Animal Venom in Modern Medicine: A Review of Therapeutic Applications
Abstract
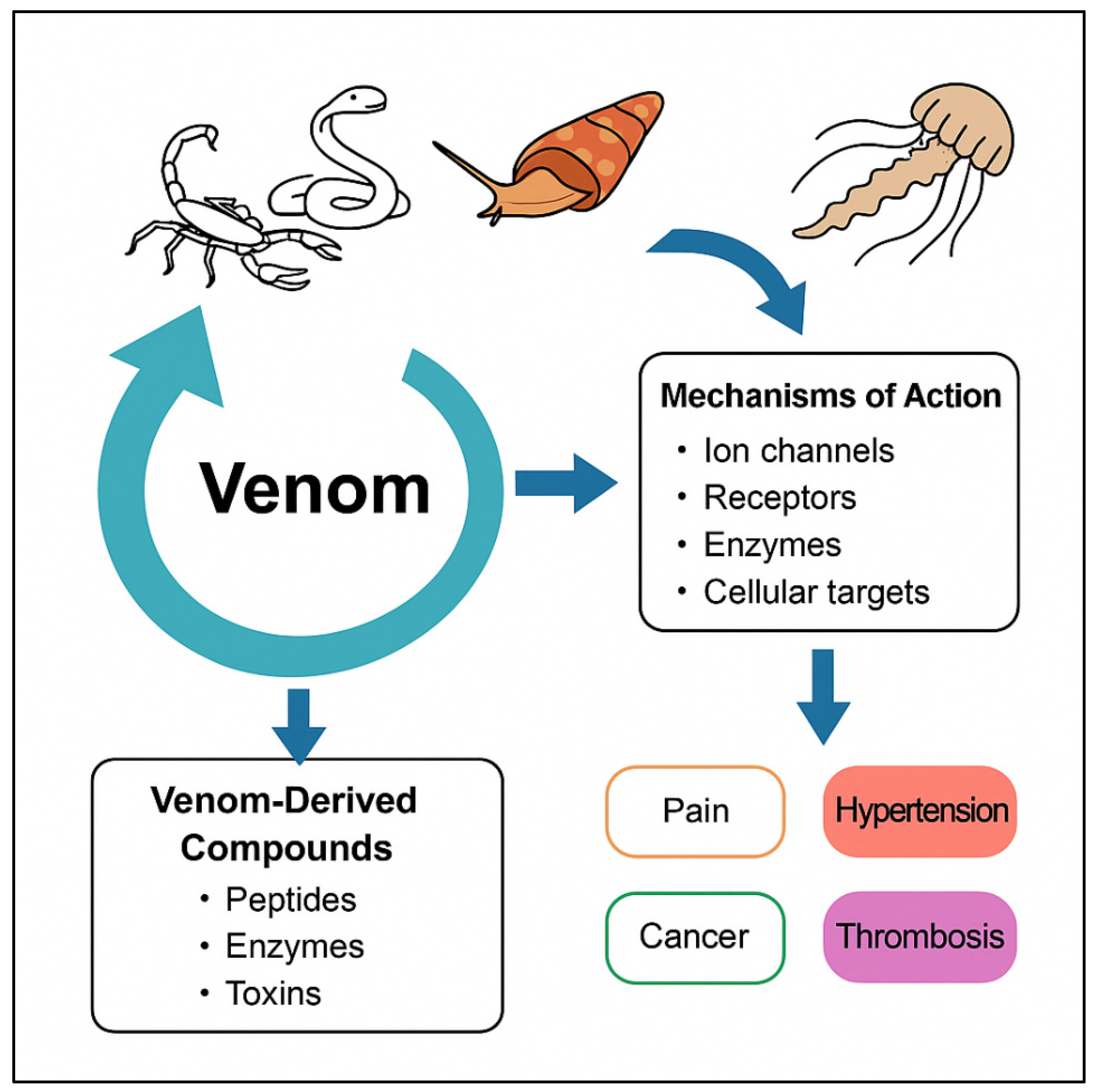
1. Introduction
2. Biochemical Composition of Animal Venoms
3. Mechanisms of Action of Venom Components
4. Therapeutic Applications of Venom-Derived Compounds
| Drug Name | Source Toxin | Origin Animal | Target/Mechanism | Indication | Clinical Status | Refs. |
|---|---|---|---|---|---|---|
| Captopril | Bradykinin-potentiating peptide | Bothrops jararaca (Viper) | ACE inhibitor | Hypertension, heart failure | FDA-Approved | [4,56,57,58,76] |
| Ziconotide (Prialt®) | ω-Conotoxin MVIIA | Conus magus (Cone snail) | N-type Ca2+ channel blocker | Chronic neuropathic pain | FDA-Approved | [45,59,60] |
| Eptifibatide (Integrilin®) | Barbourin analog (Disintegrin) | Sistrurus miliarius (Viper) | GPIIb/IIIa inhibitor | ACS, PCI | FDA-Approved | [61,62] |
| Tirofiban (Aggrastat®) | Echistatin analog | Echis carinatus (Saw-scaled viper) | GPIIb/IIIa antagonist | Coronary artery disease | FDA-Approved | [77] |
| Exenatide (Byetta®) | Exendin-4 | Heloderma suspectum (Gila monster) | GLP-1 receptor Agonist | Type 2 diabetes, weight loss | FDA-Approved (tirzepatide) | [63,64] |
| Desmoteplase | DSPA α1 | Desmodus rotundus (Vampire bat) | Clot-specific plasminogen activator | Ischemic stroke | Completed Phase II | [78] |
| Chlorotoxin-based imaging agents (e.g., BLZ-100) | Chlorotoxin | Leiurus quinquestriatus (Scorpion) | MMP-2 binding | Glioma imaging | Phase I/II | [65,66,67] |
| Dalazatide (ShK-186) | ShK toxin | Stichodactyla helianthus (Sea anemone) | Kv1.3 channel blocker | Psoriasis, MS | Completed Phase I | [79] |
| Heparin mimetics (e.g., Ancrod) | Ancrod | Calloselasma rhodostoma (Pit viper) | Defibrinogenating agent | Stroke, thrombosis | Withdrawn after Phase III | [80] |
| Huwentoxin-IV analogs | Huwentoxin-IV | Ornithoctonus huwena (Spider) | Nav1.7 sodium channel blocker | Analgesia | Preclinical | [81] |
| D-Melittin–nanoparticle conjugates | Melittin | Apis mellifera (Bee) | Membrane disruption | Cancer | Preclinical | [68,82] |
| Apimol/Apitoxin | Bee venom extract containing melittin | Apis mellifera (Bee) | Anti-inflammatory and analgesic pathway | Rheumatoid arthritis pain | Phase II (clinical trials, bee venom extract containing melittin) | [70] |
5. Technological Advances Facilitating Venom-Based Drug Discovery
6. Venom-Derived Therapeutics for Unmet Medical Needs
7. Challenges and Future Directions
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schendel, V.; Rash, L.D.; Jenner, R.A.; Undheim, E.A.B. The Diversity of Venom: The Importance of Behavior and Venom System Morphology in Understanding Its Ecology and Evolution. Toxins 2019, 11, 666. [Google Scholar] [CrossRef]
- Chen, N.; Xu, S.; Zhang, Y.; Wang, F. Animal protein toxins: Origins and therapeutic applications. Biophys. Rep. 2018, 4, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.J.; Garcia, M.L. Therapeutic potential of venom peptides. Nat. Rev. Drug Discov. 2003, 2, 790–802. [Google Scholar] [CrossRef]
- Smith, C.G.; Vane, J.R. The discovery of captopril. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2003, 17, 788–789. [Google Scholar] [CrossRef]
- Cushman, D.W.; Ondetti, M.A. Design of angiotensin converting enzyme inhibitors. Nat. Med. 1999, 5, 1110–1113. [Google Scholar] [CrossRef] [PubMed]
- Utkin, Y.N. Modern trends in animal venom research—Omics and nanomaterials. World J. Biol. Chem. 2017, 8, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Modahl, C.M.; Brahma, R.K.; Koh, C.Y.; Shioi, N.; Kini, R.M. Omics Technologies for Profiling Toxin Diversity and Evolution in Snake Venom: Impacts on the Discovery of Therapeutic and Diagnostic Agents. Annu. Rev. Anim. Biosci. 2020, 8, 91–116. [Google Scholar] [CrossRef] [PubMed]
- Bordon, K.C.F.; Cologna, C.T.; Fornari-Baldo, E.C.; Pinheiro-Júnior, E.L.; Cerni, F.A.; Amorim, F.G.; Anjolette, F.A.P.; Cordeiro, F.A.; Wiezel, G.A.; Cardoso, I.A.; et al. From Animal Poisons and Venoms to Medicines: Achievements, Challenges, and Perspectives in Drug Discovery. Front. Pharmacol. 2020, 11, 1132. [Google Scholar] [CrossRef]
- Utkin, Y.N. Animal Venom Studies: Current Benefits and Future Developments. World J. Biol. Chem. 2015, 6, 28–33. [Google Scholar] [CrossRef]
- Vidya, V.; Achar, R.R.; Himathi, M.U.; Akshita, N.; Kameshwar, V.H.; Byrappa, K.; Ramadas, D. Venom peptides—A comprehensive translational perspective in pain management. Curr. Res. Toxicol. 2021, 2, 329–340. [Google Scholar] [CrossRef]
- Ageitos, L.; Torres, M.D.; de la Fuente-Nunez, C. Biologically Active Peptides from Venoms: Applications in Antibiotic Resistance, Cancer, and Beyond. Int. J. Mol. Sci. 2022, 23, 15437. [Google Scholar] [CrossRef]
- Moga, M.A.; Dimienescu, O.G.; Arvătescu, C.A.; Ifteni, P.; Pleş, L. Anticancer Activity of Toxins from Bee and Snake Venom—An Overview on Ovarian Cancer. Molecules 2018, 23, 692. [Google Scholar] [CrossRef]
- Coulter-Parkhill, A.; McClean, S.; Gault, V.A.; Irwin, N. Therapeutic Potential of Peptides Derived from Animal Venoms: Current Views and Emerging Drugs for Diabetes. Clin. Med. Insights Endocrinol. Diabetes 2021, 14, 11795514211006071. [Google Scholar] [CrossRef]
- Kalia, J.; Milescu, M.; Salvatierra, J.; Wagner, J.; Klint, J.K.; King, G.F.; Olivera, B.M.; Bosmans, F. From foe to friend: Using animal toxins to investigate ion channel function. J. Mol. Biol. 2015, 427, 158–175. [Google Scholar] [CrossRef]
- Oliveira, A.L.; Viegas, M.F.; da Silva, S.L.; Soares, A.M.; Ramos, M.J.; Fernandes, P.A. The chemistry of snake venom and its medicinal potential. Nat. Rev. Chem. 2022, 6, 451–469. [Google Scholar] [CrossRef] [PubMed]
- Michálek, O.; King, G.F.; Pekár, S. Prey specificity of predatory venoms. Biol. Rev. Camb. Philos. Soc. 2024, 99, 2253–2273. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Escalante, T.; Rucavado, A. Experimental Pathophysiology of Systemic Alterations Induced by Bothrops asper Snake Venom. Toxicon 2009, 54, 976–987. [Google Scholar] [CrossRef]
- Kini, R.M.; Koh, C.Y. Metalloproteases Affecting Blood Coagulation, Fibrinolysis and Platelet Aggregation from Snake Venoms: Definition and Nomenclature of Interaction Sites. Toxins 2016, 8, 284. [Google Scholar] [CrossRef]
- Serrano, S.M.T. The Long Road of Research on Snake Venom Serine Proteinases. Toxicon 2013, 62, 19–26. [Google Scholar] [CrossRef]
- AlShammari, A.K.; Abd El-Aziz, T.M.; Al-Sabi, A. Snake Venom: A Promising Source of Neurotoxins Targeting Voltage-Gated Potassium Channels. Toxins 2023, 16, 12. [Google Scholar] [CrossRef]
- Kalita, B.; Utkin, Y.N.; Mukherjee, A.K. Current Insights in the Mechanisms of Cobra Venom Cytotoxins and Their Complexes in Inducing Toxicity: Implications in Antivenom Therapy. Toxins 2022, 14, 839. [Google Scholar] [CrossRef]
- Aird, S.D.; Aggarwal, S.; Villar-Briones, A.; Tin, M.M.; Terada, K.; Mikheyev, A.S. Snake venoms are integrated systems, but abundant venom proteins evolve more rapidly. BMC Genom. 2015, 16, 647. [Google Scholar] [CrossRef]
- Modahl, C.M.; Mrinalini Frietze, S.; Mackessy, S.P. Adaptive evolution of distinct prey-specific toxin genes in rear-fanged snake venom. Proc. Biol. Sci. 2018, 285, 20181003. [Google Scholar] [CrossRef]
- Utkin, Y.N. Three-finger toxins, a deadly weapon of elapid venom—Milestones of discovery. Toxicon 2013, 62, 50–55. [Google Scholar] [CrossRef]
- Harvey, A.L. Twenty years of dendrotoxins. Toxicon 2001, 39, 15–26. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Escalante, T.; Rucavado, A.; Herrera, C. Hemorrhage caused by snake venom metalloproteinases: A journey of discovery and understanding. Toxins 2016, 8, 93. [Google Scholar] [CrossRef]
- Kini, R.M. Serine proteases affecting blood coagulation and fibrinolysis from snake venoms. Pathophysiol. Haemost. Thromb. 2005, 34, 200–204. [Google Scholar] [CrossRef]
- Lebbe, E.K.M.; Tytgat, J. In the picture: Disulfide-poor conopeptides, a class of pharmacologically interesting compounds. J. Venom. Anim. Toxins Incl. Trop. Dis. 2016, 22, 30. [Google Scholar] [CrossRef]
- McGivern, J.G. Ziconotide: A review of its pharmacology and use in the treatment of pain. Neuropsychiatr. Dis. Treat. 2007, 3, 69–85. [Google Scholar] [CrossRef]
- King, G.F. Venoms as a platform for human drugs: Translating toxins into therapeutics. Expert Opin. Biol. Ther. 2011, 11, 1469–1484. [Google Scholar] [CrossRef]
- Mariottini, G.L.; Pane, L. Cytotoxic and Cytolytic Cnidarian Venoms. A Review on Health Implications and Possible Therapeutic Applications. Toxins 2013, 6, 108–151. [Google Scholar] [CrossRef]
- Lee, H.; Jung, E.-S.; Kang, C.; Yoon, W.D.; Kim, J.-S.; Kim, E. Scyphozoan jellyfish venom metalloproteinases and their role in the cytotoxicity. Toxicon 2011, 58, 277–284. [Google Scholar] [CrossRef]
- Xie, B.; Huang, Y.; Baumann, K.; Fry, B.G.; Shi, Q. From Marine Venoms to Drugs: Efficiently Supported by a Combination of Transcriptomics and Proteomics. Mar. Drugs 2017, 15, 103. [Google Scholar] [CrossRef]
- Anderluh, G.; Macek, P. Dissecting the actinoporin pore-forming mechanism. Structure 2003, 11, 1312–1313. [Google Scholar] [CrossRef]
- Bittenbinder, M.A.; van Thiel, J.; Cardoso, F.C.; Casewell, N.R.; Gutiérrez, J.M.; Kool, J.; Vonk, F.J. Tissue damaging toxins in snake venoms: Mechanisms of action, pathophysiology and treatment strategies. Commun. Biol. 2024, 7, 358. [Google Scholar] [CrossRef]
- Utkin, Y.N. Animal Venoms and Their Components: Molecular Mechanisms of Action. Toxins 2021, 13, 415. [Google Scholar] [CrossRef]
- Bosmans, F.; Tytgat, J. Voltage-gated sodium channel modulation by scorpion α-toxins. Toxicon 2007, 49, 142–158. [Google Scholar] [CrossRef]
- Moran, Y.; Gordon, D.; Gurevitz, M. Sea anemone toxins affecting voltage-gated sodium channels—Molecular and evolutionary features. Toxicon 2009, 54, 1089–1101. [Google Scholar] [CrossRef]
- Adams, M.E. Agatoxins: Ion channel specific toxins from the American funnel web spider, Agelenopsis aperta. Toxicon 2004, 43, 509–525. [Google Scholar] [CrossRef]
- Chow, C.Y.; Absalom, N.; Biggs, K.; King, G.F.; Ma, L. Venom-derived modulators of epilepsy-related ion channels. Biochem. Pharmacol. 2020, 181, 114043. [Google Scholar] [CrossRef]
- Souza, A.C.N.; Binda, N.S.; Almeida, H.Y.; de Castro Jünior, C.J.; Gomez, M.V.; Ribeiro, F.M.; Da Silva, J.F. Ion Channels-related Neuroprotection and Analgesia Mediated by Spider Venom Peptides. Curr. Protein Pept. Sci. 2023, 24, 365–379. [Google Scholar] [CrossRef]
- Ferreira, S.H. A bradykinin-potentiating factor (BPF) present in the venom of Bothrops jararaca. Br. J. Pharmacol. Chemother. 1965, 24, 163–169. [Google Scholar] [CrossRef]
- Ondetti, M.A.; Rubin, B.; Cushman, D.W. Design of specific inhibitors of angiotensin-converting enzyme: New class of orally active antihypertensive agents. Science 1977, 196, 441–444. [Google Scholar] [CrossRef]
- Satkunanathan, N.; Livett, B.; Gayler, K.; Sandall, D.; Down, J.; Khalil, Z. Alpha-conotoxin Vc1.1 alleviates neuropathic pain and accelerates functional recovery of injured neurons. Brain Res. 2005, 1059, 149–158. [Google Scholar] [CrossRef]
- Schmidtko, A.; Lötsch, J.; Freynhagen, R.; Geisslinger, G. Ziconotide for treatment of severe chronic pain. Lancet 2010, 375, 1569–1577. [Google Scholar] [CrossRef]
- Calvete, J.J. Snake venomics: From the inventory of toxins to biology. Toxicon 2013, 75, 44–62. [Google Scholar] [CrossRef]
- Lazarovici, P.; Marcinkiewicz, C.; Lelkes, P.I. From Snake Venom’s Disintegrins and C-Type Lectins to Anti-Platelet Drugs. Toxins 2019, 11, 303. [Google Scholar] [CrossRef]
- McLane, M.A.; Sanchez, E.E.; Wong, A.; Paquette-Straub, C.; Perez, J.C. Disintegrins. Current Drug Targets. Cardiovasc. Hematol. Disord. 2004, 4, 327–355. [Google Scholar] [CrossRef]
- Williams, J.A.; Day, M.; Heavner, J.E. Ziconotide: An update and review. Expert Opin. Pharmacother. 2008, 9, 1575–1583. [Google Scholar] [CrossRef]
- Cardoso, F.C.; Lewis, R.J. Structure-Function and Therapeutic Potential of Spider Venom-Derived Cysteine Knot Peptides Targeting Sodium Channels. Front. Pharmacol. 2019, 10, 366. [Google Scholar] [CrossRef]
- King, G.F.; Vetter, I. No gain, no pain: NaV1.7 as an analgesic target. ACS Chem. Neurosci. 2014, 5, 749–751. [Google Scholar] [CrossRef]
- Harvey, A.L. Toxins and drug discovery. Toxicon 2014, 92, 193–200. [Google Scholar] [CrossRef]
- Kini, R.M.; Koh, C.Y. Snake venom three-finger toxins and their potential in drug development targeting cardiovascular diseases. Biochem. Pharmacol. 2020, 181, 114105. [Google Scholar] [CrossRef]
- Munawar, A.; Ali, S.A.; Akrem, A.; Betzel, C. Snake Venom Peptides: Tools of Biodiscovery. Toxins 2018, 10, 474. [Google Scholar] [CrossRef]
- Sadeghi, M.; McArthur, J.R.; Finol-Urdaneta, R.K.; Adams, D.J. Analgesic conopeptides targeting G protein-coupled receptors reduce excitability of sensory neurons. Neuropharmacology 2017, 127, 116–123. [Google Scholar] [CrossRef]
- Cushman, D.W.; Ondetti, M.A. History of the design of captopril and related inhibitors of angiotensin converting enzyme. Hypertension 1991, 17, 589–592. [Google Scholar] [CrossRef]
- Bader, M.; Ganten, D. Update on tissue renin-angiotensin systems. J. Mol. Med. 2008, 86, 615–621. [Google Scholar] [CrossRef]
- Rella, M.; Rushworth, C.A.; Guy, J.L.; Turner, A.J.; Langer, T.; Jackson, R.M. Structure-based pharmacophore design and virtual screening for novel angiotensin converting enzyme 2 inhibitors. J. Chem. Inf. Model. 2006, 46, 708–716. [Google Scholar] [CrossRef]
- Staats, P.S.; Yearwood, T.; Charapata, S.G.; Presley, R.W.; Wallace, M.S.; Byas-Smith, M.; Fisher, R.; Bryce, D.A.; Mangieri, E.A.; Luther, R.R.; et al. Intrathecal ziconotide in the treatment of refractory pain in patients with cancer or AIDS: A randomized controlled trial. JAMA 2004, 291, 63–70. [Google Scholar] [CrossRef]
- Wallace, M.S.; Rauck, R.; Fisher, R.; Charapata, S.G.; Ellis, D.; Dissanayake, S.; Ziconotide 98-022 Study Group. Intrathecal ziconotide for severe chronic pain: Safety and tolerability results of an open-label, long-term trial. Anesth. Analg. 2008, 106, 628–637. [Google Scholar] [CrossRef]
- Scarborough, R.M.; Naughton, M.A.; Teng, W.; Rose, J.W.; Phillips, D.R.; Nannizzi, L.; Arfsten, A.; Campbell, A.M.; Charo, I.F. Design of potent and specific integrin antagonists: Peptide antagonists with high specificity for glycoprotein IIb/IIIa. J. Biol. Chem. 1993, 268, 1066–1073. [Google Scholar] [CrossRef]
- Estevez, B.; Shen, B.; Du, X. Targeting integrin and integrin signaling in treating thrombosis. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 24–29. [Google Scholar] [CrossRef]
- DeMarsilis, A.; Reddy, N.; Boutari, C.; Filippaios, A.; Sternthal, E.; Katsiki, N.; Mantzoros, C. Pharmacotherapy of type 2 diabetes: An update and future directions. Metabolism 2022, 137, 155332. [Google Scholar] [CrossRef]
- Dahl, D.; Onishi, Y.; Norwood, P.; Huh, R.; Bray, R.; Patel, H.; Rodríguez, Á. Effect of Subcutaneous Tirzepatide vs Placebo Added to Titrated Insulin Glargine on Glycemic Control in Patients with Type 2 Diabetes. JAMA 2022, 327, 534–545. [Google Scholar] [CrossRef] [PubMed]
- Soroceanu, L.; Gillespie, Y.; Khazaeli, M.B.; Sontheimer, H. Use of chlorotoxin for targeting of primary brain tumors. Cancer Res. 1998, 58, 4871–4879. [Google Scholar] [PubMed]
- Veiseh, M.; Gabikian, P.; Bahrami, S.-B.; Veiseh, O.; Zhang, M.; Hackman, R.C.; Ravanpay, A.C.; Stroud, M.R.; Kusuma, Y.; Hansen, S.J.; et al. Tumor paint: A chlorotoxin:Cy5.5 bioconjugate for intraoperative visualization of cancer foci. Cancer Res. 2007, 67, 6882–6888. [Google Scholar] [CrossRef]
- Wiranowska, M. Advances in the use of chitosan and chlorotoxin—Functionalized chitosan polymers in drug delivery and detection of glioma—A review. Carbohydr. Polym. Technol. Appl. 2024, 7, 100427. [Google Scholar] [CrossRef]
- Yu, X.; Jia, S.; Yu, S.; Chen, Y.; Zhang, C.; Chen, H.; Dai, Y. Recent advances in melittin-based nanoparticles for antitumor treatment: From mechanisms to targeted delivery strategies. J. Nanobiotechnol. 2023, 21, 454. [Google Scholar] [CrossRef]
- Oršolić, N. Bee venom in cancer therapy. Cancer Metastasis Rev. 2012, 31, 173–194. [Google Scholar] [CrossRef]
- Son, D.J.; Lee, J.W.; Lee, Y.H.; Song, H.S.; Lee, C.K.; Hong, J.T. Therapeutic application of anti-arthritis, pain-releasing, and anti-cancer effects of bee venom and its constituent compounds. Pharmacol. Ther. 2007, 115, 246–270. [Google Scholar] [CrossRef]
- Ayroza, G.; Ferreira, I.L.C.; Sayegh, R.S.R.; Tashima, A.K.; da Silva Junior, P.I. Juruin: An antifungal peptide from the venom of the Amazonian Pink Toe spider, Avicularia juruensis, which contains the inhibitory cystine knot motif. Front. Microbiol. 2012, 3, 324. [Google Scholar] [CrossRef] [PubMed]
- Cesa-Luna, C.; Muñoz-Rojas, J.; Saab-Rincon, G.; Baez, A. Structural characterization of scorpion peptides and their bactericidal activity against clinical isolates of multidrug-resistant bacteria. PLoS ONE 2019, 14, e0222438. [Google Scholar] [CrossRef] [PubMed]
- Vinhote, J.F.C.; Lima, D.B.; Mello, C.P.; de Souza, B.M.; Havt, A.; Palma, M.S.; Martins, A.M.C. Trypanocidal activity of mastoparan from Polybia paulista wasp venom by interaction with TcGAPDH. Toxicon 2017, 137, 168–172. [Google Scholar] [CrossRef]
- Akondi, K.B.; Muttenthaler, M.; Dutertre, S.; Kaas, Q.; Craik, D.J.; Lewis, R.J.; Alewood, P.F. Discovery, synthesis and development of structure-activity relationships of Conotoxins. Chem. Rev. 2014, 114, 5815–5847. [Google Scholar] [CrossRef]
- Akcan, M.; Craik, D.J. Chapter 11: Engineering Venom Peptides to Improve Their Stability and Bioavailability. In Venoms to Drugs; Series: Drug Discovery Series; Royal Society of Chemistry: Cambridge, UK, 2015; pp. 275–289. [Google Scholar] [CrossRef]
- Messadi, E. Snake Venom Components as Therapeutic Drugs in Ischemic Heart Disease. Biomolecules 2023, 13, 1539. [Google Scholar] [CrossRef] [PubMed]
- The RESTORE Investigators. Effects of platelet glycoprotein IIb/IIIa blockade with tirofiban on adverse cardiac events in patients with unstable angina or acute myocardial infarction undergoing coronary angioplasty. Circulation 1997, 96, 1445–1453. [Google Scholar] [CrossRef]
- Hacke, W.; Albers, G.; Al-Rawi, Y.; Bogousslavsky, J.; Davalos, A.; Eliasziw, M.; Fischer, M.; Furlan, A.; Kaste, M.; Lees, K.R.; et al. The Desmoteplase in Acute Ischemic Stroke Trial (DIAS): A phase II MRI-based 9-hour window acute stroke thrombolysis trial with intravenous desmoteplase. Stroke 2005, 36, 66–73. [Google Scholar] [CrossRef]
- Tarcha, E.J.; Olsen, C.M.; Probst, P.; Peckham, D.; Muñoz-Elías, E.J.; Kruger, J.G.; Iadonato, S.P. Safety and pharmacodynamics of dalazatide, a Kv1.3 channel inhibitor, in the treatment of plaque psoriasis: A randomized phase 1b trial. PLoS ONE 2017, 12, e0180762. [Google Scholar] [CrossRef]
- Sherman, D.G.; Atkinson, R.P.; Chippendale, T.; Levin, K.A.; Ng, K.; Futrell, N.; Hsu, C.Y.; Levy, D.E. Intravenous ancrod for treatment of acute ischemic stroke: The STAT study: A randomized controlled trial. Stroke Treatment with Ancrod Trial. JAMA 2000, 283, 2395–2403. [Google Scholar] [CrossRef]
- Lopez, L.; Montnach, J.; Oliveira-Mendes, B.; Khakh, K.; Thomas, B.; Lin, S.; Caumes, C.; Wesolowski, S.; Nicolas, S.; Servent, D.; et al. Synthetic Analogues of Huwentoxin-IV Spider Peptide with Altered Human NaV1.7/NaV1.6 Selectivity Ratios. Front. Cell Dev. Biol. 2021, 9, 798588. [Google Scholar] [CrossRef]
- Lv, S.; Sylvestre, M.; Song, K.; Pun, S.H. Development of D-melittin polymeric nanoparticles for anti-cancer treatment. Biomaterials 2021, 277, 121076. [Google Scholar]
- King, G.F. Venoms to Drugs: Venom as a Source for the Development of Human Therapeutics; Royal Society of Chemistry: Cambridge, UK, 2015; ISBN 978-1-84973-663-3. [Google Scholar] [CrossRef]
- Slagboom, J.; Derks, R.J.; Sadighi, R.; Somsen, G.W.; Ulens, C.; Casewell, N.R.; Kool, J. High-Throughput Venomics. J. Proteome Res. 2023, 22, 1734–1746. [Google Scholar] [CrossRef]
- Espín-Angulo, J.; Vela, D. Exploring the venom gland transcriptome of Bothrops asper and Bothrops jararaca: De novo assembly and analysis of novel toxic proteins. Toxins 2024, 16, 511. [Google Scholar] [CrossRef]
- Suryamohan, K.; Krishnankutty, S.P.; Guillory, J.; Jevit, M.; Schröder, M.S.; Wu, M.; Kuriakose, B.; Mathew, O.K.; Perumal, R.C.; Koludarov, I.; et al. The Indian cobra reference genome and transcriptome enables comprehensive identification of venom toxins. Nat. Genet. 2020, 52, 106–117. [Google Scholar] [CrossRef]
- Escoubas, P.; Sollod, B.; King, G.F. Venom landscapes: Mining the complexity of spider venoms via a combined cDNA and mass spectrometric approach. Toxicon 2006, 47, 650–663. [Google Scholar] [CrossRef]
- Yang, C.; Chen, E.A.; Zhang, Y. Protein-Ligand Docking in the Machine-Learning Era. Molecules 2022, 27, 4568. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Hasan, M.M.; Wang, D. In Silico Conotoxin Studies: Progress and Prospects. Molecules 2024, 29, 6061. [Google Scholar] [CrossRef] [PubMed]
- Ravi, D.A.; Hwang, D.H.; Mohan Prakash, R.L.; Kang, C.; Kim, E. Indian Medicinal Plant-Derived Phytochemicals as Potential Antidotes for Snakebite: A Pharmacoinformatic Study of Atrolysin Inhibitors. Int. J. Mol. Sci. 2024, 25, 12675. [Google Scholar] [CrossRef]
- Clement, H.; Flores, V.; Diego-Garcia, E.; Corrales-Garcia, L.; Villegas, E.; Corzo, G. A comparison between the recombinant expression and chemical synthesis of a short cysteine-rich insecticidal spider peptide. J. Venom. Anim. Toxins Incl. Trop. Dis. 2015, 21, 19. [Google Scholar] [CrossRef] [PubMed]
- Budisa, N. Expanded genetic code for the engineering of ribosomally and post-translationally modified peptide natural products (RiPPs). Curr. Opin. Biotechnol. 2013, 24, 591–598. [Google Scholar] [CrossRef]
- Gordon, D.; Chen, R.; Chung, S.-H. Computational Methods of Studying the Binding of Toxins from Venomous Animals to Biological Ion Channels: Theory and Applications. Physiol. Rev. 2013, 93, 767–802. [Google Scholar] [CrossRef]
- Giribaldi, J.; Haufe, Y.; Evans, E.R.J.; Amar, M.; Durner, A.; Schmidt, C.; Faucherre, A.; Moha Ou Maati, H.; Enjalbal, C.; Molgó, J.; et al. Backbone Cyclization Turns a Venom Peptide into a Stable and Equipotent Ligand at Both Muscle and Neuronal Nicotinic Receptors. J. Med. Chem. 2020, 63, 12682–12692. [Google Scholar] [CrossRef]
- Sunagar, K.; Undheim, E.A.B.; Scheib, H.; Gren, E.C.; Cochran, C.; Person, C.E.; Koludarov, I.; Kelln, W.; Hayes, W.K.; King, G.F.; et al. Intraspecific venom variation in the medically significant Southern Pacific Rattlesnake (Crotalus oreganus helleri): Biodiscovery, clinical and evolutionary implications. J. Proteom. 2014, 99, 68–83. [Google Scholar] [CrossRef] [PubMed]
- Zancolli, G.; Casewell, N.R. Venom systems as models for studying the origin and regulation of evolutionary novelties. Mol. Biol. Evol. 2020, 37, 2777–2790. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.D.; Li, Q.; Bandyopadhyay, P.K.; Gajewiak, J.; Yandell, M.; Papenfuss, A.T.; Purcell, A.W.; Norton, R.S.; Olivera, B.M. Diversity of conotoxin gene superfamilies in the venomous snail Conus victoriae. PLoS ONE 2017, 9, e87648. [Google Scholar] [CrossRef]
- Dowell, D.; Tamara, M.; Chou, H.R. CDC Guideline for Prescribing Opioids for Chronic Pain—United States, 2016. JAMA 2016, 315, 1624–1645. [Google Scholar] [CrossRef]
- Carey, R.M.; Calhoun, D.A.; Bakris, G.L.; Brook, R.D.; Daugherty, S.L.; Dennison-Himmelfarb, C.R.; Egan, B.M.; Flack, J.M.; Gidding, S.S.; Judd, E.; et al. Resistant Hypertension: Detection, Evaluation, and Management: A Scientific Statement from the American Heart Association. Hypertension 2018, 72, e53–e90. [Google Scholar] [CrossRef] [PubMed]
- Tasoulis, T.; Isbister, G.K. A review and database of snake venom proteomes. Toxins 2017, 9, 290. [Google Scholar] [CrossRef]
- Casewell, N.R.; Wüster, W.; Vonk, F.J.; Harrison, R.A.; Fry, B.G. Complex cocktails: The evolutionary novelty of venoms. Trends Ecol. Evol. 2013, 28, 219–229. [Google Scholar] [CrossRef]
- Dutertre, S.; Jin, A.H.; Vetter, I.; Hamilton, B.; Sunagar, K.; Lavergne, V.; Lewis, R.J. Evolution of separate predation- and defence-evoked venoms in carnivorous cone snails. Nat. Commun. 2014, 5, 3521. [Google Scholar] [CrossRef]
- Golden, D.B.K.; Demain, J.G.; Freeman, T.M.; Graft, D.F.; Tankersley, M.S.; Tracy, J.M.; Blessing-Moore, J.; Bernstein, D.; Dinakar, C.; Greenhawt, M.; et al. Stinging insect hypersensitivity: A practice parameter update 2016. Ann. Allergy Asthma Immunol. 2017, 118, 28–54. [Google Scholar] [CrossRef]
- Stevens, W.W.; Kraft, M.; Eisenbarth, S.C. Recent Insights into the Mechanisms of Anaphylaxis. Curr. Opin. Immunol. 2023, 81, 102288. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.M.; Chess, R.B. Effect of pegylation on pharmaceuticals. Nat. Rev. Drug Discov. 2003, 2, 214–221. [Google Scholar] [CrossRef]
- Zamani, A.; Sääksjärvi, E.; Prendini, L. Venom-extraction and exotic pet trade may hasten the extinction of scorpions. Arachnol. Mitteilungen Arachnol. Lett. 2021, 61, 20–23. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Nong, F.T.; Wang, Y.Z.; Yan, C.X.; Gu, Y.; Song, P.; Sun, X.M. Strategies for efficient production of recombinant proteins in Escherichia coli: Alleviating the host burden and enhancing protein activity. Microb. Cell Fact. 2022, 21, 191. [Google Scholar] [CrossRef] [PubMed]
- Guan, C.; Torres, M.D.T.; Li, S.; de la Fuente-Nunez, C. Venomics AI: A computational exploration of global venoms for antibiotic discovery. bioRxiv 2024. bioRxiv:2024.12.17.628923. [Google Scholar] [CrossRef]
- Ma, R.; Mahadevappa, R.; Kwok, H.F. Venom-based peptide therapy: Insights into anti-cancer mechanism. Oncotarget 2017, 8, 100908–100930. [Google Scholar] [CrossRef]
| Therapeutic Area | Limitations of Current Treatments | Advantages of Venom-Derived Therapeutics |
|---|---|---|
| Chronic Pain | Opioid tolerance, dependence, limited efficacy in neuropathic pain | Novel ion channel blockers (e.g., ziconotide) with high specificity |
| Hypertension | Refractory cases, side effects with polytherapy | ACE inhibitors derived from snake venom (e.g., captopril) |
| Cancer Pain | Limited efficacy, opioid-related issues | Crotoxin showing analgesic and potential antitumor effects |
| Diabetes | Suboptimal glycemic control, hypoglycemia risk | GLP-1 receptor agonists from Gila monster venom (e.g., exenatide) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, E.; Hwang, D.H.; Mohan Prakash, R.L.; Asirvatham, R.D.; Lee, H.; Heo, Y.; Munawir, A.; Seyedian, R.; Kang, C. Animal Venom in Modern Medicine: A Review of Therapeutic Applications. Toxins 2025, 17, 371. https://doi.org/10.3390/toxins17080371
Kim E, Hwang DH, Mohan Prakash RL, Asirvatham RD, Lee H, Heo Y, Munawir A, Seyedian R, Kang C. Animal Venom in Modern Medicine: A Review of Therapeutic Applications. Toxins. 2025; 17(8):371. https://doi.org/10.3390/toxins17080371
Chicago/Turabian StyleKim, Euikyung, Du Hyeon Hwang, Ramachandran Loganathan Mohan Prakash, Ravi Deva Asirvatham, Hyunkyoung Lee, Yunwi Heo, Al Munawir, Ramin Seyedian, and Changkeun Kang. 2025. "Animal Venom in Modern Medicine: A Review of Therapeutic Applications" Toxins 17, no. 8: 371. https://doi.org/10.3390/toxins17080371
APA StyleKim, E., Hwang, D. H., Mohan Prakash, R. L., Asirvatham, R. D., Lee, H., Heo, Y., Munawir, A., Seyedian, R., & Kang, C. (2025). Animal Venom in Modern Medicine: A Review of Therapeutic Applications. Toxins, 17(8), 371. https://doi.org/10.3390/toxins17080371






