Case Series and Literature Review on Botulinum Toxin Efficacy in Axial Extensor Truncal Dystonia
Abstract
1. Introduction
2. Results
3. New Case Reports
3.1. Case No. 1
3.2. Case No. 2
3.3. Case No. 3
3.4. Case No. 4
4. Discussion
5. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Comella, C.L.; Shannon, K.M.; Jaglin, J. Extensor truncal dystonia: Successful treatment with botulinum toxin injections: Extensor Truncal Dystonia. Mov. Disord. Off. J. Mov. Disord. Soc. 1998, 13, 552–555. [Google Scholar] [CrossRef]
- Mehta, S.; Ray, S.; Chakravarty, K.; Lal, V. Spectrum of truncal dystonia and response to treatment: A retrospective analysis. Ann. Indian Acad. Neurol. 2020, 23, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Pana, A.; Saggu, B. Dystonia. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar] [PubMed]
- Tassorelli, C.; De Iccoa, R.; Alfonsib, E.; Bartoloe, M.; Serraof, M.; Avenalia, M.; De Paolia, I.; Conteg, C.; Pozzic, N.G.; Bramanti, P.; et al. Botulinum toxin type A potentiates the effect of Neuromotor Rehabilitation of Pisa syndrome in parkinson disease: A Placebo controlled study. Park. Relat. Disord. 2014, 20, 1140–1144. [Google Scholar] [CrossRef] [PubMed]
- Sławek, J.; Derejko, M.; Lass, P.; Dubaniewicz, M. Camptocormia or Pisa syndrome in multiple system atrophy. Clin. Neurol. Neurosurg. 2006, 108, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, D.J.; Frucht, S.J. The phenomenology and treatment of idiopathic adult-onset truncal dystonia: A retrospective review. J. Clin. Mov. Disord. 2016, 3, 15. [Google Scholar] [CrossRef]
- Simpson, D.M.; Hallett, M.; Ashman, E.J.; Comella, C.L.; Green, M.W.; Gronseth, G.S.; Armstrong, M.J.; Gloss, D.; Potrebic, S.; Jankovic, J.; et al. Practice guideline update summary: Botulinum neurotoxin for the treatment of blepharospasm, cervical dystonia, adult spasticity, and headache: Report of the Guideline Development Subcommittee of the American Academy of Neurology: Report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology 2016, 86, 1818–1826. [Google Scholar] [CrossRef]
- Mezaki, T.; Kaji, R.; Hamano, T.; Nagamine, T.; Shibasaki, H.; Shimizu, T.; Kimura, J. Optimisation of botulinum treatment for cervical and axial dystonias: Experience with a Japanese type A toxin. J. Neurol. Neurosurg. Psychiatry 1994, 57, 1535–1537. [Google Scholar] [CrossRef] [PubMed]
- Lagueny, A.; Burbaud, P.; Le Masson, G.; Bergouignan, F.X.; Ferrer, X.; Julien, J. Involvement of respiratory muscles in adult-onset dystonia: A clinical and electrophysiological study. Mov. Disord. Off. J. Mov. Disord. Soc. 1995, 10, 708–713. [Google Scholar] [CrossRef]
- Quirk, J.A.; Sheean, G.L.; Marsden, C.D.; Lees, A.J. Treatment of nonoccupational limb and trunk dystonia with botulinum toxin. Mov. Disord. Off. J. Mov. Disord. Soc. 1996, 11, 377–383. [Google Scholar] [CrossRef]
- Bonanni, L.; Thomas, A.; Varanese, S.; Scorrano, V.; Onofrj, M. Botulinum toxin treatment of lateral axial dystonia in Parkinsonism. Mov. Disord. Off. J. Mov. Disord. Soc. 2007, 22, 2097–2103. [Google Scholar] [CrossRef]
- Rosales, R.L.; Ng, A.R.; Santos, M.M.D.-D.; Fernandez, H.H. The broadening application of chemodenervation in X-linked dystonia-parkinsonism (Part II): An open-label experience with botulinum toxin-A (Dysport®) injections for oromandibular, lingual, and truncal-axial dystonias. Int. J. Neurosci. 2011, 121 (Suppl. S1), 44–56. [Google Scholar] [CrossRef]
- Voos, M.C.; de Paula Oliveira, T.; Piemonte, M.E.P.; Barbosa, E.R. Case report: Physical therapy management of axial dystonia. Physiother. Theory Pract. 2014, 30, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.; Lal, V. Neurodegeneration with brain iron accumulation: Two additional cases with dystonic opisthotonos. Tremor Other Hyperkinetic Mov. 2019, 9, 10-7916. [Google Scholar] [CrossRef]
- Hull, M.; Parnes, M.; Jankovic, J. Botulinum neurotoxin injections in childhood opisthotonos. Toxins 2021, 13, 137. [Google Scholar] [CrossRef] [PubMed]
- Kupsch, A.; Benecke, R.; Müller, J.; Trottenberg, T.; Schneider, G.-H.; Poewe, W.; Eisner, W.; Wolters, A.; Müller, J.-U.; Deuschl, G.; et al. Pallidal deep-brain stimulation in primary generalized or segmental dystonia. N. Engl. J. Med. 2006, 355, 1978–1990. [Google Scholar] [CrossRef]
- Albanese, A.; Asmus, F.; Bhatia, K.P.; Elia, A.E.; Elibol, B.; Filippini, G.; Gasser, T.; Krauss, J.K.; Nardocci, N.; Newton, A.; et al. EFNS guidelines on diagnosis and treatment of primary dystonias: EFNS dystonia guidelines. Eur. J. Neurol. Off. J. Eur. Fed. Neurol. Soc. 2011, 18, 5–18. [Google Scholar] [CrossRef]
- Aleid, A.; Aleid, M.; Alehaiwi, G.; Alharbi, H.; Alhuthayli, A.; Al Rebih, Z.M.; Alhumaidi, N.; Albashrawi, W.; Bazarah, R.S.; Alharbi, A.; et al. Advancements in the clinical outcomes of functional neurosurgery with deep brain stimulation for movement disorders: A literature review. Cureus 2023, 15, e40350. [Google Scholar] [CrossRef]
- Albanese, A.; Wissel, J.; Jost, W.H.; Castagna, A.; Althaus, M.; Comes, G.; Scheschonka, A.; Vacchelli, M.; Jinnah, H.A. Pain Reduction in Cervical Dystonia Following Treatment with IncobotulinumtoxinA: A Pooled Analysis. Toxins 2023, 15, 333. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jabbari, B.; Richardson, D. Treatment of stiff-person syndrome with botulinum toxin. In Manual of Botulinum Toxin Therapy; Trung, D., Dressler, D., Hallett, M., Eds.; Cambridge University Press: Cambridge, UK, 2009; pp. 189–194. [Google Scholar]
- Tyślerowicz, M.; Kiedrzyńska, W.; Adamkiewicz, B.; Jost, W.H.; Sławek, J. Cervical dystonia—Improving the effectiveness of botulinum toxin therapy. Neurol. Neurochir. Pol. 2020, 54, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Tyślerowicz, M.; Dulski, J.; Gawryluk, J.; Sławek, J. Does Ultrasound Guidance Improve the Effectiveness of Neurotoxin Injections in Patients with Cervical Dystonia? (A Prospective, Partially-Blinded, Clinical Study). Toxins 2022, 14, 674. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Livneh, V.; Faust-Socher, A.; Cohen, M.E.; Schechter, Y.; Israel, I.; Eichel, R.; Gurevich, T.; Yahalom, G. Comparison of Guided and Unguided Botulinum Injections for Cervical Dystonia: EMG, Ultrasound, and Anatomic Landmarks. CNS Neurol. Disord. Drug Targets 2025. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.D.; Yun, S.I.; Ryoo, D.; Chung, M.E.; Park, J. Accuracy of Ultrasound-Guided and Non-guided Botulinum Toxin Injection Into Neck Muscles Involved in Cervical Dystonia: A Cadaveric Study. Ann. Rehabil. Med. 2020, 44, 370–377. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Carda, S.; Reebye, R. A practical booklet for ultrasound-guided botulinum toxin injections. Toxicon 2025, 256, 108287. [Google Scholar] [CrossRef] [PubMed]
- Lungu, C.; Nmashie, A.; George, M.C.; Karp, B.I.; Alter, K.; Shin, S.; Tse, W.; Frucht, S.J.; Wu, T.; Koo, V.; et al. Comparison of Ultrasound and Electrical Stimulation Guidance for Onabotulinum Toxin-A Injections: A Randomized Crossover Study. Mov. Disord. Clin. Pract. 2022, 9, 1055–1061. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gandolfi, M.; Artusi, C.A.; Imbalzano, G.; Camozzi, S.; Crestani, M.; Lopiano, L.; Tinazzi, M.; Geroin, C. Botulinum Toxin for Axial Postural Abnormalities in Parkinson’s Disease: A Systematic Review. Toxins 2024, 16, 228. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
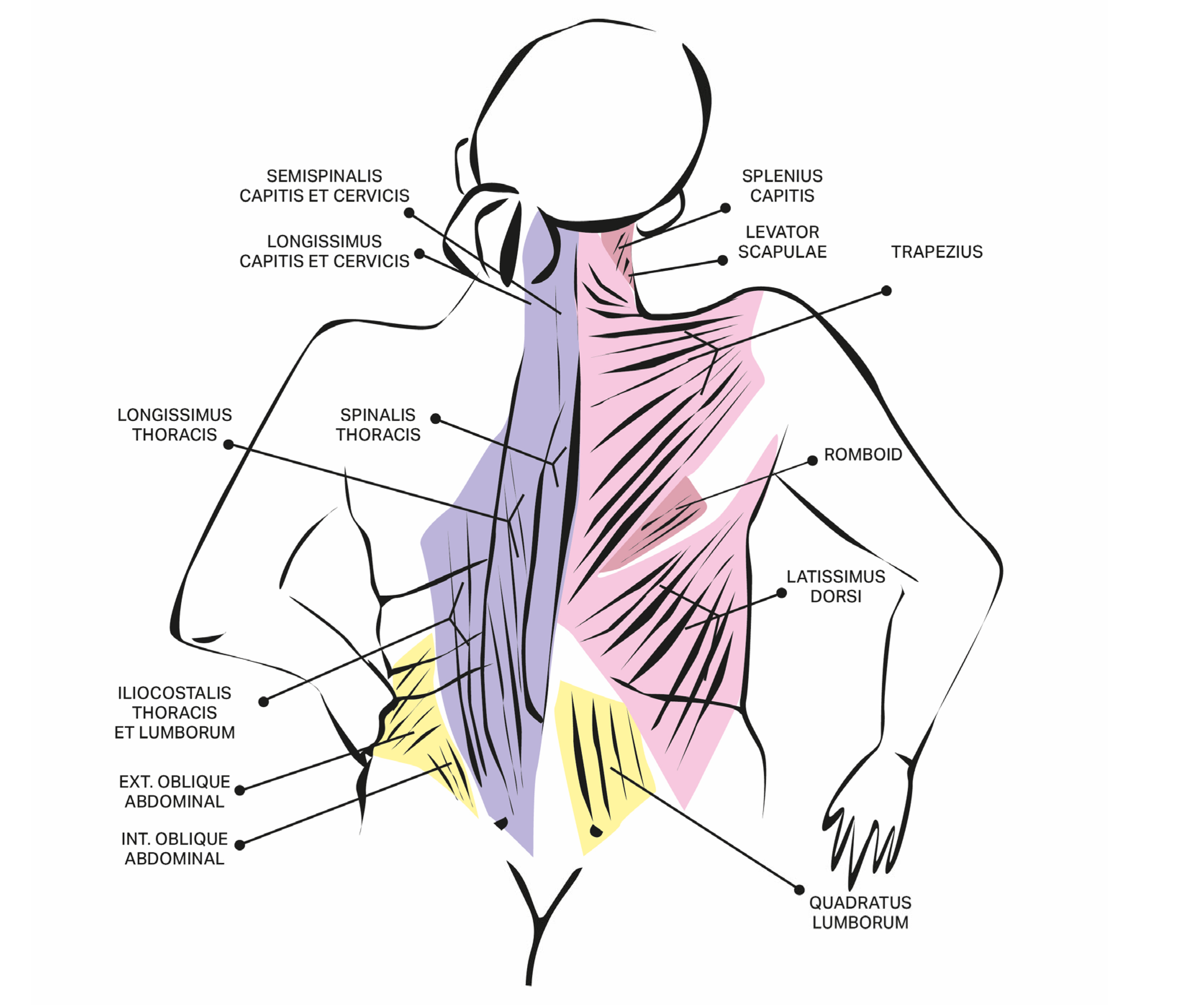

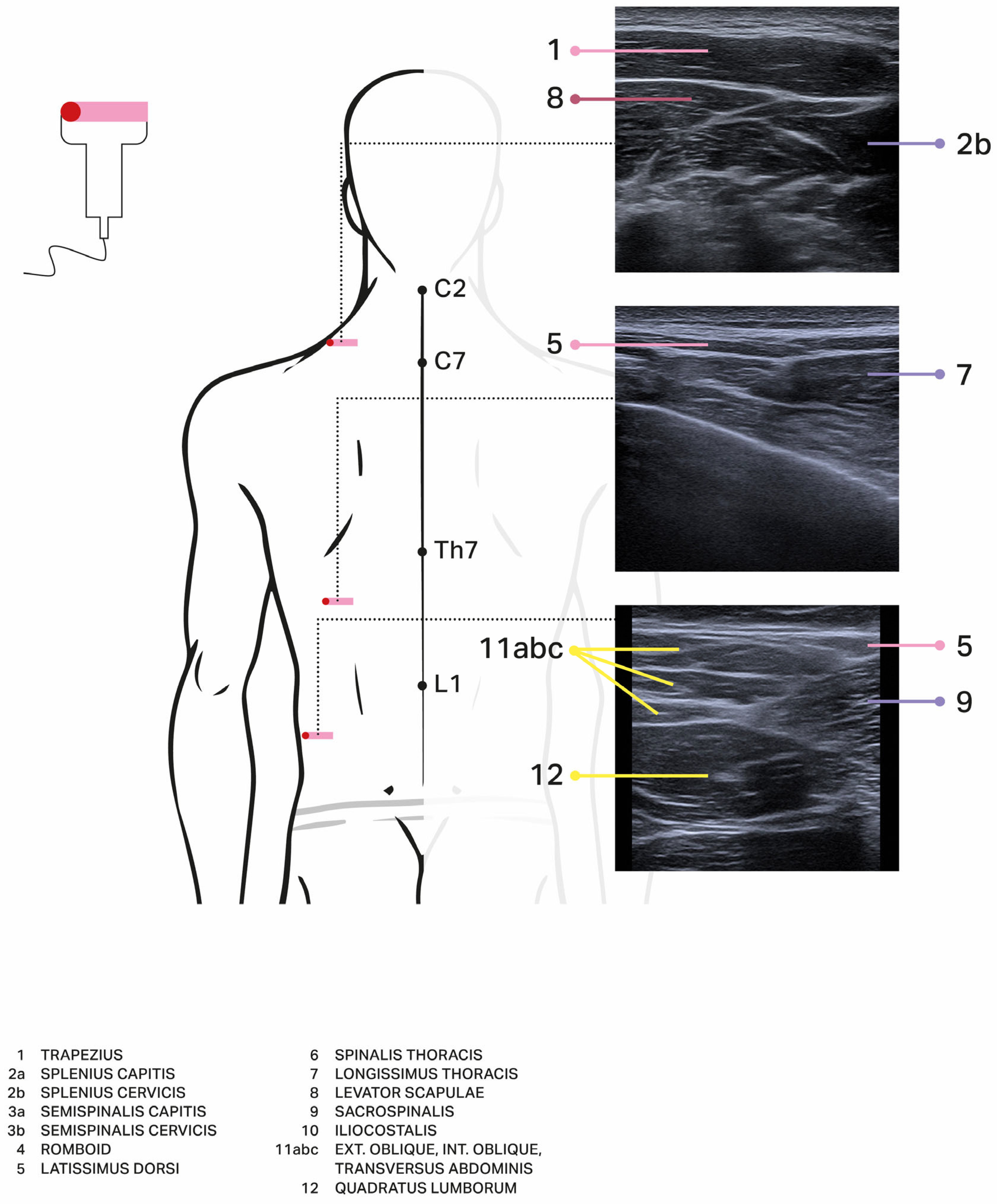
| Author, Year, Title | No of Patients with Extensor Trunk Dystonia in the Whole Group | Injected Muscles (as Specified by Authors) | Drug, Dosage [Units] | Clinical Effect (as Specified by Authors) | Adverse Effects | Method of Assessment/Scale | Etiology of Dystonia |
|---|---|---|---|---|---|---|---|
| Mezaki, 1994 Optimisation of botulinum treatment for cervical and axial dystonias: experience with a Japanese type A toxin [8] | 2 | Cervical and thoracic paravertebral muscles | Crystallised type A botulinum toxin (CS-BOT; Chiba Serum Institute, Chiba, Japan) The maximal dose: 150 units/muscle, 300 units/session | 1st patient: Regained the ability to walk after eight injections (cumulative dose 2100 units) 2nd patient: Abnormal posture was corrected (cumulative dose 700 units) | No data | Subjective ranged from 0 (no improvement) to 100 (complete recovery), as rated by the patient objective scores (videotape assessed by neurologist) | 1st patient: Tardive generalised dystonia for 12 years 2nd patient: Idiopathic axial dystonia for 5 years |
| Lagueny, 1995 Involvement of Respiratory Muscles in Adult-Onset Dystonia: A Clinical and Electrophysiological Study [9] | 1 | Splenius and paraspinal muscles | AbobotulinumtoxinA (Dysport®) Single dose per splenius muscle was 100 units for one injection (3 injections through the year, cumulative dose for both splenius muscles during one year was 600 u). Single dose per paraspinal muscles was 200 u for one injection (3 injections throughout the year, cumulative dose for both paraspinal muscles during one year was 1200 u). | Improvement of axial dystonia and respiratory problems More regular pattern of EMG activity | No data | Clinical general assessment EMG activity | Idiopathic axial dystonia following isolated significant retrocollis |
| Quirk, 1996 Treatment of Nonoccupational Limb and Trunk Dystonia with Botulinum Toxin [10] | 1 | T4-T10 paraspinal muscles | AbobotulinumtoxinA (Dysport®) Paraspinal muscles—750 u | Patients’ assessment: very good Clinical assessment: increased mobility | No data | Patients graded their overall assessment of response to treatment as: no benefit, fair, good, very good, or excellent. Pain assessment: 0 = no improvement and 3 = complete pain relief Improvement in posture assessed clinically by two neurologists and scored on a 5-point scale (where 0 = no change and 4 = normal posture), and functional benefits were recorded descriptively. | Idiopathic |
| Comella, et al., 1998 Extensor Truncal Dystonia: Successful Treatment With Botulinum Toxin Injections [1] | 5 | longissimus and spinalis muscle groups The muscles were injected in the lower back from the level of the tenth thoracic vertebrae to the second lumbar vertebrae. | Onabotulinum toxinA (BOTOX®) Patients with moderate hypertrophy received 150–200 u; patients with marked hypertrophy received of 200–300 u; 2 or 3 sites on each side, with 25–50 u per site; | Objective improvement on videotaped scores (37%); subjective improvement (46%); improvement in pain (30–80%) all patients reported less pain and increased range of motion | no adverse effects | Modified from the Burke-Fahn-Marsden videotape protocol examiner blinded to the treatment order, scale using 0 (no dystonia) to 10 (extreme arching or bending). Patients’ self-assessment: from −100% (worse) to +100% (improved) on position, pain, and mobility | Adult-onset; no data on etiology |
| Bonnani, 2007 Botulinum Toxin Treatment of Lateral Axial Dystonia in Parkinsonism [11] | 9 | paraspinal muscles 2 to 2.5 cm lateral to spinous processes at level L2-L5 on the side of the trunk | AbobotulinumtoxinA (Dysport®) total dose of 500 u | 6/9 patients showed improvement in grading of function (TDDS) and pain (VAS); two patients did not benefit The improvement of lateral bending responding to the treatment: 50% to 85.7% Marked improvement of posture. 7/9: a remarkable improvement of pain | No adverse effects | Videotaping by an examiner blind to treatment and assessed with the Trunk Dystonia Disability scale (TDDS). A mobile wall goniometer used to calculate the degrees of trunk inclination; Pain rated with a visual analogue scale (VAS: 0–10) | L-dopa responsive parkinsonism |
| Rosales, 2011 The Broadening Application of Chemodenervation in X-Linked Dystonia-Parkinsonism (Part II): An Open-Label Experience With Botulinum Toxin-A (Dysport) Injections for Oromandibular, Lingual, and Truncal-Axial Dystonias [12] | 7 | erector spinae, bilaterally | AbobotulinumtoxinA (Dysport®) Median total dose: 750 u (500–1000 u) | Global Dystonia rating scale median (range): 2 (1–3) Pain VAS reduction at week 4—median (range): 50% (25–75) in 10 out of 12 cases with pain | No adverse effects | The global dystonia rating scale (DRS; ranging from 0 = no effect to 4 = complete dystonia abolition) visual analog scale (VAS: 0–100%) | Adult-onset X-linked Dystonia-Parkinsonism (XDP) |
| Voos, 2013 Case Report: Physical therapy management of axial dystonia [13] | 1 | right superior and medium trapezius, scalene and erector spinae; | Botulinum toxin type A (preparation not specified) 1st year: 400 u four times a year After one year of treatment: 300 u four times a year | pain relief, no functional improvement | No data | Parts I, II and III of Toronto Western Spasmodic Torticollis Rating Scale (TWSTRS-I, TWSTRS-II and TWSTRS-III), Berg Balance Scale (BBS), Six-Minute Walk Test (6-MWT), and the motor domain of Functional Independence Measure (FIM-motor) before and after the two-year treatment and after the one-year follow-up | Idiopathic |
| Ehrlich, 2016 The phenomenology and treatment of idiopathic adult-onset truncal dystonia: a retrospective review [6] | 2 | injected different muscle groups -not specified | OnabotulinumtoxinA (BOTOX®) botulinum toxin injections were not standardized, different injectors employed different techniques; a total dose of 700 u per patient | No improvement | No data | Videotapes evaluated by senior movement disorder physicians in the outpatient clinic of an urban academic center | Idiopathic |
| Mehta, Lal, 2019 Neurodegeneration with Brain Iron Accumulation: Two Additional cases with Dystonic Opisthotonus [14] | 2 | paraspinal muscles | Abobotulinum toxinA (DYSPORT®);
| 1st patient: Poor response to the treatment 2nd patient: Regained ability to walk | No data |
| 1st patient: pathogenic variant in Exon 1 and 3 of PANK2 gene 2nd patient: compound heterozygous gene mutations of uncertain significance in PANK2 gene |
| Mehta et al., 2020 Spectrum of Truncal Dystonia and Response to Treatment: A Retrospective Analysis [2] | 12 | Paraspinal muscles | AbobotulinumtoxinA (DYSPORT®); Unilateral → median (SD, range) dose 143.33 u ± 54.32 (100–250 u) Bilateral → median dose 286.67 u ± 108.65 (200–500 u) | Average subjective response of improvement was 30.8 ± 21.8% (0–70%) | No adverse effects | Single question evaluating improvement in pain, dystonia and functional status scored 0–100 Improvement assessed only for truncal dystonia in patients with multifocal, segmental, or generalized distribution Response measured only for the first injection in patients who underwent multiple sessions | 2 cases of Parkinson’s Disease, 4 tardive dystonia, 4 idiopathic, 2 PKAN (Pantothenate kinase-associated neurodegeneration) |
| Hull, 2021 Botulinum Neurotoxin Injections in Childhood Opisthotonus [15] | 7 | paraspinal muscles | Onabotulinumtoxin A (BOTOX®); Paraspinals—between 120 to 300 u (average 215.7 u)/divided into three to five injections on each side. Total dose administered 150–650 u (average 341.4 u) or 16.7 to 23.8 u/kg (average 19.6 u/kg). | All patients showed an improvement in opisthotonus within 3–14 days (average 6.1 days). | no adverse effects; except for one: neck extensor weakness, resolved over a period of weeks with no recurrence | Improvement was assessed on the basis on “to be crossed out examination of each child, and improvement deemed to be of a meaningful and sufficient degree by caregivers of all injected children” | 3 acquired (Post-infectious, Perinatal HIE, Tardive dystonia), 4 genetic (Hypomyelinating leukodystrophy-14, NBIA/PKAN, CASK-related disorder, presumed) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sławek, J.; Łobińska, I.A.; Schinwelski, M.; Kopcewicz-Wiśniewska, J.; Castagna, A. Case Series and Literature Review on Botulinum Toxin Efficacy in Axial Extensor Truncal Dystonia. Toxins 2025, 17, 375. https://doi.org/10.3390/toxins17080375
Sławek J, Łobińska IA, Schinwelski M, Kopcewicz-Wiśniewska J, Castagna A. Case Series and Literature Review on Botulinum Toxin Efficacy in Axial Extensor Truncal Dystonia. Toxins. 2025; 17(8):375. https://doi.org/10.3390/toxins17080375
Chicago/Turabian StyleSławek, Jarosław, Iga Alicja Łobińska, Michał Schinwelski, Joanna Kopcewicz-Wiśniewska, and Anna Castagna. 2025. "Case Series and Literature Review on Botulinum Toxin Efficacy in Axial Extensor Truncal Dystonia" Toxins 17, no. 8: 375. https://doi.org/10.3390/toxins17080375
APA StyleSławek, J., Łobińska, I. A., Schinwelski, M., Kopcewicz-Wiśniewska, J., & Castagna, A. (2025). Case Series and Literature Review on Botulinum Toxin Efficacy in Axial Extensor Truncal Dystonia. Toxins, 17(8), 375. https://doi.org/10.3390/toxins17080375





