Investigation into Paralytic Shellfish Toxins and Microcystins in Seabirds from Portugal
Abstract
1. Introduction
2. Results and Discussion
2.1. Biotoxin Levels in Different Species and Tissues
2.2. Biotoxin Transfer (Putative Toxin Reservoirs), Bioaccumulation, and Biotransformation
2.3. Clinical Signs and Etiology
3. Conclusions
4. Materials and Methods
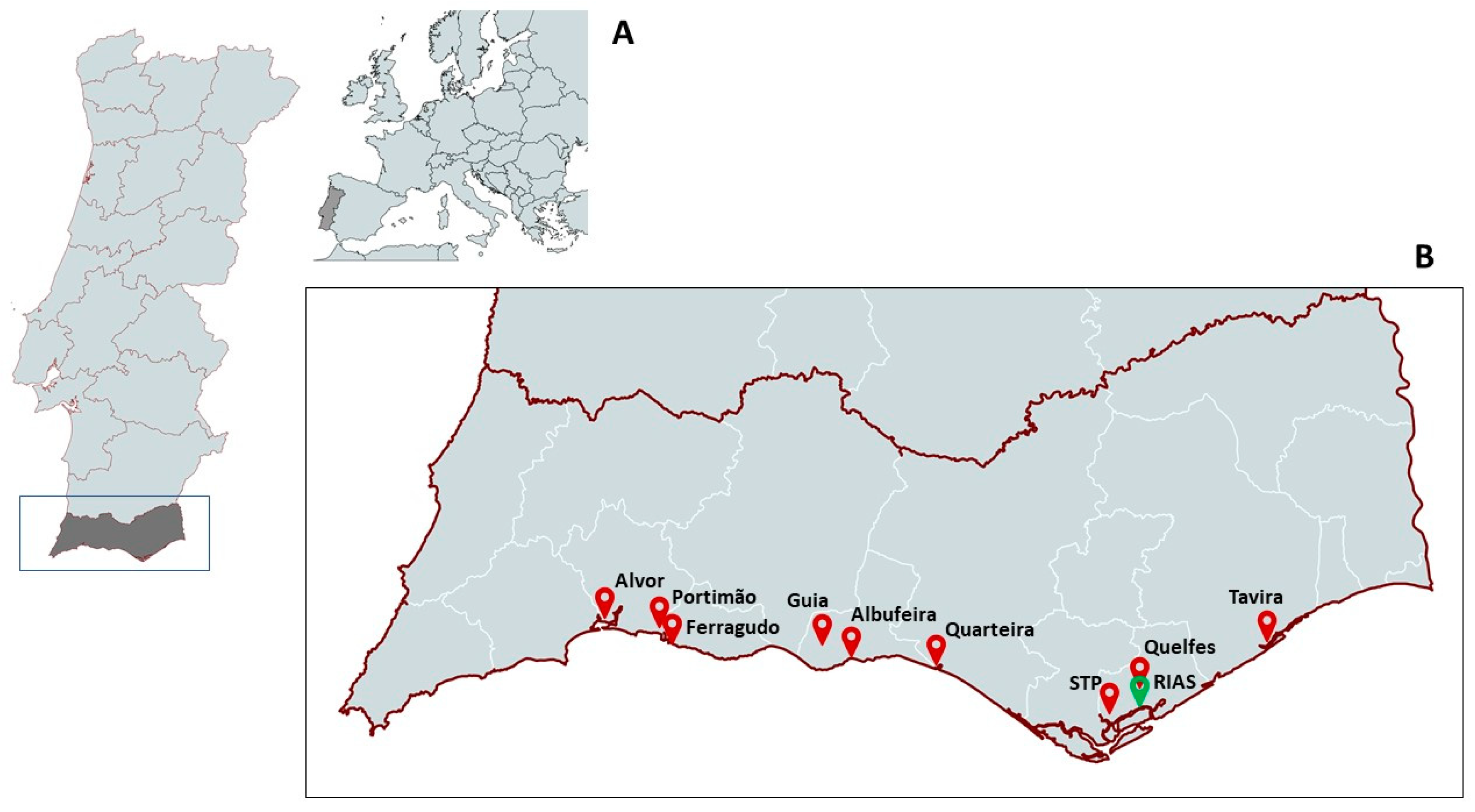
4.1. Wildlife Rehabilitation Centre and Admission Protocol
4.2. Marine and Freshwater Biotoxin Analysis
4.3. Bibliographic Search
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Turner, J.T.; Tester, P.A. Toxic Marine Phytoplankton, Zooplankton Grazers, and Pelagic Food Webs. Limnol. Ocean. 1997, 42, 1203–1213. [Google Scholar] [CrossRef]
- Granéli, E.; Turner, J.T. An Introduction to Harmful Algae. In Ecology of Harmful Algae; Springer: Berlin/Heidelberg, Germany, 2006; pp. 3–7. [Google Scholar]
- Jonsson, P.R.; Pavia, H.; Toth, G. Formation of Harmful Algal Blooms Cannot Be Explained by Allelopathic Interactions. Proc. Natl. Acad. Sci. USA 2009, 106, 11177–11182. [Google Scholar] [CrossRef]
- Cembella, A.D. Chemical Ecology of Eukaryotic Microalgae in Marine Ecosystems. Phycologia 2003, 42, 420–447. [Google Scholar] [CrossRef]
- Foss, A.J.; Miles, C.O.; Samdal, I.A.; Løvberg, K.E.; Wilkins, A.L.; Rise, F.; Jaabæk, J.A.H.; McGowan, P.C.; Aubel, M.T. Analysis of Free and Metabolized Microcystins in Samples Following a Bird Mortality Event. Harmful Algae 2018, 80, 117–129. [Google Scholar] [CrossRef]
- Ben-Gigirey, B.; Soliño, L.; Bravo, I.; Rodríguez, F.; Casero, M.V.M. Paralytic and Amnesic Shellfish Toxins Impacts on Seabirds, Analyses and Management. Toxins 2021, 13, 454. [Google Scholar] [CrossRef]
- Broadwater, M.H.; Van Dolah, F.M.; Fire, S.E. Vulnerabilities of Marine Mammals to Harmful Algal Blooms. In Harmful Algal Blooms; Wiley: Hoboken, NJ, USA, 2018; pp. 191–222. ISBN 9781118994672. [Google Scholar]
- Turner, A.D.; Dhanji-Rapkova, M.; Dean, K.; Milligan, S.; Hamilton, M.; Thomas, J.; Poole, C.; Haycock, J.; Spelman-Marriott, J.; Watson, A.; et al. Fatal Canine Intoxications Linked to the Presence of Saxitoxins in Stranded Marine Organisms Following Winter Storm Activity. Toxins 2018, 10, 94. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xu, C.; Liu, Y.; Jeppesen, E.; Svenning, J.C.; Wu, J.; Zhang, W.; Zhou, T.; Wang, P.; Nangombe, S.; et al. From Unusual Suspect to Serial Killer: Cyanotoxins Boosted by Climate Change May Jeopardize Megafauna. Innovation 2021, 2, 100092. [Google Scholar] [CrossRef]
- García, C.; Bravo, M.D.C.; Lagos, M.; Lagos, N. Paralytic Shellfish Poisoning: Post-Mortem Analysis of Tissue and Body Fluid Samples from Human Victims in the Patagonia Fjords. Toxicon 2004, 43, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Vale, P. Shellfish Contamination with Marine Biotoxins in Portugal and Spring Tides: A Dangerous Health Coincidence. Environ. Sci. Pollut. Res. 2020, 27, 41143–41156. [Google Scholar] [CrossRef]
- Roberts, V.A.; Vigar, M.; Backer, L.; Veytsel, G.E.; Hilborn, E.D.; Hamelin, E.I.; Vanden, K.L.; Lively, J.Y.; Cope, J.R.; Hlavsa, M.C.; et al. Surveillance for Harmful Algal Bloom Events and Associated Human and Animal Illnesses-One Health Harmful Algal Bloom System, United States, 2016-2018; Morbidity and Mortality Weekly Report; CDC: Atlanta, GA, USA, 2020; Volume 69. [Google Scholar]
- European Union. Regulation (EC) No 853/2004 of the European Parliament and of the Council of 29 April 2004 Laying Down Specific Hygiene Rules for Food of Animal Origin; European Union: Brussels, Belgium, 2004. [Google Scholar]
- Wiese, M.; D’Agostino, P.M.; Mihali, T.K.; Moffitt, M.C.; Neilan, B.A. Neurotoxic Alkaloids: Saxitoxin and Its Analogs. Mar. Drugs 2010, 8, 2185–2211. [Google Scholar] [CrossRef]
- Pérez-Gómez, A.; Tasker, R.A. Domoic Acid as a Neurotoxin. In Handbook of Neurotoxicity, 2nd ed.; Springer International Publishing: Cham, Switzerland, 2023; Volume 2, pp. 873–897. ISBN 9783031150807. [Google Scholar]
- Valdiglesias, V.; Prego-Faraldo, M.V.; Paśaro, E.; Meńdez, J.; Laffon, B. Okadaic Acid: More than a Diarrheic Toxin. Mar. Drugs 2013, 11, 4328–4349. [Google Scholar] [CrossRef]
- Doucette, G.; Maneiro, I.; Riveiro, I.; Svensen, C. Phycotoxin Pathways in Aquatic Food Webs: Transfer, Accumulation and Degradation. In Ecology of Harmful Algae; Springer: Berlin/Heidelberg, Germany, 2006; pp. 283–295. [Google Scholar]
- Bargu, S.; Silver, M.W.; Ohman, M.D.; Benitez-Nelson, C.R.; Garrison, D.L. Mystery behind Hitchcock’s Birds. Nat. Geosci. 2012, 5, 2–3. [Google Scholar] [CrossRef]
- Reis Costa, P. Impact and Effects of Paralytic Shellfish Poisoning Toxins Derived from Harmful Algal Blooms to Marine Fish. Fish Fish. 2016, 17, 226–248. [Google Scholar] [CrossRef]
- Corriere, M.; Soliño, L.; Costa, P.R. Effects of the Marine Biotoxins Okadaic Acid and Dinophysistoxins on Fish. J. Mar. Sci. Eng. 2021, 9, 293. [Google Scholar] [CrossRef]
- Turner, A.D.; Powell, A.; Schofield, A.; Lees, D.N.; Baker-Austin, C. Detection of the Pufferfish Toxin Tetrodotoxin in European Bivalves, England, 2013 to 2014. Eurosurveillance 2015, 20, 21009. [Google Scholar] [CrossRef]
- Antonelli, P.; Salerno, B.; Bordin, P.; Peruzzo, A.; Orsini, M.; Arcangeli, G.; Barco, L.; Losasso, C. Tetrodotoxin in Live Bivalve Mollusks from Europe: Is It to Be Considered an Emerging Concern for Food Safety? Compr. Rev. Food Sci. Food Saf. 2022, 21, 719–737. [Google Scholar] [CrossRef] [PubMed]
- Soliño, L.; Gouveia, N.; Timóteo, V.; Costa, P.R. New Insights into the Occurrence of Paralytic Shellfish Toxins in the Oceanic Pufferfish Lagocephalus lagocephalus (Linnaeus, 1758) from Madeira Island, Portugal. Reg. Stud. Mar. Sci. 2021, 42, 101657. [Google Scholar] [CrossRef]
- Kosker, A.R.; Özogul, F.; Ayas, D.; Durmus, M.; Ucar, Y.; Regenstein, J.M.; Özogul, Y. Tetrodotoxin Levels of Three Pufferfish Species (Lagocephalus sp.) Caught in the North-Eastern Mediterranean Sea. Chemosphere 2019, 219, 95–99. [Google Scholar] [CrossRef]
- Guardone, L.; Gasperetti, L.; Maneschi, A.; Ricci, E.; Susini, F.; Guidi, A.; Armani, A. Toxic Invasive Pufferfish (Tetraodontidae Family) along Italian Coasts: Assessment of an Emerging Public Health Risk. Food Control 2018, 91, 330–338. [Google Scholar] [CrossRef]
- Pinto, E.P.; Rodrigues, S.M.; Gouveia, N.; Timóteo, V.; Costa, P.R. Tetrodotoxin and Saxitoxin in Two Native Species of Puffer Fish, Sphoeroides marmoratus and Lagocephalus lagocephalus, from NE Atlantic Ocean (Madeira Island, Portugal). Mar. Environ. Res. 2019, 151, 104780. [Google Scholar] [CrossRef]
- Katikou, P.; Gokbulut, C.; Kosker, A.R.; Campàs, M.; Ozogul, F. An Updated Review of Tetrodotoxin and Its Peculiarities. Mar. Drugs 2022, 20, 47. [Google Scholar] [CrossRef] [PubMed]
- Carmichael, W.W. Health Effects of Toxin-Producing Cyanobacteria: “The CyanoHABs”. Hum. Ecol. Risk Assess. 2001, 7, 1393–1407. [Google Scholar] [CrossRef]
- Thawabteh, A.M.; Naseef, H.A.; Karaman, D.; Bufo, S.A.; Scrano, L.; Karaman, R. Understanding the Risks of Diffusion of Cyanobacteria Toxins in Rivers, Lakes, and Potable Water. Toxins 2023, 15, 582. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, J.S.; Codd, G.A. Cyanotoxins. In Ecology of Cyanobacteria II: Their Diversity in Space and Time; Springer: Dordrecht, The Netherlands, 2012; pp. 651–675. [Google Scholar]
- Cheung, M.Y.; Liang, S.; Lee, J. Toxin-Producing Cyanobacteria in Freshwater: A Review of the Problems, Impact on Drinking Water Safety, and Efforts for Protecting Public Health. J. Microbiol. 2013, 51, 1–10. [Google Scholar] [CrossRef]
- Stewart, I.; Seawright, A.A.; Shaw, G.R. Cyanobacterial Poisoning in Livestock, Wild Mammals and Birds—An Overview. In Cyanobacterial Harmful Algal Blooms: State of the Science and Research Needs; Hudnell, H.K., Ed.; Springer: New York, NY, USA, 2008; pp. 613–637. ISBN 978-0-387-75865-7. [Google Scholar]
- Livesay, H.N.; Vance, P.H.; Trevino, E.; Weissfeld, A.S. Algae-Associated Illnesses in Humans and Dogs and Presence of Algae on Buildings and Other Structures. Clin. Microbiol. Newsl. 2021, 43, 9–13. [Google Scholar] [CrossRef]
- Paerl, H.W.; Scott, J.T. Throwing Fuel on the Fire: Synergistic Effects of Excessive Nitrogen Inputs and Global Warming on Harmful Algal Blooms. Environ. Sci. Technol. 2010, 44, 7756–7758. [Google Scholar] [CrossRef]
- Trainer, V.L.; Moore, S.K.; Hallegraeff, G.; Kudela, R.M.; Clement, A.; Mardones, J.I.; Cochlan, W.P. Pelagic Harmful Algal Blooms and Climate Change: Lessons from Nature’s Experiments with Extremes. Harmful Algae 2020, 91, 101591. [Google Scholar] [CrossRef]
- Tester, P.A.; Litaker, R.W.; Berdalet, E. Climate Change and Harmful Benthic Microalgae. Harmful Algae 2020, 91, 101655. [Google Scholar] [CrossRef]
- Burford, M.A.; Carey, C.C.; Hamilton, D.P.; Huisman, J.; Paerl, H.W.; Wood, S.A.; Wulff, A. Perspective: Advancing the Research Agenda for Improving Understanding of Cyanobacteria in a Future of Global Change. Harmful Algae 2020, 91, 101601. [Google Scholar] [CrossRef]
- Dias, M.P.; Martin, R.; Pearmain, E.J.; Burfield, I.J.; Small, C.; Phillips, R.A.; Yates, O.; Lascelles, B.; Borboroglu, P.G.; Croxall, J.P. Threats to Seabirds: A Global Assessment. Biol. Conserv. 2019, 237, 525–537. [Google Scholar] [CrossRef]
- Griffith, A.W.; Gobler, C.J. Harmful Algal Blooms: A Climate Change Co-Stressor in Marine and Freshwater Ecosystems. Harmful Algae 2020, 91, 101590. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.; Parrish, J.K.; Punt, A.E.; Trainer, V.L.; Kudela, R.; Lang, J.; Brancato, M.S.; Odell, A.; Hickey, B. Mass Mortality of Marine Birds in the Northeast Pacific Caused by Akashiwo sanguinea. Mar. Ecol. Prog. Ser. 2017, 579, 111–127. [Google Scholar] [CrossRef]
- Starr, M.; Lair, S.; Michaud, S.; Scarratt, M.; Quilliam, M.; Lefaivre, D.; Robert, M.; Wotherspoon, A.; Michaud, R.; Ménard, N.; et al. Multispecies Mass Mortality of Marine Fauna Linked to a Toxic Dinoflagellate Bloom. PLoS ONE 2017, 12, e0176299. [Google Scholar] [CrossRef] [PubMed]
- Silvagni, P.A.; Lowenstine, L.J.; Spraker, T.; Lipscomb, T.P.; Gulland, F.M.D. Pathology of Domoic Acid Toxicity in California Sea Lions (Zalophus californianus). Vet. Pathol. 2005, 42, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Lugomela, C.; Pratap, H.B.; Mgaya, Y.D. Cyanobacteria Blooms—A Possible Cause of Mass Mortality of Lesser Flamingos in Lake Manyara and Lake Big Momela, Tanzania. Harmful Algae 2006, 5, 534–541. [Google Scholar] [CrossRef]
- Fernández, A.; Sierra, E.; Arbelo, M.; Gago-Martínez, A.; Leao Martins, J.M.; García-Álvarez, N.; Bernaldo de Quiros, Y.; Arregui, M.; Vela, A.I.; Díaz-Delgado, J. First Case of Brevetoxicosis Linked to Rough-Toothed Dolphin (Steno bredanensis) Mass-Mortality Event in Eastern Central Atlantic Ocean: A Climate Change Effect? Front. Mar. Sci. 2022, 9, 834051. [Google Scholar] [CrossRef]
- Rattner, B.A.; Wazniak, C.E.; Lankton, J.S.; McGowan, P.C.; Drovetski, S.V.; Egerton, T.A. Review of Harmful Algal Bloom Effects on Birds with Implications for Avian Wildlife in the Chesapeake Bay Region. Harmful Algae 2022, 120, 102319. [Google Scholar] [CrossRef]
- Shumway, S.E.; Allen, S.M.; Dee Boersma, P. Marine Birds and Harmful Algal Blooms: Sporadic Victims or under-Reported Events? Harmful Algae 2003, 2, 1–17. [Google Scholar] [CrossRef]
- Sistema Nacional de Monitorização de Moluscos Bivalves. Available online: https://www.Ipma.Pt/Pt/Bivalves/Index.Jsp (accessed on 3 March 2025).
- Casero, M.V.M.; Ramos, J.A.; Pereira, L. Seabirds and Biotoxins. In Volume 1: Seabird Biodiversity and Human Activities; CRC Press: Boca Raton, FL, USA, 2022; pp. 126–134. [Google Scholar]
- Mena, M.V.; Turner, A.D.; Ben-Gigirey, B.; Alexander, R.P.; Dean, K.J.; Hatfield, R.G.; Maskrey, B.H.; Mazuef, C.; Karamendin, K.; Mateo, R. Identifying Causative Agents of a Paretic Syndrome in Waterbirds in Southern Portugal. Toxins 2025, 17, 62. [Google Scholar] [CrossRef]
- Turner, A.D.; McNabb, P.S.; Harwood, D.T.; Selwood, A.I.; Boundy, M.J. Single-Laboratory Validation of a Multitoxin Ultra-Performance LC-Hydrophilic Interaction LC-MS/MS Method for Quantitation of Paralytic Shellfish Toxins in Bivalve Shellfish. J. AOAC Int. 2015, 98, 609–621. [Google Scholar] [CrossRef]
- Turner, A.D.; Waack, J.; Lewis, A.; Edwards, C.; Lawton, L. Development and Single-Laboratory Validation of a UHPLC-MS/MS Method for Quantitation of Microcystins and Nodularin in Natural Water, Cyanobacteria, Shellfish and Algal Supplement Tablet Powders. J. Chromatogr. B 2018, 1074–1075, 111–123. [Google Scholar] [CrossRef]
- Dusek, R.J.; Smith, M.M.; Van Hemert, C.; Shearn-Bochsler, V.I.; Hall, S.; Ridge, C.D.; Hardison, D.R.; Kaler, R.S.A.; Bodenstein, B.L.; Hofmeister, E.K.; et al. Acute Oral Toxicity and Tissue Residues of Saxitoxin in the Mallard (Anas platyrhynchos). Harmful Algae 2020, 109, 102109. [Google Scholar] [CrossRef] [PubMed]
- Van Hemert, C.; Dusek, R.J.; Smith, M.M.; Kaler, R.; Sheffield, G.; Divine, L.M.; Kuletz, K.J.; Knowles, S.; Lankton, J.S.; Hardison, D.R.; et al. Investigation of Algal Toxins in a Multispecies Seabird Die-Off in the Bering and Chukchi Seas. J. Wildl. Dis. 2021, 57, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Van Hemert, C.; Harley, J.R.; Baluss, G.; Smith, M.M.; Dusek, R.J.; Lankton, J.S.; Hardison, D.R.; Schoen, S.K.; Kaler, R.S.A. Paralytic Shellfish Toxins Associated with Arctic Tern Mortalities in Alaska. Harmful Algae 2022, 117, 102270. [Google Scholar] [CrossRef] [PubMed]
- Cadaillon, A.M.; Mattera, B.; Albizzi, A.; Montoya, N.; Maldonado, S.; Raya Rey, A.; Riccialdelli, L.; Almandoz, G.O.; Schloss, I.R. Multispecies Mass Mortality in the Beagle Channel Associated with Paralytic Shellfish Toxins. Harmful Algae 2024, 132, 102581. [Google Scholar] [CrossRef]
- Shearn-Bochsler, V.; Lance, E.W.; Corcoran, R.; Piatt, J.; Bodenstein, B.; Frame, E.; Lawonn, J. Fatal Paralytic Shellfish Poisoning in Kittlitz’s Murrelet (Brachyramphus brevirostris) Nestlings, Alaska, USA. J. Wildl. Dis. 2014, 50, 933–937. [Google Scholar] [CrossRef]
- Van Hemert, C.; Schoen, S.K.; Litaker, R.W.; Smith, M.M.; Arimitsu, M.L.; Piatt, J.F.; Holland, W.C.; Ransom Hardison, D.; Pearce, J.M. Algal Toxins in Alaskan Seabirds: Evaluating the Role of Saxitoxin and Domoic Acid in a Large-Scale Die-off of Common Murres. Harmful Algae 2020, 92, 101730. [Google Scholar] [CrossRef]
- Gibble, C.M.; Kudela, R.M.; Knowles, S.; Bodenstein, B.; Lefebvre, K.A. Domoic Acid and Saxitoxin in Seabirds in the United States between 2007 and 2018. Harmful Algae 2021, 103, 101981. [Google Scholar] [CrossRef]
- Levasseur, M.; Michaud, S.; Bonneau, E.; Cantin, G.; Auger, F.; Gagne, A.; Claveau, R. Overview of the August 1996 Red Tide Event in the St. Lawrence: Effects of a Storm Surge. In Canadian Technical Report of Fisheries and Aquatic Sciences No. 2138, Proceedings of the Fifth Canadian Workshop on Harmful Marine Algae, St. John’s, NF, USA, 11–13 September 1996; Minister of Public Works and Government Services: St. John’s, NB, Canada, 1996; p. 76. [Google Scholar]
- Uhart, M.; Karesh, W.; Cook, R.; Huin, N.; Lawrence, K.; Guzman, L.; Pacheco, H.; Pizarro, G.; Mattsson, R.; Mörner, T. Paralytic Shellfish Poisoning in Gentoo Penguins (Pygoscelis papua) from the Falkland (Malvinas) Islands. In Proceedings of the AAZV/AAWV/WDA Joint Conference; American Association of Zoo Veterinarians: Yulee, FL, USA, 2004; pp. 481–486. [Google Scholar]
- Greenwald, K.M.; Gibble, C.M.; Miller, M.A.; Donnelly-Greenan, E.; Kudela, R.M. Investigation of a Mass Stranding Event Reveals a Novel Pattern of Cascading Comorbidities in Northern Fulmars (Fulmarus glacialis). J. Wildl. Dis. 2024, 60, 171–178. [Google Scholar] [CrossRef]
- Piatt, J.F.; Parrish, J.K.; Renner, H.M.; Schoen, S.K.; Jones, T.T.; Arimitsu, M.L.; Kuletz, K.J.; Bodenstein, B.; García-Reyes, M.; Duerr, R.S.; et al. Extreme Mortality and Reproductive Failure of Common Murres Resulting from the Northeast Pacific Marine Heatwave of 2014–2016. PLoS ONE 2020, 15, e0226087. [Google Scholar] [CrossRef]
- Jones, T.; Divine, L.M.; Renner, H.; Knowles, S.; Lefebvre, K.A.; Burgess, H.K.; Wright, C.; Parrish, J.K. Unusual Mortality of Tufted Puffins (Fratercula cirrhata) in the Eastern Bering Sea. PLoS ONE 2019, 14, e0216532. [Google Scholar] [CrossRef] [PubMed]
- Montoya, N.G. Paralyzing Shellfish Toxins in the Argentine Sea: Impact, Trophic Transfer and Perspective. Mar. Fish. Sci. (MAFIS) 2019, 32, 47–69. [Google Scholar] [CrossRef]
- Montoya, N.G.; Carignan, M.O.; Carreto, J.I. Alexandrium tamarense/catenella Blooms in the Southwestern Atlantic: Paralytic Shellfish Toxin Production and Its Trophic Transference. In Plankton Ecology of the Southwestern Atlantic: From the Subtropical to the Subantarctic Realm; Springer International Publishing: Cham, Switzerland, 2018; pp. 453–476. ISBN 9783319778693. [Google Scholar]
- Papadimitriou, T.; Katsiapi, M.; Vlachopoulos, K.; Christopoulos, A.; Laspidou, C.; Moustaka-Gouni, M.; Kormas, K. Cyanotoxins as the “Common Suspects” for the Dalmatian Pelican (Pelecanus crispus) Deaths in a Mediterranean Reconstructed Reservoir. Environ. Pollut. 2018, 234, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Fischer, W.J.; Altheimer, S.; Cattori, V.; Meier, P.J.; Dietrich, D.R.; Hagenbuch, B. Organic Anion Transporting Polypeptides Expressed in Liver and Brain Mediate Uptake of Microcystin. Toxicol. Appl. Pharmacol. 2005, 203, 257–263. [Google Scholar] [CrossRef]
- Hinojosa, M.G.; Gutiérrez-Praena, D.; Prieto, A.I.; Guzmán-Guillén, R.; Jos, A.; Cameán, A.M. Neurotoxicity Induced by Microcystins and Cylindrospermopsin: A Review. Sci. Total Environ. 2019, 668, 547–565. [Google Scholar] [CrossRef]
- Alonso-Andicoberry, C.; García-Viliada, L.; Lopez-Rodas, V.; Costas, E. Catastrophic Mortality of Flamingos in a Spanish National Park Caused by Cyanobacteria. Vet. Rec. 2002, 151, 706. [Google Scholar]
- Krienitz, L.; Ballot, A.; Kotut, K.; Wiegand, C.; Pütz, S.; Metcalf, J.S.; Codd, G.A.; Stephan, P. Contribution of Hot Spring Cyanobacteria to the Mysterious Deaths of Lesser Flamingos at Lake Bogoria, Kenya. FEMS Microbiol. Ecol. 2003, 43, 141–148. [Google Scholar] [CrossRef]
- Pašková, V.; Adamovský, O.; Pikula, J.; Skočovská, B.; Band’ouchová, H.; Horáková, J.; Babica, P.; Maršálek, B.; Hilscherová, K. Detoxification and Oxidative Stress Responses along with Microcystins Accumulation in Japanese Quail Exposed to Cyanobacterial Biomass. Sci. Total Environ. 2008, 398, 34–47. [Google Scholar] [CrossRef]
- Nonga, H.E.; Sandvik, M.; Miles, C.O.; Lie, E.; Mdegela, R.H.; Mwamengele, G.L.; Semuguruka, W.D.; Skaare, J.U. Possible Involvement of Microcystins in the Unexplained Mass Mortalities of Lesser Flamingo (Phoeniconaias minor Geoffroy) at Lake Manyara in Tanzania. Hydrobiologia 2011, 678, 167–178. [Google Scholar] [CrossRef]
- Metcalf, J.S.; Morrison, L.F.; Krienitz, L.; Ballot, A.; Krause, E.; Kotut, K.; Pütz, S.; Wiegand, C.; Pflugmacher, S.; Codd, G.A. Analysis of the Cyanotoxins Anatoxin-a and Microcystins in Lesser Flamingo Feathers†. Toxicol. Environ. Chem. 2006, 88, 159–167. [Google Scholar] [CrossRef]
- Carmichael, W.W.; Li, R. Cyanobacteria Toxins in the Salton Sea. Saline Syst. 2006, 2, 5. [Google Scholar] [CrossRef] [PubMed]
- Skocovska, B.; Hilscherova, K.; Babica, P.; Adamovsky, O.; Bandouchova, H.; Horakova, J.; Knotkova, Z.; Marsalek, B.; Paskova, V.; Pikula, J. Effects of Cyanobacterial Biomass on the Japanese Quail. Toxicon 2007, 49, 793–803. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Rodas, V.; Maneiro, E.; Lanzarot, M.P.; Perdigones, N.; Costas, E. Mass Wildlife Mortality Due to Cyanobacteria in the Doñana National Park, Spain. Vet. Rec. 2008, 162, 317–318. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.Ipma.Pt/En/Bivalves/Index.Jsp (accessed on 3 March 2025).
- Garthe, S.; Peschko, V.; Fifield, D.A.; Borkenhagen, K.; Nyegaard, T.; Dierschke, J. Migratory Pathways and Winter Destinations of Northern Gannets Breeding at Helgoland (North Sea): Known Patterns and Increasing Importance of the Baltic Sea. J. Ornithol. 2024, 165, 869–880. [Google Scholar] [CrossRef]
- Mendes, R.F.; Ramos, J.A.; Paiva, V.H.; Calado, J.G.; Matos, D.M.; Ceia, F.R. Foraging Strategies of a Generalist Seabird Species, the Yellow-Legged Gull, from GPS Tracking and Stable Isotope Analyses. Mar. Biol. 2018, 165, 168. [Google Scholar] [CrossRef]
- Oshima, Y. Postcolumn Derivatization Liquid Chromatographic Method for Paralytic Shellfish Toxins. J. AOAC Int. 1995, 78, 528–532. [Google Scholar] [CrossRef]
- EFSA. Marine Biotoxins in Shellfish—Saxitoxin Group. EFSA J. 2009, 7, 1019. [Google Scholar] [CrossRef]
- FAO; WHO. Toxicity Equivalency Factors for Marine Biotoxins Associated with Bivalve Molluscs; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Costa, P.R.; Robertson, A.; Quilliam, M.A. Toxin Profile of Gymnodinium catenatum (Dinophyceae) from the Portuguese Coast, as Determined by Liquid Chromatography Tandem Mass Spectrometry. Mar. Drugs 2015, 13, 2046–2062. [Google Scholar] [CrossRef]
- Lage, S.; Costa, P.R.; Canário, A.V.M.; Da Silva, J.P. LC-HRMS Profiling of Paralytic Shellfish Toxins in Mytilus galloprovincialis after a Gymnodinium catenatum Bloom. Mar. Drugs 2022, 20, 680. [Google Scholar] [CrossRef]
- Leal, J.F.; Bombo, G.; Pereira, H.; Vicente, B.; Amorim, A.; Cristiano, M.L.S. Toxin Profile of Two Gymnodinium catenatum Strains from Iberian Coastal Waters. Toxins 2022, 14, 762. [Google Scholar] [CrossRef]
- Vale, C.; Alfonso, A.; Vieytes, M.R.; Romarís, X.M.; Arevalo, F.; Botana, A.M.; Botana, L.M. In Vitro and in Vivo Evaluation of Paralytic Shellfish Poisoning Toxin Potency and the Influence of the PH of Extraction. Anal. Chem. 2008, 80, 1770–1776. [Google Scholar] [CrossRef]
- Costa, P.R.; Botelho, M.J.; Lefebvre, K.A. Characterization of Paralytic Shellfish Toxins in Seawater and Sardines (Sardina pilchardus) during Blooms of Gymnodinium catenatum. Hydrobiologia 2010, 655, 89–97. [Google Scholar] [CrossRef]
- Costa, P.R.; Pereira, P.; Guilherme, S.; Barata, M.; Nicolau, L.; Santos, M.A.; Pacheco, M.; Pousão-Ferreira, P. Biotransformation Modulation and Genotoxicity in White Seabream upon Exposure to Paralytic Shellfish Toxins Produced by Gymnodinium catenatum. Aquat. Toxicol. 2012, 106–107, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.R.; Lage, S.; Barata, M.; Pousão-Ferreira, P. Uptake, Transformation, and Elimination Kinetics of Paralytic Shellfish Toxins in White Seabream (Diplodus sargus). Mar. Biol. 2011, 158, 2805–2811. [Google Scholar] [CrossRef]
- Paerl, H.W.; Otten, T.G.; Kudela, R. Mitigating the Expansion of Harmful Algal Blooms Across the Freshwater-to-Marine Continuum. Environ. Sci. Technol. 2018, 52, 5519–5529. [Google Scholar] [CrossRef] [PubMed]
- Preece, E.P.; Hardy, F.J.; Moore, B.C.; Bryan, M. A Review of Microcystin Detections in Estuarine and Marine Waters: Environmental Implications and Human Health Risk. Harmful Algae 2017, 61, 31–45. [Google Scholar] [CrossRef]
- BirdLife International. BirdLife International (2024) Species Factsheet: Calidris Alba. 2024. Available online: https://datazone.birdlife.org/search?search=Calidris%20alba (accessed on 8 May 2024).
- De Pace, R.; Valeria, V.; Silvia, B.M.; Pasquale, G.; Milena, B. Microcystin Contamination in Sea Mussel Farms from the Italian Southern Adriatic Coast Following Cyanobacterial Blooms in an Artificial Reservoir. J. Ecosyst. 2014, 2014, 374027. [Google Scholar] [CrossRef]
- Sonne, C.; Alstrup, A.K.O.; Therkildsen, O.R. A Review of the Factors Causing Paralysis in Wild Birds: Implications for the Paralytic Syndrome Observed in the Baltic Sea. Sci. Total Environ. 2012, 416, 32–39. [Google Scholar] [CrossRef]
- Soares, S.; Lopes, H.; Azevedo, F.; Valkenburg, T.; Ventura, T.; Nunes, T.; Madeira de Carvalho, L. Paretic Syndrome in Gulls (Laridae) in the South of Portugal. Master’s Thesis, Universidade de Lisboa, Lisbon, Portugal, 2014. [Google Scholar]
- Li, X.-Y. Exposure to Crude Microcystins via Intraperitoneal Injection, but Not Oral Gavage, Causes Hepatotoxicity in Ducks. Afr. J. Biotechnol. 2012, 11, 10894–10898. [Google Scholar] [CrossRef]
- Rocke, T.E.; Bollinger, T.K. Avian Botulism. In Infectious Diseases of Wild Birds; Blackwell Publishing: Hoboken, NJ, USA, 2007; pp. 377–416. [Google Scholar]
- Landsberg, J.H.; Vargo, G.A.; Flewelling, L.J.; Wiley, F.E. Algal Biotoxins. In Infectious and Parasitic Diseases of Wild Birds; Blackwell Press: Oxford, UK, 2007; pp. 431–455. [Google Scholar]
- Murphy, T.; Lawson, A.; Nalewajko, C.; Murkin, H.; Ross, L.; Oguma, K.; McIntyre, T. Algal Toxins—Initiators of Avian Botulism? Environ. Toxicol. 2000, 15, 558–567. [Google Scholar] [CrossRef]
- Majó, N.; Dolz, R. Atlas de La Necropsia Aviar: Diagnóstico Macroscópico: Toma de Muestras; Servet: Bellaterra, Barcelona, Spain, 2011; ISBN 8492569360. [Google Scholar]
- Boundy, M.J.; Selwood, A.I.; Harwood, D.T.; McNabb, P.S.; Turner, A.D. Development of a Sensitive and Selective Liquid Chromatography–Mass Spectrometry Method for High Throughput Analysis of Paralytic Shellfish Toxins Using Graphitised Carbon Solid Phase Extraction. J. Chromatogr. A 2015, 1387, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Turner, A.D.; Dhanji-Rapkova, M.; Fong, S.Y.T.; Hungerford, J.; McNabb, P.S.; Boundy, M.J.; Harwood, D.T.; Collaborators. Ultrahigh-Performance Hydrophilic Interaction Liquid Chromatography with Tandem Mass Spectrometry Method for the Determination of Paralytic Shellfish Toxins and Tetrodotoxin in Mussels, Oysters, Clams, Cockles, and Scallops: Collaborative Study. J. AOAC Int. 2020, 103, 533–562. [Google Scholar] [CrossRef] [PubMed]
- Rourke, W.A.; Murphy, C.J.; Pitcher, G.; van de Riet, J.M.; Burns, B.G.; Thomas, K.M.; Quilliam, M.A. Rapid Postcolumn Methodology for Determination of Paralytic Shellfish Toxins in Shellfish Tissue. J. AOAC Int. 2008, 91, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, F.; Garrido, J.L.; Sobrino, C.; Johnsen, G.; Riobó, P.; Franco, J.; Aamot, I.; Ramilo, I.; Sanz, N.; Kremp, A. Divinyl Chlorophyll a in the Marine Eukaryotic Protist Alexandrium ostenfeldii (Dinophyceae). Environ. Microbiol. 2016, 18, 627–643. [Google Scholar] [CrossRef]
| Admission Number | Admission Date | Place | Species (Latin Name/Common Name) | Tissue | dcSTX (µg/kg) | MCs (µg/kg) |
|---|---|---|---|---|---|---|
| V0048/20/A | 8 January 2020 | Portimão | Larus michahellis yellow-legged gull | Liver | 6.5 | <LOD |
| Kidney | <LOD | <LOD | ||||
| M1719/20 | 21 July 2020 | STP | Ichthyaetus audouinii Audouin’s gull | Liver | 6.6 | <LOD |
| Kidney | <LOD | <LOD | ||||
| M2527/20 | 12 October2020 | Albufeira | Morus bassanus Northern gannet | Liver | 8.7 | <LOD |
| Kidney | <LOD | <LOD | ||||
| V2626/20/A | 24 October 2020 | Quarteira | L. michahellis yellow-legged gull | Liver | 5.5 | <LOD |
| Kidney | <LOD | <LOD | ||||
| V2641/20/A | 26 October 2020 | Quarteira | Larus fuscus lesser black-backed gull | Liver | 5.6 | <LOD |
| M2693/20/A | 2 November 2020 | Portimão | L. michahellis yellow-legged gull | Liver | 5.7 | <LOD |
| Kidney | <LOD | <LOD | ||||
| M0183/19/A | 12 March 2019 | Quarteira | L. fuscus lesser black-backed gull | Liver | <LOD | 5.8 |
| M1997/19/A | 13 September 2019 | Albufeira | L. michahellis yellow-legged gull | Liver | <LOD | 5.2 |
| Kidney | <LOD | <LOD | ||||
| V0427/20/A | 28 February 2020 | Quelfes | L. michahellis yellow-legged gull | Liver | <LOD | 7.4 |
| M0441/20/A | 2 March 2020 | Albufeira | L. michahellis yellow-legged gull | Liver | <LOD | 7.4 |
| M1001/20/A | 15 May 2020 | Portimão | L. michahellis yellow-legged gull | Liver | <LOD | 7.2 |
| Kidney | <LOD | <LOD | ||||
| M1897/20/A | 31 July 2020 | STP | I. audouinii Audouin’s gull | Liver | <LOD | 1.6 |
| M2528/20/A | 12 October 2020 | Ferragudo | L. michahellis yellow-legged gull | Cloaca | <LOD | 4.9 |
| V2603/20/A | 22 October 2020 | Guia | M. bassanus Northern gannet | Kidney | <LOD | 9.6 |
| Liver | <LOD | <LOD | ||||
| V2617/20/A | 23 October 2020 | Tavira | M. bassanus Northern gannet | Liver | <LOD | 30.2 |
| Kidney | <LOD | <LOD | ||||
| V2787/20/A | 15 November 2020 | Albufeira | L. michahellis yellow-legged gull | Liver | <LOD | 4.8 |
| Kidney | <LOD | <LOD | ||||
| M2811/20/A | 16 November 2020 | Alvor | Calidris alba sanderling | Liver | <LOD | 27.2 |
| Kidney | <LOD | <LOD |
| Species (Latin Name/Common Name) | N° Individuals | PST Concentration Range (Minimum < LOQ) | Units | Analogues/Total Toxicity | Tissue | Symptoms | Necropsy | Observations | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Alca torda/Razorbill | 5 | <LOD (5.8)–15 | µg STXeq/100 g | Total toxicity, C1, C2, GTX2, GTX3, STX | Liver | Death | Good body condition. No other significant findings | ELISA and LC-FLD | [41] |
| Anas platyrhynchos/Mallard | 14 | <LOD (2)–106.3/ 3.6 | µg STXeq/100 g | Total toxicity/ dcSTX | Small intestine/Liver | Weight loss, head shaking, excessive drinking, regurgitating, wing twitching and settling, tail wagging, death | No specific abnormalities or tissue pathology | STX orally administered. dcSTX detected in the liver. Lowest lethal dose = 110 µg/kg STX, LD50% = 167 µg/kg | [53] |
| Brachyramphus brevirostris/Kittlitz’s Murrelet | 9 | <LOD (56.3)–106.4 | ng/g STXeq | Total toxicity | Liver | Chick dead shortly after consuming sand lance | Good body condition. Nematode infestation in five birds. No other significant findings | ELISA, samples with lowest levels likely due to improper preservation (ethanol) | [56] |
| Caracara plancus/Southern or crested caracara | 1 | 29.59 | µg STXeq/kg | GTX2/3, STX | Liver | Death | Good body condition. No other significant findings | HPLC-FLD | [55] |
| Cepphus grylle/Black guillemot | 8 | <LOD (20)–41 | µg STXeq/100 g | Total toxicity | Liver | Death | Good body condition. No other significant findings | ELISA | [41] |
| Fratercula cirrhata/Tufted puffin | 4 | 3.1–9.5 | ng/g STXeq | Total toxicity | Stomach and cloaca contents | Death | Emaciation. No other significant findings | ELISA. Most birds in flight feather moult. Toxins considered not the primary cause of death | [63] |
| Fulmarus glacialis/Northern fulmar | 18 | <LOD (1.4)–5.9 | µg STXeq/100 g | Total toxicity | Liver | Death. Dying animals showed weakness, lethargy, drooping heads, staggering, and lack of predador avoidance | Most in poor body condition and evidence of drowning. Some with evidence of blood in gastrointestinal tract | ELISA and HPLC, C1, 2; GTX5; STX; GTX1, 4; NEO in stomach contents | [53] |
| Fulmarus glacialis/Northern fulmar | 2 | 6.87 | ng/g STXeq | Total toxicity | Liver | Death | Emaciation, renal coccidiosis, bacterial pyelonephritis, dehydration with urate stasis, ureteral rupture | ELISA, highest level in bile | [58,61] |
| Gavia immer/Comon loon | 2 | <LOD-7.7 | µg STXeq/100 g | Total toxicity | Liver | Death | One thin, the other in good body condition. No other significant findings | ELISA | [41] |
| Gavia stellate/Red-throated loon | 1 | 6.1 | µg STXeq/100 g | Total toxicity | Digestive tract | Death | Thin. No other significant findings | ELISA, <LOD in the liver | [41] |
| Gull not identified | 2 | <LOD-33.7 | µg STXeq/100 g | Total toxicity | Liver | Death | One thin, other in good body condition. No other significant findings | ELISA. A liver with <LOD analyzed by LC-FLD; toxins not detected | [41] |
| Larus argentatus/Herring gull | 7 | <LOD-10 | µg STXeq/100 g | Total toxicity | Liver | Death | Good body condition, pancreatitis in one individual. No other significant findings | ELISA. A liver with <LOD analyzed by LC-FLD; toxins not detected | [41] |
| Larus argentatus/Herring gull | - | 110 | µg STXeq/100 g | Total toxicity | Intestine | Death | - | HPLC | [59] |
| Larus delawarensis/Ring-billed gull | 2 | 42 | µg STXeq/100 g | Total toxicity | Digestive tract | Death | Good nutritional condition. No other apparent pathological lesions | ELISA, toxins in the liver <LOD | [41] |
| Larus dominicus/Kelp gull | - | 39 | nmol/g | GTX1/4 | Intestine | Death | - | HPLC-FLD. GTX4 was present in all studied tissues (intestine, stomach, liver, and kidney) | [64,65] |
| Larus dominicus/Kelp gull | 8 | 15.46 | µg/kg STXeq | GTX3/2, trace levels of STX | Liver (pooled) | Death | Good body condition. No other significant findings | HPLC–FLD. Selected animals in good nutritional condition and with stomach contents for PST analysis | [55] |
| Larus philadelphia/Bonaparte’s gull | 1 | 0.01, 0.02, 2.8 | µg 100 g | C1, C2, STX | Gastrointestinal contents | Death | Good nutritional condition. No other apparent pathological lesions | LC–FLD | [41] |
| Melanita deglandi/White–winged scoter | 4 | <LOD (4.68)–6.4 | ng/g STXeq | Total toxicity | Liver | Death | - | ELISA | [58] |
| Melanita Perspicillata/Surf scoter | 3 | <LOD-4.68 | ng/g STXeq | Total toxicity | Intestinal contents | Death | - | ELISA | [58] |
| Morus bassanus/Northern gannet | 5 | <LOD (4.7)–85 | µg STXeq/100 g | Total toxicity | Liver | Death | Two of them thin, no significant findings. Highest toxin content in those with good body condition | ELISA | [41] |
| Pelecanus crispus/Dalmatian pelican | 10 | 0~25 | ng/g | Total toxicity | Liver | Decreased movement before death, not opisthotonus | - | ELISA. Cylindrospermopsins and MCs also present. The highest concentration in stomach contents | [66] |
| Phalacrocorax auritus/Double–crested cormorant | 19 | <LOD (4.6)–9.8 | µg STXeq/100 g | Total toxicity | Liver | Death | Good body condition for most of them, some thin. Pneumonia and aspergillosis observed in a thin hatch-year female individual. No other significant findings | ELISA. Only STX detected by LC-FLD in GI contents | [41] |
| Phalacrocorax penicillatus/Brandt’s cormorant | 2 | <LOD-2.0 | ng/g STXeq | Total toxicity | Stomach contents | Death | Emaciated | ELISA. DA also detected | [58] |
| Pygoscelis papua/Papua or gentoo penguin | 1 | 43 | µg STXeq/kg | GTX2/3, STX | Liver | Death | Good body condition. No other significant findings | HPLC-FLD. Selected animals in good nutritional condition and with stomach contents for PST analysis | [55] |
| Rissa tridactyla/Black-legged kittiwake | 52 | <LOD (4.2)–8.8 | µg STXeq/100 g | Total toxicity, only STX | Liver | Death | Some of them were thin. No other significant findings | ELISA, LC-FLD | [41] |
| Rissa tridactyla/Black-legged kittiwake | 59 | <LOD-2.7 | µg STXeq/100 g | Total toxicity | Liver | Healthy | Good nutritional condition. No other apparent pathological lesions | ELISA | [57] |
| Somateria mollissima/Common eider | 3 | <LOD (5.7)–74 | µg STXeq/100 g | Total toxicity | Digestive tract | Death | One was in good body condition. Two of them were thin; one presented granulomatous myopathy and the other, pasteurellosis | ELISA, toxins in the liver <LOD, highest values in the specimen with good body condition | [41] |
| Spheniscus magellanicus/ Magellanic penguin | 2 | 28–54 | µg STXeq/kg | GTX2 and 3, dcGTX2 and 3, STX, GTX1,4 at trace levels | Liver | Death | Good body condition. No other significant findings | HPLC–FLD. Selected animals in good nutritional condition and with stomach contents for PST analysis | [55] |
| Sterna paradisaea/Artic tern | 11 | <LOD (2)–5.9 | µg STXeq/100 g | Total toxicity | Liver | Death, convulsion | Most in fair body condition, no significant gross or microscopic abnormalities | ELISA, HPLC. Three nestling, one adult. C1,2, dcSTX, GTX2 and 3, GTX5 also found in liver | [54] |
| Thalasseus máxima/Royal tern | - | 37 | nmol/g | GTX1/4 | Intestine | Death | - | HPLC-FLD | [64,65] |
| Uria aalge/Common murre | 44 | <LOD-10.8 | µg STXeq/100 g | Total toxicity | Liver | Death, reproductive failure | Emaciation. No other apparent pathological lesions | ELISA | [57] |
| Uria aalge/Common murre | 16 | <LOD-1.3 | µg STXeq/100 g | Total toxicity | Upper gastrointestinal content | Healthy | Good nutritional condition. No other apparent pathological lesions | ELISA, no toxins detected in liver | [57] |
| Uria aalge/Common murre | 8 | 1.4–3.9 | ppb STXeq | Total toxicity | Proventriculus or cloaca | Death | Emaciated | ELISA. Toxins not considered the primary cause of death | [62] |
| Species (Latin Name/Common Name) | N° Individuals | MC Concentration Range (Minimum > LOD) | Units | Analogues | Tissue | Symptoms | Necropsy | Observations | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Anas platyrhynchos/Mallard | 2 | 172–218 | Total MCs ng/g (dry weight) | MC-LR, [D-Leu 1]MC-LR | Liver | Lethargy, dehydration, difficulty holding head up, dry eyelids | NA | LC-MS 2 MCs may not have been the primary cause of death (botulism?) | [5] |
| Anas platyrhynchos/Mallard | 3 | 31.1 | mg/g MC-LR eq | - | Liver | Depression, ataxia and paresis, rapid death | Intrahepatic hemorrhage, edema, and hepatomegaly. No other evidence of infectious disease | Mouse bioassay and commercial kit | [76] |
| Coturnix japonica/Japanese quail | 5 indiv × 5 groups | 2.2–43.7 (10 days), 0.47–7.5 (30 days) | ng/g (fresh weight) | NA | Liver | No mortality or clinical signs of pathology. Increased activities of lactate dehydrogenase and a drop in blood glucose | No gross pathological changes in inner organs. Hepatic changes with the highest doses | Birds exposed to daily dose of 0.2–224.46 ng/MCs for 10 or 30 days | [71,75] |
| Chroicocephalus ridibundus/Black-headed gull | 3 | 34.5 | mg/g MC-LR eq | - | Liver | Depression, ataxia and paresis, rapid death | Intrahepatic hemorrhage, edema, and hepatomegaly. No other evidence of infectious disease | Mouse bioassay and commercial kit | [76] |
| Fulica atra/Coot | 9 | 75.9 | mg/g MC-LR eq | - | Liver | Depression, ataxia and paresis, rapid death | Intrahepatic hemorrhage, edema, and hepatomegaly. No other evidence of infectious disease | Mouse bioassay and commercial kit | [76] |
| Pelecanus crispus/Dalmatian pelican | 10 | 0~300 | ng/g | - | Liver | Decreased movement before death, not opisthotonus | - | ELISA. Also cylindrospermopsins and STX | [66] |
| Phoeniconaias minor/Lesser flamingo | 2 | 0.196 | µg/g MC-LR eq (fresh weight) | MC-LR, MC-RR, MC-LF, MC-YR | Stomach contents | Ophistotonus behaviour, convulsed position of extremities and neck in the dying phase, death | NA | Also, anatoxin-a was found | [70] |
| Phoeniconaias minor/Lesser flamingo | 11 | 0.3-54.1 | 6 µg/g (wet weight) | MC-LR MC-YR MC-RR | Liver | Starvation and struggles prior to death | Emaciation, hemorrhagic lesions in the liver and muscles, enlargement of visceral organs | LC–MS/MS | [72] |
| Phoenicopterus roseus/Greater flamingo | 8 | 31,100–75,900 | ng/g | - | Liver | Death | No significant findings | Mouse bioassay and commercial kit. Values corrected from original manuscript in [5], | [5,69] |
| Podiceps cristatus/Great crested grebe | 6 | 53.2 | mg/g MC-LR eq | - | Liver | Depression, ataxia and paresis, rapid death | Intrahepatic hemorrhage, edema, and hepatomegaly. No other evidence of infectious disease | Mouse bioassay and commercial kit | [76] |
| Podiceps nigricollis/Eared grebe | 27 | <LOD (0.06)–110 | ng/g dry weight | - | Liver | Death | - | ELISA | [74] |
| Biotoxins | Samples Analyzed | Positives | Range (µg/kg) | Prevalence | Positive Organs |
|---|---|---|---|---|---|
| Paralytic Shellfish Toxins | 329 | 6 | 5.5–8.7 | 1.82% | Liver |
| Domoic Acid | 335 | 0 | - | 0% | - |
| Anatoxin-a | 315 | 0 | - | 0% | - |
| Cylindrospermins | 315 | 0 | - | 0% | - |
| Tetrodotoxins | 315 | 0 | - | 0% | - |
| Microcystins | 315 | 11 | 1.6–30.2 | 3.49% | Liver, cloaca content, and kidney |
| Month-Year | PST-Producing Phytoplankton (Alert and Interdiction Levels 500 and 1500 cells/L) | PSP Biotoxins | Observations | ||
|---|---|---|---|---|---|
| Density (Cells/L) | Harvesting Area | Concentration (µg STX Equiv/kg), Vector | Harvesting Area | ||
| March-2019 | 40 | TAV | 84, mussel | OLH3 | >2400 (µg STX equiv/kg) determined in Venus verrucosa and Donax trunculus from Portuguese central coast (Costa de Caparica, Comporta) |
| September-2019 | 160 | L7c2 | 36, mussel | L7c1 | >1500 µg STX equiv/kg) determined in Donax trunculus from Portuguese central coast (Costa de Caparica, Comporta) |
| January-2020 | 160 | TAV, FUZ | 33, mussel | L7c2 | |
| February-2020 | 1340 * | L7c2 | NQ | L8 | |
| March-2020 | 5880 ** | TAV | 39, mussel | L7c2 | >2400 µg STX equiv/kg determined in Donax trunculus from Portuguese central coast (Costa de Caparica) |
| May-2020 | 160 | LAG | NQ | NA | |
| July-2020 | ND | NA | NQ | NA | |
| October-2020 | ND | NA | NQ | NA | |
| November-2020 | ND | NA | NQ | NA | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soliño, L.; Turner, A.D.; Ben-Gigirey, B.; Alexander, R.P.; Dean, K.J.; Hatfield, R.G.; Maskrey, B.H.; Casero, M.V.M. Investigation into Paralytic Shellfish Toxins and Microcystins in Seabirds from Portugal. Toxins 2025, 17, 135. https://doi.org/10.3390/toxins17030135
Soliño L, Turner AD, Ben-Gigirey B, Alexander RP, Dean KJ, Hatfield RG, Maskrey BH, Casero MVM. Investigation into Paralytic Shellfish Toxins and Microcystins in Seabirds from Portugal. Toxins. 2025; 17(3):135. https://doi.org/10.3390/toxins17030135
Chicago/Turabian StyleSoliño, Lucía, Andrew D. Turner, Begoña Ben-Gigirey, Ryan P. Alexander, Karl J. Dean, Robert G. Hatfield, Benjamin H. Maskrey, and María V. Mena Casero. 2025. "Investigation into Paralytic Shellfish Toxins and Microcystins in Seabirds from Portugal" Toxins 17, no. 3: 135. https://doi.org/10.3390/toxins17030135
APA StyleSoliño, L., Turner, A. D., Ben-Gigirey, B., Alexander, R. P., Dean, K. J., Hatfield, R. G., Maskrey, B. H., & Casero, M. V. M. (2025). Investigation into Paralytic Shellfish Toxins and Microcystins in Seabirds from Portugal. Toxins, 17(3), 135. https://doi.org/10.3390/toxins17030135






