (EU) 2023/2782 of 14 December 2023 down the Methods of Sampling and Analysis for the Control of the Levels of Mycotoxins in food and Repealing Regulation (EC) No 401/2006. Off. J. Eur. Union 2023, 2782, 1–44. [Google Scholar]
- ISO 5725-1:2023; International Organization for Standardization Accuracy (Trueness and Precision) of Measurement Methods and Results. ISO: Geneva, Switzerland, 2023.
- Di Bucchianico, A. Coefficient of Determination (R 2). In Encyclopedia of Statistics in Quality and Reliability; Wiley: Hoboken, NJ, USA, 2007. [Google Scholar]
- Vidal, A.; Belova, L.; Stove, C.; De Boevre, M.; De Saeger, S.; Vidal, A.; Belova, L.; Stove, C.; De Boevre, M.; De Saeger, S. Volumetric Absorptive Microsampling as an Alternative Tool for Biomonitoring of Multi-Mycotoxin Exposure in Resource-Limited Areas. Toxins 2021, 13, 345. [Google Scholar] [CrossRef] [PubMed]
- Lu, E.H.; Grimm, F.A.; Rusyn, I.; De Saeger, S.; De Boevre, M.; Chiu, W.A. Advancing Probabilistic Risk Assessment by Integrating Human Biomonitoring, New Approach Methods, and Bayesian Modeling: A Case Study with the Mycotoxin Deoxynivalenol. Environ. Int. 2023, 182, 108326. [Google Scholar] [CrossRef]
- Gelman, A.; Bois, F.; Jiang, J. Physiological Pharmacokinetic Analysis Using Population Modeling and Informative Prior Distributions. J. Am. Stat. Assoc. 1996, 91, 1400–1412. [Google Scholar] [CrossRef]
- Meerpoel, C.; Vidal, A.; Huybrechts, B.; Tangni, E.K.; De Saeger, S.; Croubels, S.; Devreese, M. Comprehensive Toxicokinetic Analysis Reveals Major Interspecies Differences in Absorption, Distribution and Elimination of Citrinin in Pigs and Broiler Chickens. Food Chem. Toxicol. 2020, 141, 111365. [Google Scholar] [CrossRef]
- Bois, F.Y. GNU MCSim: Bayesian Statistical Inference for SBML-Coded Systems Biology Models. Bioinformatics 2009, 25, 1453–1454. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R Foundation for Statistical Computing, Vienna, Austria. R: A Language and Environment for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 15 October 2024).
- Gelman, A.; Carlin, J.B.; Stern, H.S.; Dunson, D.B.; Vehtari, A.; Rubin, D.B. Bayesian Data Analysis, 3rd ed.; Chapman & Hall/CRC Texts in Statistical Science; Taylor & Francis: Abingdon, UK, 2013; ISBN 9781439840955. [Google Scholar]
- WHO-IPCS. Assessing Human Health Risks of Chemicals: Derivation of Guidance Values for Health- Based Exposure Limits; Vsemirnaia Organizatsiia Zdravookhraneniia: Geneve, Switzerland, 1994. [Google Scholar]




| Matrix | R2 | LOD | Calibration Range | SSE | RA | Re | Intra-Day Bias | Inter-Day Bias | RSDr | RSDR | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ng/mL * | LLOQ-ULOQ | % | Without IS | With IS | % | LLOQ | ULOQ | LLOQ | ULOQ | LLOQ | ULOQ | LLOQ | ULOQ | ||
| ng/mL * | % | % | % | % | % | % | % | % | % | % | |||||
| Urine | 0.9951 | 0.005 | 0.01–10 | 175.4 | 129.4 | 101.3 | 57.8 | 7.5 | 1.8 | 9.1 | 1.7 | 11.3 | 4.1 | 15.4 | 4.5 |
| Blood | 0.9926 | 0.027 | 0.05–2.5 | 113.1 | 122.7 | 94.7 | 108.5 | −9.4 | −0.1 | −9.3 | 0.22 | 14.6 | 9.1 | 15.1 | 16.9 |
| Feces | 0.9989 | 0.002 | 0.01–5 | 47.7 | 36.5 | 93.0 | 76.6 | −0.1 | 0.3 | 1.1 | 0.3 | 5.7 | 2.5 | 5.9 | 2.4 |
| Mean Difference Urine (%) | Mean Difference Capillary Blood (%) | ||||||
|---|---|---|---|---|---|---|---|
| 0.01 ng/mL | 10 ng/mL | 0.01 ng/mL | 10 ng/mL | 0.1 ng/mL | 2.5 ng/mL | 0.1 ng/mL | 2.5 ng/mL |
| −20 °C 21 Days | −20 °C 21 Days | 4 °C 5 Days | 4 °C 5 Days | 4 °C 21 Days | 4 °C 21 Days | 20 °C 5 Days | 20 °C 5 Days |
| 15.00 | 0.10 | −1.73 | 0.03 | 6.87 | 14.12 | 4.84 | −8.55 |
| Parameter | Unit | Preliminary TK Parameters (Degen et al., 2018) [16] | Population Posterior Distributions Median [90% CI] | |
|---|---|---|---|---|
| GM | GSD | |||
| t1/2 | h | 7.5–13.8 | 9.33 [6.43–13.53] | 1.29 [1.20–1.52] |
| kel | h−1 | n.a. | 0.074 [0.051–0.108] | 1.29 [1.20–1.52] |
| Tmax * | h | n.a. | 0.63 [0.35–1.15] | 1.92 [1.60–2.93] |
| Cmax * | ng/mL | n.a. | 0.28 [0.15–0.52] | 1.99 [1.64–3.09] |
| AUC ** | ng/(L·kg bw) | n.a. | 3654.2 [2290.8–5829.1] | 1.49 [1.34–1.94] |
| Cltot | L/(h·kg bw) | 0.005–0.007 | 0.025 [0.020–0.030] | 1.51 [1.32–1.71] |
| Clmet | L/(h·kg bw) | n.a. | 0.033 [0.005–0.239] | 1.19 [0.98–1.45] |
| Vdist | L/kg bw | 0.052–0.123 | 0.330 [0.254–0.428] | 1.18 [0.95–1.47] |
| Vdistmet | L/kg bw | n.a. | 0.836 [0.136–5.079] | 1.72 [1.53–1.95] |
| Fgutabs | - | n.a. | 0.246 [0.093–0.651] | 1.17 [0.97–1.41] |
| kgutelim | h−1 | n.a. | 4.141 [2.565–6.686] | 1.96 [1.61–2.40] |
| kufrac | - | 0.076–0.456 | 0.351 [0.273–0.451] | 1.69 [1.49–1.92] |
| Analyte | Rt | Cone | [M+CH3OH-H]− m/z | CE | Product Ion m/z |
|---|---|---|---|---|---|
| (min) | (V) | (eV) | |||
| CIT | 7.30 | 30 | 281.30 | 25 | 249.20 (Q) |
| 15 | 205.15 * | ||||
| 13C13-CIT | 7.30 | 30 | 294.30 | 25 | 262.20 (Q) |
| 15 | 217.20 |
| Parameter | Description (Unit) | Central Value | Prior Distribution for Population Geometric Mean (Natural Logarithm) |
|---|---|---|---|
| Cltot | Total clearance of CIT (L/Kg·h) | 0.04 | LogNormal (−3.33, 1.15) |
| Clmet | Clearance of HO-CIT (L/Kg·h) | 0.04 | LogNormal (−3.33, 1.15) |
| Vdist | Volume of distribution of CIT (L/kg) | 0.90 | LogNormal (−0.10, 1.15) |
| Vdistmet | Volume of distribution of HO-CIT (L/kg) | 0.90 | LogNormal (−0.10, 1.15) |
| kufrac | Fraction of CIT eliminated in urine | 0.50 | TruncLogNormal (−0.7, 1.15, −4.61, −0.01) |
| kumet | Elimination rate of HO-CIT in urine (h−1) | 0.50 | LogNormal (−0.7, 1.15) |
| ktot | Total elimination rate of CIT (h−1) | 0.02 | LogNormal (−3.96, 1.15) |
| kgutelim | Gut elimination rate (h−1) | 0.50 | LogNormal (−0.7, 1.15) |
| Fgutabs | Fraction absorbed | 0.17 | TruncLogNormal (−1.76, 1.15, −2.3, 0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Visintin, L.; Martino, C.; De Saeger, S.; Alladio, E.; De Boevre, M.; Chiu, W.A. Derivation of Human Toxicokinetic Parameters and Chemical-Specific Adjustment Factor of Citrinin Through a Human Intervention Trial and Hierarchical Bayesian Population Modeling. Toxins 2025, 17, 382. https://doi.org/10.3390/toxins17080382
Visintin L, Martino C, De Saeger S, Alladio E, De Boevre M, Chiu WA. Derivation of Human Toxicokinetic Parameters and Chemical-Specific Adjustment Factor of Citrinin Through a Human Intervention Trial and Hierarchical Bayesian Population Modeling. Toxins. 2025; 17(8):382. https://doi.org/10.3390/toxins17080382
Chicago/Turabian StyleVisintin, Lia, Camilla Martino, Sarah De Saeger, Eugenio Alladio, Marthe De Boevre, and Weihsueh A. Chiu. 2025. "Derivation of Human Toxicokinetic Parameters and Chemical-Specific Adjustment Factor of Citrinin Through a Human Intervention Trial and Hierarchical Bayesian Population Modeling" Toxins 17, no. 8: 382. https://doi.org/10.3390/toxins17080382
APA StyleVisintin, L., Martino, C., De Saeger, S., Alladio, E., De Boevre, M., & Chiu, W. A. (2025). Derivation of Human Toxicokinetic Parameters and Chemical-Specific Adjustment Factor of Citrinin Through a Human Intervention Trial and Hierarchical Bayesian Population Modeling. Toxins, 17(8), 382. https://doi.org/10.3390/toxins17080382








