Multiple Mycotoxin Contamination in Livestock Feed: Implications for Animal Health, Productivity, and Food Safety
Abstract
1. Introduction
2. Mycotoxins of Economic Importance: Regulatory Framework and Global (Co-)Occurrence
2.1. Key Mycotoxins in Animal Agriculture
2.2. Regulatory Framework for Mycotoxin in Animal Feed
2.3. Prevalence of Multi-Mycotoxin Co-Occurrence in Livestock Feed
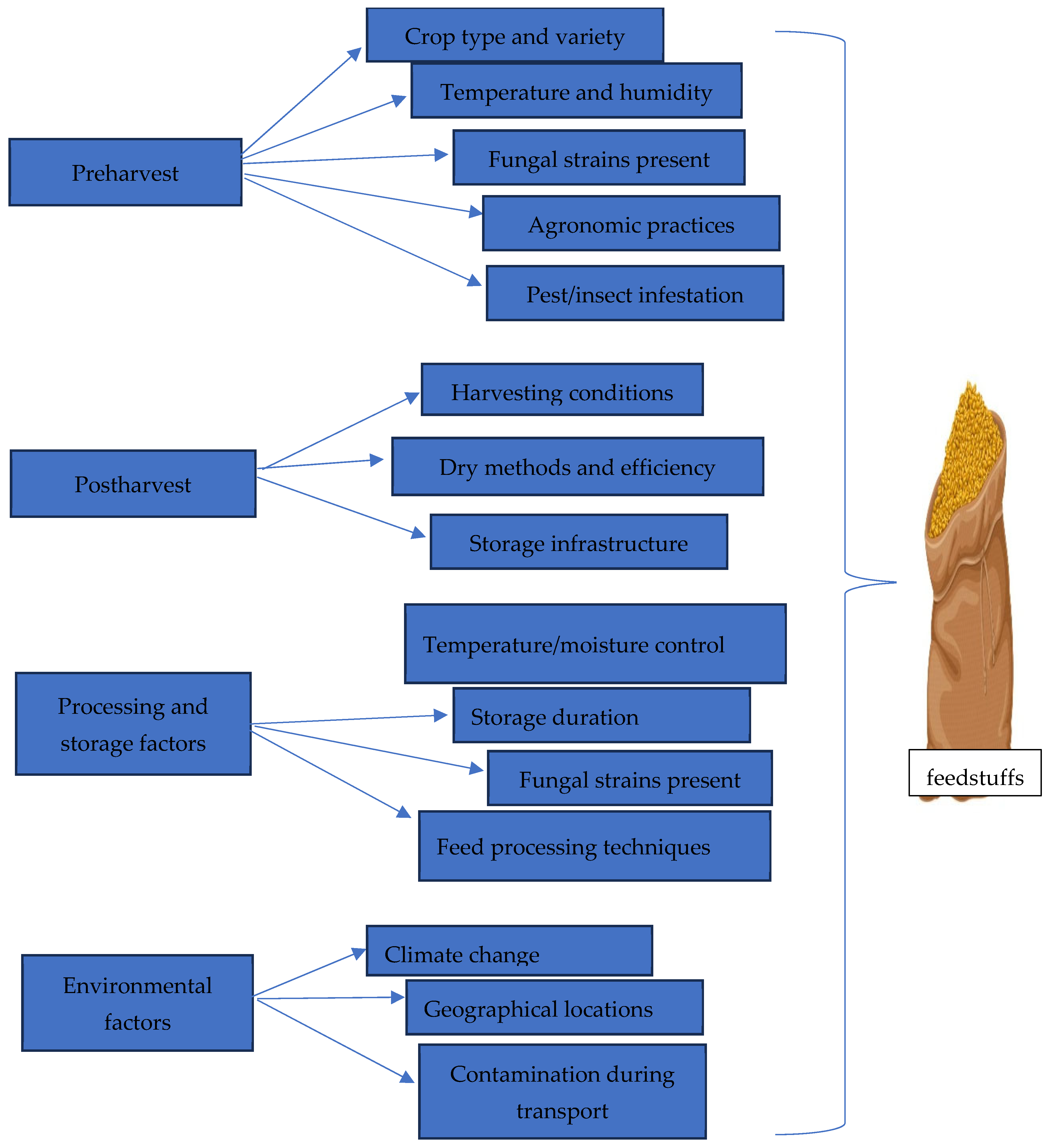
2.3.1. Factors Influencing the Spread of Mycotoxin in Feed
2.3.2. Global Occurrence of Multi-Mycotoxin in Feed
2.4. Common Mycotoxin Combinations in Livestock Feed
3. Mechanisms of Mycotoxin Action
3.1. Cellular and Metabolic Effects of Mycotoxins
- AFB1 Toxicity
- OTA Toxicity
- FUMs Toxicity
- T-2 Toxin and DON Toxicity
- ZEN Toxicity
3.2. Toxicological Interaction of Mycotoxins in Animal Production
3.2.1. Types of Mycotoxin Interactions
3.2.2. Factors Influencing Mycotoxin Interactions
4. Health Impacts on Livestock
4.1. Effect on Growth Performance
4.2. Immune Function, Antioxidant Status, and Reproductive Effects
4.3. Effect on Production Indices
5. Conclusions
6. Future Research Direction
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Neme, K.; Mohammed, A. Mycotoxin occurrence in grains and the role of postharvest management as a mitigation strategy. A review. Food Control 2017, 78, 412–425. [Google Scholar] [CrossRef]
- Maphaisa, T.C.; Akinmoladun, O.F.; Adelusi, O.A.; Mwanza, M.; Fon, F.; Tangni, E.; Njobeh, P.B. Advances in mycotoxin detection techniques and the crucial role of reference material in ensuring food safety. A review. Food Chem. Toxicol. 2025, 200, 115387. [Google Scholar] [CrossRef] [PubMed]
- Siri-Anusornsak, W.; Kolawole, O.; Mahakarnchanakul, W.; Greer, B.; Petchkongkaew, A.; Meneely, J.; Elliott, C.; Vangnai, K. The occurrence and co-occurrence of regulated, emerging, and masked mycotoxins in rice bran and maize from Southeast Asia. Toxins 2022, 14, 567. [Google Scholar] [CrossRef]
- Gruber-Dorninger, C.; Jenkins, T.; Schatzmayr, G. Global mycotoxin occurrence in feed: A ten-year survey. Toxins 2019, 11, 375. [Google Scholar] [CrossRef]
- Njobeh, P.B.; Dutton, M.F.; Åberg, A.T.; Haggblom, P. Estimation of multi-mycotoxin contamination in South African compound feeds. Toxins 2012, 4, 836–848. [Google Scholar] [CrossRef] [PubMed]
- European Commission (EC). Commission Regulation (EU) No 574/2011 of 16 June 2011 amending Annex I to Directive 2002/32/EC of the European Parliament and of the Council as regards maximum levels for nitrite, melamine, Ambrosia spp. and carry-over of certain coccidiostats and histomonostats and consolidating Annexes I and II thereto. Off. J. Eur. Union 2011, L 159, 7–24. [Google Scholar]
- European Commission (EC). Commission Recommendation 2013/165/EU of 27 March 2013 on the presence of T-2 and HT-2 toxins in cereals and cereal products. Off. J. Eur. Union 2013, L 91, 12–15. [Google Scholar]
- Battilani, P.; Palumbo, R.; Giorni, P.; Dall’Asta, C.; Dellafiora, L.; Gkrillas, A.; Toscano, P.; Crisci, A.; Brera, C.; De Santis, B.; et al. Mycotoxin mixtures in food and feed: Holistic, innovative, flexible risk assessment modelling approach: MYCHIF. EFSA Support. Publ. 2020, 17, 1757E. [Google Scholar] [CrossRef]
- Hajnal, E.J.; Kos, J.; Malachová, A.; Steiner, D.; Stranska, M.; Krska, R.; Sulyok, M. Mycotoxins in maize harvested in Serbia in the period 2012–2015. Part 2: Non-regulated mycotoxins and other fungal metabolites. Food Chem. 2020, 317, 126409. [Google Scholar] [CrossRef]
- Santos Pereira, C.; Cunha, C.S.; Fernandes, J.O. Prevalent mycotoxins in animal feed: Occurrence and analytical methods. Toxins 2019, 11, 290. [Google Scholar] [CrossRef]
- Kumar, P.; Mahato, D.K.; Kamle, M.; Mohanta, T.K.; Kang, S.G. Aflatoxins: A global concern for food safety, human health and their management. Front. Microbiol. 2017, 7, 2170. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, N.E.; Cheng, L.W.; Palumbo, J.D. Fate of aflatoxins during almond oil processing. J. Food Protect. 2021, 84, 106–112. [Google Scholar] [CrossRef]
- Ellis, W.O.; Smith, J.P.; Simpson, B.K.; Oldham, J.H. Aflatoxins in food: Occurrence, biosynthesis, effects on organisms, detection, and methods of control. Crit. Rev. Food Sci. Nutr. 1991, 30, 403–439. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhang, Q.; Zhang, D.; Guan, D.; Liu, D.X.; Fang, S.; Wang, X.; Zhang, W. Aflatoxin measurement and analysis. In Aflatoxins-Detection, Measurement and Control; IntechOpen: London, UK, 2011; pp. 183–208. [Google Scholar]
- Zentai, A.; Jóźwiak, Á.; Süth, M.; Farkas, Z. Carry-Over of Aflatoxin B1 from Feed to Cow Milk-A Review. Toxins 2023, 15, 195. [Google Scholar] [CrossRef]
- Marasas, W.F.O.; Miller, J.D.; Riley, R.T.; Visconti, A.; Voss, K.A. Fumonisins—Occurrence, toxicology, metabolism and risk assessment. In Mycotoxins in Agriculture and Food Safety; Bhatnagar, D., Sinha, K.K., Naik, G.S., Eds.; Marcel Dekker, Inc.: New York, NY, USA, 2001; pp. 227–235. [Google Scholar]
- Li, T.; Su, X.; Qu, H.; Duan, X.; Jiang, Y. Biosynthesis, regulation, and biological significance of fumonisins in fungi: Current status and prospects. Crit. Rev. Microbiol. 2022, 48, 450–462. [Google Scholar] [CrossRef]
- Abdul, N.S.; Chuturgoon, A.A. Fumonisin B1 regulates LDL receptor and ABCA1 expression in an LXR dependent mechanism in liver (HepG2) cells. Toxicon 2021, 190, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Morgavi, D.P.; Riley, R.T. An historical overview of field disease outbreaks known or suspected to be caused by consumption of feeds contaminated with Fusarium toxins. Anim. Feed Sci. Technol. 2007, 137, 201–212. [Google Scholar] [CrossRef]
- Zinedine, A.; Soriano, J.M.; Moltó, J.C.; Mañes, J. Review on the toxicity, occurrence, metabolism, detection and control of zearalenone in food and feed. Food Chem. Toxicol. 2007, 45, 1–18. [Google Scholar] [CrossRef]
- Balló, A.; Busznyákné Székvári, K.; Czétány, P.; Márk, L.; Török, A.; Szántó, Á.; Máté, G. Estrogenic and non-estrogenic disruptor effect of zearalenone on male reproduction: A review. Int. J. Mol. Sci. 2023, 24, 1578. [Google Scholar] [CrossRef]
- Zhou, J.; Zhao, L.; Huang, S.; Liu, Q.; Ao, X.; Lei, Y.; Ji, C.; Ma, Q. Zearalenone toxicosis on reproduction as estrogen receptor selective modulator and alleviation of zearalenone biodegradative agent in pregnant sows. J. Anim. Sci. Biotechnol. 2022, 13, 36. [Google Scholar] [CrossRef]
- Gajęcka, M.; Zielonka, Ł.; Gajęcki, M. Activity of zearalenone in the porcine intestinal tract. Molecules 2016, 22, 18. [Google Scholar] [CrossRef]
- Liu, J.; Applegate, T. Zearalenone (ZEN) in livestock and poultry: Dose, toxicokinetics, toxicity and estrogenicity. Toxins 2020, 12, 377. [Google Scholar] [CrossRef] [PubMed]
- Pestka, J.J. Deoxynivalenol: Toxicity, mechanisms and animal health risks. Anim. Feed. Sci. Technol. 2007, 137, 283–298. [Google Scholar] [CrossRef]
- Perrone, G.; Gallo, A. Aspergillus species and their associated mycotoxins. Methods Mol. Biol. 2017, 1542, 33–49. [Google Scholar]
- Przybylska-Gornowicz, B.; Lewczuk, B.; Prusik, M.; Hanuszewska, M.; Petrusewicz- Kosinska, M.; Gajecka, M.; Zielonka, L.; Gajecki, M. The effects of deoxynivalenol and zearalenone on the pig large intestine. A light and electron microscopy study. Toxins 2018, 10, 148. [Google Scholar] [CrossRef]
- Luo, K.; Guo, J.; He, D.; Li, G.; Ouellet, T. Deoxynivalenol accumulation and detoxification in cereals and its potential role in wheat–Fusarium graminearum interactions. Abiotech 2023, 4, 155–171. [Google Scholar] [CrossRef]
- Adhikari, M.; Negi, B.; Kaushik, N.; Adhikari, A.; Al-Khedhairy, A.A.; Kaushik, N.K.; Choi, E.H. T-2 mycotoxin: Toxicological effects and decontamination strategies. Oncotarget 2017, 8, 33933. [Google Scholar] [CrossRef]
- Li, C.; Liu, X.; Wu, J.; Ji, X.; Xu, Q. Research progress in toxicological effects and mechanism of aflatoxin B1 toxin. PeerJ 2022, 10, e13850. [Google Scholar] [CrossRef]
- Bennett, J.W.; Klich, M. Mycotoxins. Clin. Microbiol. Rev. 2003, 16, 497–516. [Google Scholar] [CrossRef]
- Agriopoulou, S.; Stamatelopoulou, E.; Varzakas, T. Advances in occurrence, importance, and mycotoxin control strategies: Prevention and detoxification in foods. Foods 2020, 9, 137. [Google Scholar] [CrossRef]
- Pfohl-Leszkowicz, A.; Hadjeba-Medjdoub, K.; Ballet, N.; Schrickx, J.; Fink-Gremmels, J. Assessment and characterisation of yeast-based products intended to mitigate ochratoxin exposure using in vitro and in vivo models. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2015, 32, 604–616. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wen, J.; Tang, Y.; Shi, J.; Mu, G.; Yan, R.; Cai, J.; Long, M. Research progress on fumonisin B1 contamination and toxicity: A review. Molecules 2021, 26, 5238. [Google Scholar] [CrossRef]
- Schertz, H.; Dänicke, S.; Frahm, J.; Schatzmayr, D.; Dohnal, I.; Bichl, G.; Schwartz-Zimmermann, H.E.; Colicchia, S.; Breves, G.; Teifke, J.P.; et al. Biomarker evaluation and toxic effects of an acute oral and systemic fumonisin exposure of pigs with a special focus on dietary fumonisin esterase supplementation. Toxins 2018, 10, 296. [Google Scholar] [CrossRef]
- Li, S.J.; Zhang, G.; Xue, B.; Ding, Q.; Han, L.; Huang, J.C.; Wu, F.; Yang, C. Toxicity and detoxification of T-2 toxin in poultry. Food Chem. Toxicol. 2022, 169, 113392. [Google Scholar] [CrossRef] [PubMed]
- Monson, M.S.; Coulombe, R.A.; Reed, K.M. Aflatoxicosis: Lessons from toxicity and responses to aflatoxin B1 in poultry. Agriculture 2015, 5, 742–777. [Google Scholar] [CrossRef]
- Klein, P.J.; Van Vleet, T.R.; Hall, J.O.; Coulombe, R.A., Jr. Biochemical factors underlying the age-related sensitivity of turkeys to aflatoxin B1. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2002, 132, 193–201. [Google Scholar] [CrossRef]
- Liu, W.C.; Pushparaj, K.; Meyyazhagan, A.; Arumugam, V.A.; Pappuswamy, M.; Bhotla, H.K.; Baskaran, R.; Khaneghah, A.M. Ochratoxin A as an alarming health threat for livestock and human: A review on molecular interactions, mechanism of toxicity, detection, detoxification, and dietary prophylaxis. Toxicon 2022, 213, 59–75. [Google Scholar] [CrossRef]
- Heussner, A.H.; Bingle, L.E. Comparative ochratoxin toxicity: A review of the available data. Toxins 2015, 7, 4253–4282. [Google Scholar] [CrossRef]
- Song, B.; Ma, T.; Prévéraud, D.P.; Zhang, K.; Wang, J.; Ding, X.; Zeng, Q.; Peng, H.; Bai, J.; Lv, L.; et al. Research Note: Effects of feeding corn naturally contaminated with aflatoxin B1, deoxynivalenol, and zearalenone on reproductive performance of broiler breeders and growth performance of their progeny chicks. Poult. Sci. 2023, 102, 103024. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations. Worldwide Regulations for Mycotoxins in Food and Feed in 2003; FAO Food and Nutrition Paper No. 81; FAO: Rome, Italy, 2004. [Google Scholar]
- van Egmond, H.P.; Jonker, M.A. Worldwide regulations on aflatoxins—The situation in 2002. Toxin Rev. 2004, 23, 273–293. [Google Scholar] [CrossRef]
- Food and Drug Administration (FDA). Guidance for Industry #186: Advisory Levels for Deoxynivalenol (DON) in Finished Wheat Products for Human Consumption and Grains and Grain By-Products Used for Animal Feed; FDA: Rockwell, MD, USA, 2010. [Google Scholar]
- Food and Drug Administration (FDA). Guidance for Industry #221: Recommended Maximum Levels for Fumonisins in Human Foods and Animal Feeds; FDA: Rockwell, MD, USA, 2011. [Google Scholar]
- Food and Drug Administration (FDA). Compliance Policy Guide Sec. 683.100: Action Levels for Aflatoxins in Animal Feeds; FDA: Rockwell, MD, USA, 2022. [Google Scholar]
- Joint FAO/WHO Expert Committee on Food Additives (JECFA). Safety Evaluation of Certain Mycotoxins in Food; WHO Food Additives Series 47; World Health Organization: Geneva, Switzerland, 2001; Available online: https://inchem.org/documents/jecfa/jecmono/v47je01.htm (accessed on 5 April 2025).
- European Commission (EC). Directive 2002/32/EC of the European Parliament and of the Council of 7 May 2002 on undesirable substances in animal feed. Off. J. Eur. Communities 2002, L140, 10–22. [Google Scholar]
- European Commission (EC). Commission Recommendation of 17 August 2006 on the presence of deoxynivalenol, zearalenone, ochratoxin A, T-2 and HT-2 toxin and fumonisins in products intended for animal feeding (2006/576/EC). Off. J. Eur. Union 2006, L 229, 7–9. [Google Scholar]
- Codex Alimentarius Commission (CAC). General Principles of Food Hygiene CXC 1-1969. (Rev. 5). FAO/WHO. 2020. Available online: https://www.fao.org/fao-who-codexalimentarius/sh-proxy/it/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXC%2B1-1969%252FCXC_001e.pdf (accessed on 15 May 2025).
- Casu, A.; Camardo Leggieri, M.; Toscano, P.; Battilani, P. Changing climate, shifting mycotoxins: A comprehensive review of climate change impact on mycotoxin contamination. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13323. [Google Scholar] [CrossRef]
- Paterson, R.R.M.; Lima, N. Further mycotoxin effects from climate change. Food Res. Int. 2011, 44, 2555–2566. [Google Scholar] [CrossRef]
- Kosicki, R.; Błajet-Kosicka, A.; Grajewski, J.; Twaruzek, M. Multiannual mycotoxin survey in feed materials and feeding stuffs. Anim. Feed Sci. Technol. 2016, 215, 65–180. [Google Scholar] [CrossRef]
- Sumner, D.R. Crop rotation and plant productivity. In Handbook of Agricultural Productivity; CRC Press: Boca Raton, FL, USA, 2018; pp. 273–314. [Google Scholar]
- Eskola, M.; Kos, G.; Elliott, C.T.; Hajšlová, J.; Mayar, S.; Krska, R. Worldwide contamination of food-crops with mycotoxins: Validity of the widely cited ‘FAO estimate’of 25%. Crit. Rev. Food Sci. Nutr. 2020, 60, 2773–2789. [Google Scholar] [CrossRef]
- Ray, R.V. 2024. Effects of pathogens and disease on plant physiology. In Agrios’ Plant Pathology; Academic Press: Cambridge, MA, USA, 2024; pp. 63–92. [Google Scholar]
- Battilani, P.; Toscano, P.; Van der Fels-Klerx, H.J.; Moretti, A.; Camardo Leggieri, M.; Brera, C.; Rortais, A.; Goumperis, T.; Robinson, T. Aflatoxin B1 contamination in maize in Europe increases due to climate change. Sci. Rep. 2016, 6, 24328. [Google Scholar] [CrossRef]
- Palumbo, R.; Gonçalves, A.; Gkrillas, A.; Logrieco, A.; Dorne, J.L.; Dall’Asta, C.; Venâncio, A.; Battilani, P. Mycotoxins in maize: Mitigation actions, with a chain management approach. Phytopathol. Mediterr. 2020, 59, 5–28. [Google Scholar] [CrossRef]
- Schmidt-Heydt, M.; Rüfer, C.E.; Abdel-Hadi, A.; Magan, N.; Geisen, R. The production of aflatoxin B1 or G1 by Aspergillus parasiticus at various combinations of temperature and water activity is related to the ratio of aflS to aflR expression. Mycotoxin Res. 2010, 26, 241–246. [Google Scholar] [CrossRef]
- Muga, F.C.; Marenya, M.O.; Workneh, T.S. Effect of temperature, relative humidity and moisture on aflatoxin contamination of stored maize kernels. Bulg. J. Agric. Sci. 2019, 25, 271–277. [Google Scholar]
- Mohapatra, D.; Kumar, S.; Kotwaliwale, N.; Singh, K.K. Critical factors responsible for fungi growth in stored food grains and non-Chemical approaches for their control. Ind. Crops Prod. 2017, 108, 162–182. [Google Scholar] [CrossRef]
- Bhat, R.; Rai, R.V.; Karim, A.A. Mycotoxins in food and feed: Present status and future concerns. Compr. Rev. Food Sci. Food Saf. 2010, 9, 57–81. [Google Scholar] [CrossRef]
- Blacutt, A.A.; Gold, S.E.; Voss, K.A.; Gao, M.; Glenn, A.E. Fusarium verticillioides: Advancements in understanding the toxicity, virulence, and niche adaptations of a model mycotoxigenic pathogen of maize. Phytopathology 2018, 108, 312–326. [Google Scholar] [CrossRef]
- Kemboi, D.C.; Ochieng, P.E.; Antonissen, G.; Croubels, S.; Scippo, M.L.; Okoth, S.; Kangethe, E.K.; Faas, J.; Doupovec, B.; Lindahl, J.F.; et al. Multi-mycotoxin occurrence in dairy cattle and poultry feeds and feed ingredients from Machakos Town, Kenya. Toxins 2020, 12, 762. [Google Scholar] [CrossRef]
- Penagos-Tabares, F.; Mahmood, M.; Khan, M.Z.U.; Talha, H.M.A.; Sajid, M.; Rafique, K.; Naveed, S.; Faas, J.; Artavia, J.I.; Sulyok, M.; et al. Co-occurrence of mycotoxins and other fungal metabolites in total mixed rations of cows from dairy farms in Punjab, Pakistan. Mycotoxin Res. 2023, 39, 421–436. [Google Scholar] [CrossRef]
- Muñoz-Solano, B.; González-Peñas, E. Co-Occurrence of Mycotoxins in Feed for Cattle, Pigs, Poultry, and Sheep in Navarra, a Region of Northern Spain. Toxins 2023, 15, 172. [Google Scholar] [CrossRef]
- Arroyo-Manzanares, N.; Rodríguez-Estévez, V.; Arenas-Fernández, P.; García-Campaña, A.M.; Gámiz-Gracia, L. Occurrence of Mycotoxins in Swine Feeding from Spain. Toxins 2019, 11, 342. [Google Scholar] [CrossRef]
- Streit, E.; Schatzmayr, G.; Tassis, P.; Tzika, E.; Marin, D.; Taranu, I.; Tabuc, C.; Nicolau, A.; Aprodu, I.; Puel, O.; et al. Current situation of mycotoxin contamination and co-occurrence in animal feed–focus on Europe. Toxins 2012, 4, 788–809. [Google Scholar] [CrossRef]
- Biscoto, G.L.; Salvato, L.A.; Alvarenga, É.R.; Dias, R.R.; Pinheiro, G.R.; Rodrigues, M.P.; Pinto, P.N.; Freitas, R.P.; Keller, K.M. Mycotoxins in cattle feed and feed ingredients in Brazil: A five-year survey. Toxins 2022, 14, 552. [Google Scholar] [CrossRef]
- Changwa, R.; De Boevre, M.; De Saeger, S.; Njobeh, P.B. Feed-based multi-mycotoxin occurrence in smallholder dairy farming systems of South Africa: The case of Limpopo and Free State. Toxins 2021, 13, 166. [Google Scholar] [CrossRef]
- Mokubedi, S.M.; Phoku, J.Z.; Changwa, R.N.; Gbashi, S.; Njobeh, P.B. Analysis of mycotoxins contamination in poultry feeds manufactured in selected provinces of South Africa using UHPLC-MS/MS. Toxins 2019, 11, 452. [Google Scholar] [CrossRef]
- Rodrigues, I.; Naehrer, K. A three-year survey on the worldwide occurrence of mycotoxins in feedstuffs and feed. Toxins 2012, 4, 663–675. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Zhang, L.; Liu, M.; Su, Y.-T.; Xie, W.-M.; Zhang, N.-Y.; Dai, J.-F.; Wang, Y.; Rajput, S.A.; Qi, D.-S.; et al. Individual and Combined Occurrence of Mycotoxins in Feed Ingredients and Complete Feeds in China. Toxins 2018, 10, 113. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, L.; Xu, Z.; Liu, X.; Chen, L.; Dai, J.; Karrow, N.A.; Sun, L. Occurrence of Aflatoxin B1, Deoxynivalenol and Zearalenone in Feeds in China during 2018–2020. J. Anim. Sci. Biotechnol. 2021, 12, 74. [Google Scholar] [CrossRef] [PubMed]
- Twarużek, M.; Skrzydlewski, P.; Kosicki, R.; Grajewski, J. Mycotoxins survey in feed materials and feedingstuffs in years 2015–2020. Toxicon 2021, 202, 27–39. [Google Scholar] [CrossRef]
- Awapak, D.; Petchkongkaew, A.; Sulyok, M.; Krska, R. Co-occurrence and toxicological relevance of secondary metabolites in dairy cow feed from Thailand. Food Addit. Contam. Part A 2021, 38, 1013–1027. [Google Scholar] [CrossRef]
- Sarwat, A.; Rauf, W.; Majeed, S.; De Boevre, M.; De Saeger, S.; Iqbal, M. LC-MS/MS based appraisal of multi-mycotoxin co-occurrence in poultry feeds from different regions of Punjab, Pakistan. Food Addit. Contam. Part B 2022, 15, 106–122. [Google Scholar] [CrossRef]
- Mwihia, E.W.; Lyche, J.L.; Mbuthia, P.G.; Ivanova, L.; Uhlig, S.; Gathumbi, J.K.; Maina, J.G.; Eshitera, E.E.; Eriksen, G.S. Co-Occurrence and Levels of Mycotoxins in Fish Feeds in Kenya. Toxins 2020, 12, 627. [Google Scholar] [CrossRef]
- Yang, C.K.; Cheng, Y.H.; Tsai, W.T.; Liao, R.W.; Chang, C.S.; Chien, W.C.; Jhang, J.-C.; Yu, Y.H. Prevalence of mycotoxins in feed and feed ingredients between 2015 and 2017 in Taiwan. Environ. Sci. Pollut. Res. 2019, 26, 23798–23806. [Google Scholar] [CrossRef]
- Freire, L.; Sant’Ana, A.S. Modified mycotoxins: An updated review on their formation, detection, occurrence, and toxic effects. Food Chem. Toxicol. 2018, 111, 189–205. [Google Scholar] [CrossRef]
- Singh, K.; Kumari, A. Mycotoxins co-occurrence poisoning. In Mycotoxins and Mycotoxicosis; Springer: Singapore, 2022. [Google Scholar]
- Weaver, A.C.; Weaver, D.M.; Adams, N.; Yiannikouris, A. Co-occurrence of 35 mycotoxins: A seven-year survey of corn grain and corn silage in the United States. Toxins 2021, 13, 516. [Google Scholar] [CrossRef]
- Kovalsky, P.; Kos, G.; Nährer, K.; Schwab, C.; Jenkins, T.; Schatzmayr, G.; Sulyok, M.; Krska, R. Co-Occurrence of Regulated, Masked and Emerging Mycotoxins and Secondary Metabolites in Finished Feed and Maize—An Extensive Survey. Toxins 2016, 8, 363. [Google Scholar] [CrossRef]
- Romera, D.; González-Peñas, E.; Lizarraga, E. Co-occurrence of mycotoxins in feed for cattle, pigs, poultry, and sheep in Navarra, Spain. Toxins 2018, 10, 102. [Google Scholar]
- Chen, X.Y. Mycotoxin Contamination of Feed Raw Materials and Compound Feed in some Provinces and Cities of China in 2009–2010. Zhejiang J. Anim. Sci. Vet. Med. 2011, 2, 7–9. [Google Scholar]
- Marin, S.; Ramos, A.J.; Cano-Sancho, G.; Sanchis, V. Mycotoxins: Occurrence, toxicology, and exposure assessment. Food Chem. Toxicol. 2013, 60, 218–237. [Google Scholar] [CrossRef]
- Upadhaya, S.D.; Park, M.A.; Ha, J.K. Mycotoxins and their biotransformation in the rumen: A review. Asian-Australas. J. Anim. Sci. 2010, 23, 1250–1260. [Google Scholar] [CrossRef]
- Tian, Y.; Tan, Y.; Liu, N.; Yan, Z.; Liao, Y.; Chen, J.; De Saeger, S.; Yang, H.; Zhang, Q.; Wu, A. Detoxification of deoxynivalenol via glycosylation represents novel insights on antagonistic activities of Trichoderma when confronted with Fusarium graminearum. Toxins 2016, 8, 335. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Mu, P.; Deng, Y. Mycotoxins: Cytotoxicity and biotransformation in animal cells. Toxicol. Res. 2016, 5, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Guindon-Kezis, K.A.; Mulder, J.E.; Massey, T.E. In vivo treatment with aflatoxin B1 increases DNA oxidation, base excision repair activity and 8-oxoguanine DNA glycosylase levels in mouse lung. Toxicology 2014, 321, 21–26. [Google Scholar] [CrossRef]
- Eaton, D.L.; Williams, D.E.; Coulombe, R.A. Species differences in the biotransformation of aflatoxin B1: Primary determinants of relative carcinogenic potency in different animal species. Toxins 2025, 17, 30. [Google Scholar] [CrossRef]
- Tao, Y.; Xie, S.; Xu, F.; Liu, A.; Wang, Y.; Chen, D.; Pan, Y.; Huang, L.; Peng, D.; Wang, X.; et al. Ochratoxin A: Toxicity, oxidative stress and metabolism. Food Chem. Toxicol. 2018, 112, 320–331. [Google Scholar] [CrossRef]
- Corcuera, L.A.; Arbillaga, L.; Vettorazzi, A.; Azqueta, A.; De Cerain, A.L. Ochratoxin A reduces aflatoxin B1-induced DNA damage detected by the comet assay in Hep G2 cells. Food Chem. Toxicol. 2011, 49, 2883–2889. [Google Scholar] [CrossRef] [PubMed]
- Soriano, J.M.; Gonzalez, L.; Catala, A.I. Mechanism of action of sphingolipids and their metabolites in the toxicity of fumonisin B1. Prog. Lipid Res. 2005, 44, 345–356. [Google Scholar] [CrossRef]
- Rotter, B.A.; Thompson, B.K.; Prelusky, D.B.; Trenholm, H.L.; Stewart, B.; Miller, J.D.; Savard, M.E. Response of growing swine to dietary exposure to pure fumonisin B1 during an eight-week period: Growth and clinical parameters. Nat. Toxins 1996, 4, 42–50. [Google Scholar] [CrossRef]
- Altomare, C.; Logrieco, A.F.; Gallo, A. Mycotoxins and mycotoxigenic fungi: Risk and management. A challenge for future global food safety and security. Encycl. Mycol. 2021, 1, 64–93. [Google Scholar]
- Sobrova, P.; Adam, V.; Vasatkova, A.; Beklova, M.; Zeman, L.; Kizek, R. Deoxynivalenol and its toxicity. Interdiscip. Toxicol. 2010, 3, 94–99. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, J.; Zhang, H.; Wang, J.; Cai, H.; Li, C.; Li, K.; Liu, J.; Guo, X.; Zou, G.; et al. Integrated transcriptional and proteomic analysis with in vitro biochemical assay reveal the important role of CYP3A46 in T-2 toxin hydroxylation in porcine primary hepatocytes. Mol. Cell. Proteom. 2011, 10, 008748. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, C.W.; Koenitz, A.; Campbell, B.C.; Pestka, J.J.; Roth, J.A. Deoxynivalenol induces phosphorylation of multiple signaling proteins and upregulates cytokine expression in the RAW 264.7 macrophage cell line. Toxicol. Sci. 2011, 122, 510–520. [Google Scholar]
- Pan, X.; Whitten, D.A.; Wu, M.; Chan, C.; Wilkerson, C.G.; Pestka, J.J. Global protein phosphorylation dynamics during deoxynivalenol-induced ribotoxic stress response in the macrophage. Toxicol. Appl. Pharmacol. 2013, 268, 201–211. [Google Scholar] [CrossRef]
- Zorgui, L.; Ayed-Boussema, I.; Ayed, Y.; Bacha, H.; Hassen, W. The antigenotoxic activities of cactus (Opuntia ficus-indica) cladodes against the mycotoxin zearalenone in Balb/c mice: Prevention of micronuclei, chromosome aberrations and DNA fragmentation. Food Chem. Toxicol. 2009, 47, 662–667. [Google Scholar] [CrossRef]
- Minervini, F.; Giannoccaro, A.; Cavallini, A.; Visconti, A. Investigations on cellular proliferation induced by zearalenone and its derivatives in relation to the estrogenic parameters. Toxicol. Lett. 2005, 159, 272–283. [Google Scholar] [CrossRef]
- Gazzah, A.C.; Camoin, L.; Abid, S.; Bacha, H.; Ladjimi, M. iTRAQ: A method to elucidate cellular responses to mycotoxin zearalenone. J. Appl. Toxicol. 2013, 33, 566–575. [Google Scholar] [CrossRef] [PubMed]
- Grenier, B.O.I.P.; Oswald, I. Mycotoxin co-contamination of food and feed: Meta-analysis of publications describing toxicological interactions. World Mycotoxin J. 2011, 4, 285–313. [Google Scholar] [CrossRef]
- Gong, Y.Y.; Watson, S.; Routledge, M.N. Aflatoxin exposure and associated human health effects, a review of epidemiological studies. Food Saf. 2016, 4, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Gimeno, A.; Martins, M.L. Micotoxinas y Micotoxicosis en Animales y Humanos; Special Nutrients: Coconut Grove, FL, USA, 2011; pp. 50–53. [Google Scholar]
- Smith, M.C.; Madec, S.; Coton, E.; Hymery, N. Natural co-occurrence of mycotoxins in foods and feeds and their in vitro combined toxicological effects. Toxins 2016, 8, 94. [Google Scholar] [CrossRef]
- Song, C.; Wang, Z.; Cao, J.; Dong, Y.; Chen, Y. Neurotoxic mechanisms of mycotoxins: Focus on aflatoxin B1 and T-2 toxin. Environ. Pollut. 2024, 356, 124359. [Google Scholar] [CrossRef]
- Miazzo, R.; Peralta, M.F.; Magnoli, C.; Salvano, M.; Ferrero, S.; Chiacchiera, S.; Carvalho, E.C.Q.; Rosa, C.A.R.; Dalcero, A.M. Efficacy of sodium bentonite as a detoxifier of broiler feed contaminated with aflatoxin and fumonisin. Poult. Sci. 2005, 84, 1–8. [Google Scholar] [CrossRef]
- Qing, H.; Huang, S.; Zhan, K.; Zhao, L.; Zhang, J.; Ji, C.; Ma, Q. Combined toxicity evaluation of ochratoxin A and aflatoxin B1 on kidney and liver injury, immune inflammation, and gut microbiota alteration through pair-feeding pullet model. Front. Immunol. 2022, 13, 920147. [Google Scholar] [CrossRef]
- Shreeve, B.J.; Patterson, D.S.P.; Roberts, B.A. The ‘carry-over’ of aflatoxin, ochratoxin and zearalenone from naturally contaminated feed to tissues, urine and milk of dairy cows. Food Cosmet. Toxicol. 1979, 17, 151–152. [Google Scholar] [CrossRef]
- Kolawole, O.; Tech, B. Determination of Farm Animal Exposure to Multiple Mycotoxins and Assessment of Novel Mycotoxin Binders. Ph.D. Thesis, Queen’s University Belfast, Belfast, UK, 2020. [Google Scholar]
- Huff, W.E.; Kubena, L.F.; Harvey, R.B.; Hagler, W.M.J.; Swanson, S.P.; Phillips, T.D.; Creger, C.R. Individual and combined effects of aflatoxin and deoxynivalenol (DON, vomitoxin) in broiler chickens. Poult. Sci. 1986, 65, 1291–1298. [Google Scholar] [CrossRef]
- Fraeyman, S.; Croubels, S.; Devreese, M.; Antonissen, G. Emerging Fusarium and Alternaria mycotoxins: Occurrence, toxicity and toxicokinetics. Toxins 2017, 9, 228. [Google Scholar] [CrossRef]
- Sun, L.H.; Lei, M.Y.; Zhang, N.Y.; Zhao, L.; Krumm, C.S.; Qi, D.S. Hepatotoxic effects of mycotoxin combinations in mice. Food Chem. Toxicol. 2014, 74, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.C.; Timmins-Schiffman, E.; Coton, M.; Coton, E.; Hymery, N.; Nunn, B.L.; Madec, S. Differential impacts of individual and combined exposures of deoxynivalenol and zearalenone on the HepaRG human hepatic cell proteome. J. Proteom. 2018, 173, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.C.; Hymery, N.; Troadec, S.; Pawtowski, A.; Coton, E.; Madec, S. Hepatotoxicity of fusariotoxins, alone and in combination, towards the HepaRG human hepatocyte cell line. Food Chem. Toxicol. 2017, 109, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Deng, H.; Deng, Y.; Liang, Z.; Deng, J.; Zuo, Z.; Hu, Y.; Shen, L.; Yu, S.; Cao, S. Combined Effects of Deoxynivalenol and Zearalenone on Oxidative Injury and Apoptosis in Porcine Splenic Lymphocytes in Vitro. Exp. Toxicol. Pathol. 2017, 69, 612–617. [Google Scholar] [CrossRef]
- Kachlek, M.; Szabó-Fodor, J.; Blochné Bodnár, Z.; Horvatovich, K.; Kovács, M. Preliminary Results on the Interactive Effects of Deoxynivalenol, Zearalenone and Fumonisin B1 on Porcine Lymphocytes. Acta Vet. Hung. 2017, 65, 340–353. [Google Scholar] [CrossRef]
- Jia, R.; Liu, W.; Zhao, L.; Cao, L.; Shen, Z. Low Doses of Individual and Combined Deoxynivalenol and Zearalenone in Naturally Moldy Diets Impair Intestinal Functions via Inducing Inflammation and Disrupting Epithelial Barrier in the Intestine of Piglets. Toxicol. Lett. 2020, 333, 159–169. [Google Scholar] [CrossRef]
- Tolosa, J.; Rodríguez-Carrasco, Y.; Ruiz, M.-J.; Vila-Donat, P. Multi-mycotoxin occurrence in feed, metabolism and carry-over to animal-derived food products: A review. Food Chem. Chem. Toxicol. 2021, 158, 112661. [Google Scholar] [CrossRef]
- Pierron, A.; Alassane-Kpembi, I.; Oswald, I.P. Impact of two mycotoxins deoxynivalenol and fumonisin on pig intestinal health. Porc. Health Manag. 2016, 2, 21. [Google Scholar] [CrossRef]
- Abeni, F.; Migliorati, L.; Terzano, G.M.; Capelletti, M.; Gallo, A.; Masoero, F.; Pirlo, G. Effects of two different blends of naturally mycotoxin-contaminated maize meal on growth and metabolic profile in replacement heifers. Animal 2014, 8, 1667–1676. [Google Scholar] [CrossRef]
- Huang, S.; Zheng, N.; Fan, C.; Cheng, M.; Wang, S.; Jabar, A.; Wang, J.; Cheng, J. Effects of aflatoxin B1 combined with ochratoxin A and/or zearalenone on metabolism, immune function, and antioxidant status in lactating dairy goats. Asian-Australas. J. Anim. Sci. 2017, 31, 505–513. [Google Scholar] [CrossRef]
- Kiyothong, K.; Rowlinson, P.; Wanapat, M.; Khampa, S. Effect of mycotoxin deactivator product supplementation on dairy cows. Anim. Prod. Sci. 2012, 52, 832–841. [Google Scholar] [CrossRef]
- Weaver, G.A.; Kurtz, H.J.; Behrens, J.C.; Robison, T.S.; Seguin, B.E.; Bates, F.Y.; Mirocha, C.J. Effect of zearalenone on the fertility of virgin dairy heifers. Am. J. Vet. Res. 1986, 47, 1395–1397. [Google Scholar] [CrossRef]
- Höhler, D.; Südekum, K.H.; Wolffram, S.; Frohlich, A.A.; Marquardt, R.R. Metabolism and excretion of ochratoxin A fed to sheep. J. Anim. Sci. 1999, 77, 1217–1223. [Google Scholar] [CrossRef] [PubMed]
- Winkler, J.; Kersten, S.; Meyer, U.; Engelhardt, U.; Dänicke, S. Residues of zearalenone (ZEN), deoxynivalenol (DON) and their metabolites in plasma of dairy cows fed Fusarium contaminated maize and their relationships to performance parameters. Food Chem. Toxicol. 2014, 65, 196–204. [Google Scholar] [CrossRef]
- Andretta, I.; Lovatto, P.A.; Lanferdini, E.; Lehnen, C.R.; Rossi, C.A.R.; Hauschild, L.; Fraga, B.N.; Garcia, G.G.; Mallmann, C.A. Feeding of pre-pubertal gilts with diets containing aflatoxins or Zearalenona. Arch. Zootec. 2011, 60, 123–130. [Google Scholar]
- Kubena, L.F.; Edrington, T.S.; Harvey, R.B.; Phillips, T.D.; Sarr, A.B.; Rottinghaus, G.E. Individual and combined effects of fumonisin B1 present in Fusarium moniliforme culture material and diacetoxyscirpenol or ochratoxin A in turkey poults. Poult. Sci. 1997, 76, 256–264. [Google Scholar] [CrossRef]
- Kubena, L.F.; Edrington, T.S.; Kampsholtzapple, C.; Harvey, R.B.; Elissalde, M.H.; Rottinghaus, G.E. Effects of feeding fumonisin B1 present in Fusarium moniliforme culture material and aflatoxin singly and in combination to turkey poults. Poult. Sci. 1995, 74, 1295–1303. [Google Scholar] [CrossRef] [PubMed]
- Huff, W.E.; Doerr, J.A. Synergism between aflatoxin and ochratoxin A in broiler chickens. Poult. Sci. 1981, 60, 550–555. [Google Scholar] [CrossRef]
- Huff, W.E.; Doerr, J.A.; Wabeck, C.J.; Chaloupka, G.W.; May, J.D.; Merkley, J.W. Individual and combined effects of aflatoxin and ochratoxin on bruising in broiler chickens. Poult. Sci. 1983, 62, 1764–1771. [Google Scholar] [CrossRef]
- Huff, W.E.; Harvey, R.B.; Kubena, L.F.; Rottinghaus, G.E. Toxic synergism between aflatoxin and T-2 toxin in broiler chickens. Poult. Sci. 1988, 67, 1418–1423. [Google Scholar] [CrossRef]
- Kubena, L.R.; Harvey, R.B.; Huff, W.E.; Elissalde, M.H.; Yersin, A.G.; Phillips, T.D.; Rottinghaus, G.E. Efficacy of a hydrated sodium calcium aluminosilicate to reduce the toxicity of aflatoxin and diacetoxyscirpenol. Poult. Sci. 1993, 72, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Kubena, L.F.; Huff, W.E.; Harvey, R.B.; Corrier, D.E.; Phillips, T.D.; Creger, C.R. Influence of ochratoxin A and deoxynivalenol on growing broiler chicks. Poult. Sci. 1988, 67, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Prabu, P.; Dwivedi, P.; Sharma, A.K. Toxicopathological studies on the effects of aflatoxin B1, ocharatoxin A and their inter action in New Zealand White rabbits. Exp. Toxicol. Pathol. 2013, 65, 27786. [Google Scholar] [CrossRef]
- Chaytor, A.C.; See, M.T.; Hansen, J.A.; De Souza, A.L.P.; Middleton, T.F.; Kim, S.W. Effects of chronic exposure of diets with reduced concentrations of aflatoxin and deoxynivalenol on growth and immune status of pigs. J. Anim. Sci. 2011, 89, 124–135. [Google Scholar] [CrossRef]
- Tapia, M.O.; Seawright, A.A. Experimental combined aflatoxin B1 and ochratoxin A intoxication in pigs. Aust. Vet. J. 1985, 62, 33–37. [Google Scholar] [CrossRef]
- Malekinejad, H.; Schoevers, E.J.; Daemen, I.J.; Zijlstra, C.; Colenbrander, B.; Fink-Gremmels, J.; Roelen, B.A. Exposure of oocytes to the Fusarium toxins zearalenone and deoxynivalenol causes aneuploidy and abnormal embryo development in pigs. Biol. Reprod. 2007, 77, 840–847. [Google Scholar] [CrossRef]
- Verma, J.; Swain, B.K.; Johri, T.S. Effect of aflatoxin and ochratoxin A on biochemical parameters in broiler chickens. Indian J. Anim. Nutr. 2012, 29, 1048. [Google Scholar]
- Sakhare, P.S.; Harne, S.D.; Kalorey, D.R.; Warke, S.R.; Bhandarkar, A.G.; Kurkure, N.V. Effect of Toxiroak® polyherbal feed supplement during induced aflatoxicosis, ochratoxicosis and combined mycotoxicoses in broilers. Vet. Arhiv 2007, 2, 129–146. [Google Scholar]
- Tessari, E.N.; Kobashigawa, E.; Cardoso, A.L.S.; Ledoux, D.R.; Rottinghaus, G.E.; Oliveira, C.A. Effects of aflatoxin B1 and fumonisin B1 on blood biochemical parameters in broilers. Toxins 2010, 2, 453–460. [Google Scholar] [CrossRef]
- Tessari, E.N.C.; Oliveira, C.D.; Cardoso, A.L.S.P.; Ledoux, D.R.; Rottinghaus, G.E. Effects of aflatoxin B1 and fumonisin B1 on body weight, antibody titres and histology of broiler chicks. Br. Poult. Sci. 2006, 47, 357–364. [Google Scholar] [CrossRef]
- Weibking, T.S.; Ledoux, D.R.; Bermudez, A.J.; Rotttinghaus, G.E. Individual and combined effects of feeding Fusarium moniliforme culture material, containing known levels of fumonisin B1 and aflatoxin B1 in the young turkey poult. Poult. Sci. 1994, 73, 1517–1525. [Google Scholar] [CrossRef] [PubMed]
- Soyöz, M.; Özçelik, N.; Kihnç, I.; Altuntaş, I. The effects of ochratoxin A on lipid peroxidation and antioxidant enzymes: A protective role of melatonin. Cell Biol. Toxicol. 2004, 20, 213–219. [Google Scholar] [CrossRef]
- Jiang, S.Z.; Li, Z.; Wang, G.Y.; Yang, Z.B.; Yang, W.R.; Zhang, G.G.; Wu, Y.B. Effects of Fusarium mycotoxins with yeast cell wall absorbent on hematology, serum biochemistry, and oxidative stress in broiler chickens. J. Appl. Poult. Res. 2014, 23, 16573. [Google Scholar] [CrossRef]
- Dänicke, S.; Winkler, J.; Meyer, U.; Frahm, J.; Kersten, S. Haematological, clinical–chemical and immunological consequences of feeding Fusarium toxin contaminated diets to early lactating dairy cows. Mycotoxin Res. 2017, 33, 1–13. [Google Scholar] [CrossRef]
- Jovaisiene, J.; Bakutis, B.; Baliukoniene, V.; Gerulis, G. Fusarium and Aspergillus mycotoxins effects on dairy cow health, performance and the efficacy of Anti-Mycotoxin Additive. Pol. J. Vet. Sci. 2016, 19, 79–87. [Google Scholar] [CrossRef]
- Korosteleva, S.N.; Smith, T.K.; Boermans, H.J. Effects of feedborne Fusarium mycotoxins on the performance, metabolism, and immunity of dairy cows. J. Dairy Sci. 2007, 90, 3867–3873. [Google Scholar] [CrossRef] [PubMed]
- Szabó, A.; Szabó-Fodor, J.; Fébel, H.; Romvári, R.; Kovács, M. Individual and combined haematotoxic effects of fumonisin B1 and T-2 mycotoxins in rabbits. Food Chem. Toxicol. 2014, 72, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P. Himachal Pradesh rabbit farmers fear toxin in feed: Mortality, infertility cases on rise. Hindustan Times 1998, 74, 3. [Google Scholar]
- Wang, X.; Liu, Q.; Ihsan, A.; Huang, L.; Dai, M.; Hao, H.; Cheng, G.; Liu, Z.; Wang, Y.; Yuan, Z. JAK/STAT pathway plays a critical role in the proinflammatory gene expression and apoptosis of RAW264.7 cells induced by trichothecenes as DON and T-2 toxin. Toxicol. Sci. 2012, 127, 412–424. [Google Scholar] [CrossRef]
- Mohamed, M.Y.; Abd El-Hafeez, A.M.; Ibrahim, E.M.M.; Abd El Mola, A.M. Ameliorating effects of organic and inorganic mycotoxin binders on the performance of Ossimi sheep. Egypt. J. Sheep Goats Sci. 2019, 14, 33–48. [Google Scholar]
- Wu, X.; Guo, L.; Huang, G.; Tang, W.; Zhao, S.; Wang, J.; Zhang, Y. Effects of dietary natural mycotoxins exposure on performance, biochemical parameters and milk small molecule metabolic pathways of lactating cows. Agriculture 2022, 12, 420. [Google Scholar] [CrossRef]
- Applebaum, R.S.; Brackett, R.E.; Wiseman, D.W.; Marth, E.H. Responses of dairy cows to dietary aflatoxin: Feed intake and yield, toxin content, and quality of milk of cows treated with pure and impure aflatoxin. J. Dairy Sci. 1982, 65, 1503–1508. [Google Scholar] [CrossRef] [PubMed]
- Shanmugasundaram, R.; Lourenco, J.; Hakeem, W.A.; Dycus, M.M.; Applegate, T.J. Subclinical doses of dietary fumonisins and deoxynivalenol cause cecal microbiota dysbiosis in broiler chickens challenged with Clostridium perfringens. Front. Microbiol. 2023, 14, 1106604. [Google Scholar] [CrossRef] [PubMed]
- Verma, J.; Johri, T.S.; Swain, B.K. Effect of varying levels of aflatoxin, ochratoxin and their combinations on the performance and egg quality characteristics in laying hens. Asian-Australas. J. Anim. Sci. 2003, 16, 1015–1019. [Google Scholar] [CrossRef]
- Ogido, R.; Oliveira, C.A.F.; Ledoux, D.R.; Rottinghaus, G.E.; Correa, B.; Butkeraitis, P.; Reis, T.A.; Goncales, E.; Albuquerque, R. Effects of prolonged administration of aflatoxin B1 and fumoni-sin B1 in laying Japanese quail. Poult Sci. 2004, 83, 1953–1958. [Google Scholar] [CrossRef]
- Chowdhury, S.R.; Smith, T.K.; Boermans, H.J.; Woodward, B. Effects of feed-borne Fusarium mycotoxins on hematology and immunology in laying hens. Poult. Sci. 2005, 84, 1698–1706. [Google Scholar] [CrossRef]
- Dazuk, V.; Boiago, M.M.; Rolim, G.; Paravisi, A.; Copetti, P.M.; Bissacotti, B.F.; Morsch, V.M.; Vedovatto, M.; Gazoni, F.L.; Matte, F.; et al. Laying hens fed mycotoxin-contaminated feed produced by Fusarium fungi (T-2 toxin and fumonisin B1) and Saccharomyces cerevisiae lysate: Impacts on poultry health, productive efficiency, and egg quality. Microb. Pathog. 2020, 149, 104517. [Google Scholar] [CrossRef]
- Döll, S.; Dänicke, S.; Ueberschär, K.H.; Valenta, H.; Schnurrbusch, U.; Ganter, M.; Klobasa, F.; Flachowsky, G. Effects of graded levels of Fusarium toxin contaminated maize in diets for female weaned piglets. Arch. Anim. Nutr. 2003, 57, 311–334. [Google Scholar] [CrossRef]
| Mycotoxins | Targeted Organs | Order of Sensitivity | Age/Sex-Related Physiological Factors |
|---|---|---|---|
| FB1 | Liver, kidney, and small intestine [34] | Pig > poultry > ruminant > fish [35] | Females exhibit greater sensitivity [35] |
| AFB1 | Kidney and liver [36] | Poultry > rabbit > pig > ruminants [37] | Young and female animals have increased susceptibility [38] |
| OTA | Liver and kidney [39] | Pig > poultry > rabbits > ruminants [39] | Male animals have increased susceptibility [40] |
| DON | Liver, kidney, and lymphocyte [41] | Pig > poultry > ruminants [41] | Male and older animals exhibit greater sensitivity [41] |
| T-2 | Liver, kidney, and lymphocyte [41] | Poultry > pigs > ruminants [41] | Heightened susceptibility in young animals [41] |
| ZEN | Reproductive organs (ovaries, uterus, vulva and vagina, mammary glands, testes), liver, and kidney [24] | Pig > dairy cattle > poultry > ruminants [24] | Young animals, particularly prepubertal females, exhibit increased susceptibility [24] |
| Mycotoxin | Agency | Species/Feed Type | Maximum Limit (mg/kg) | Notes | References |
|---|---|---|---|---|---|
| Aflatoxins (Total/AFB1) | EU | All feed materials (AFB1) | 0.02 | Maximum content for AFB1 in feed | EC Directive 2002/32/EC |
| USA (FDA) | Dairy cattle, breeding animals | 0.020 | Enforceable legal limit | FDA Compliance Policy Guide Sec. 683.100 (2022) | |
| USA (FDA) | Finishing cattle, swine, poultry | 0.1 | Higher threshold allowed | FDA CPG Sec. 683.100 (2022) | |
| Codex | General feed | 0.050 | Recommended maximum (not binding) | Codex Alimentarius, CAC/RCP 45-1997 | |
| Ochratoxin A (OTA) | EU | Swine feed | 0.05 | Guidance value | EC Recommendation 2006/576/EC |
| EU | Poultry feed | 0.1 | Guidance value | EC Recommendation 2006/576/EC | |
| Canada | General feed (guideline) | 0.25 | Non-binding | CFIA Guidance for OTA in Livestock Feed (2009) | |
| USA | — | No official limit | Not regulated in feed | Not regulated | |
| Fumonisins (FB1 + FB2) | EU | All feed materials | 5 | Applies mainly to maize products | EC Recommendation 2006/576/EC |
| USA (FDA) | Swine | 10 | Swine are highly sensitive | FDA Guidance for Industry #221 (2011) | |
| USA (FDA) | Poultry | 30 | Includes broilers and layers | FDA Guidance #221 | |
| USA (FDA) | Cattle | 100 | Feedlot and breeding cattle | FDA Guidance #221 | |
| Codex | Maize products for feed | 60 | Proposed guideline | Codex Alimentarius (CAC/RCP 56-2004) | |
| Deoxynivalenol (DON/Vomitoxin) | EU | Pig feed | 0.9 | Guidance value | EC Recommendation 2006/576/EC |
| EU | Poultry, cattle feed | 5 | Less sensitive species | EC Recommendation 2006/576/EC | |
| USA (FDA) | Swine feed | 1 | Advisory level | FDA Guidance for Industry #186 (2010) | |
| USA (FDA) | Poultry | 5 | FDA Guidance #186 | ||
| USA (FDA) | Ruminants | 10 | For beef and dairy | FDA Guidance #186 | |
| Zearalenone (ZEN) | EU | Piglets, sows | 0.1–0.25 | Reproductive sensitivity | EC Recommendation 2006/576/EC |
| EU | Cattle and poultry | 0.5–3 | Guidance values by species | EC Recommendation 2006/576/EC | |
| USA | — | No official federal limit | Occasional guidance by states or industry | Not regulated | |
| T-2/HT-2 Toxin | EU | Cereal-based feed | 0.25–0.5 | Guidance only | EC Recommendation 2013/165/EU |
| Canada | General feed (guideline) | 0.1–1 | Based on species sensitivity | CFIA Feed Contaminants Guidelines | |
| USA | — | No limit | Not officially regulated | Not regulated |
| Country | Feed Types | Number of Samples Analyzed | % Containing Two or More | Mycotoxins Analyzed | Highest Mycotoxin Combinations | Reference |
|---|---|---|---|---|---|---|
| Brazil | Cattle feed and ingredients | 1329 | 87% (≥2); 28.6% (=3); 22.5% (=4); 11.4% (=5); 1.16% (=6) | AFs, DON, FUMs, OTA, T-2, ZEN | DON + ZEN-45.2%; AF + DON-42.1%; AF + ZEN-41.5% | [69] |
| South Africa | Dairy cattle feed and forages | 300–600 | 66% (≥2); 20% (=2) | AFs, DON [+3-DON, 15-ADON], ZEN, FUMs, OTA, T-2 toxin (T-2) and HT-2 toxin (HT-2), NIV, DAS, FUS-X, NEO, AOH, AME, ROQ-C, ENN B, STERIG | DON + FUM + ENN B | [70] |
| Poultry feeds | 105 | 100% | ZEN + metabolites, T-2, FUMs, AFs, HT-2, AME, DON, 3-ADON, 15-ADON | AFs + FUMs + ZENs + DON-42% | [71] | |
| Compounded feed for all classes of livestock | 92 | 98.9% | DON, ZEN, FUM, OTA, AF, T-2/HT-2 | DON + ZEA-99%; FB + DON + ZEN-67%; FB + DON + ZEN + AF-26% FB + DON + ZEN + AF + OTA-5.5% | [5] | |
| Global multi-country survey | Feed and feed raw materials | 74,821 | 64% | ZEN, DON, FUMs, OTA, T-2 | DON + ZEN + FUMs | [4] |
| Feedstuffs and feed | 7049 | 48% (≥2) | DON, ZEN, FUM, OTA, AF, T-2/HT-2 | Not stated | [72] | |
| China | Feed ingredients and pig finished feed | 1569 (742 feed ingredients; 827 finished pig feed) | 100% | AFB1, ZEN, DON | Not stated | [73] |
| Feed samples | 3507 (1417 complete/finished feed; 2083 feedstuffs) | ~100% (finished feed) | AFB1, ZEN, DON | AFB1 + DON (99.6%-pig feed; 99.7%-poultry feed; 99.3%-ruminant feed); AFB1 + ZEN (99.5%-pig feed; 99.7%-poultry feed; 99.3%-ruminant feed); DON + ZEN (98.2%-pig feed; 99.6%-poultry feed; 98.6%-ruminant feed); AFB1 + DON + ZEN (99.1%-pig feed; 99.6%-poultry feed; 98.6%-ruminant feed) | [74] | |
| Poland | Feed materials and feedstuffs | 3980 (642 maize, 2027 feed samples, 990 small grains, 142 maize silage, and 179 TMR samples) | AFs, FUMs, ZEN, OTA, T-2, HT-2, DON | DON + ZEN (98.7%-complete feed, 100%-TMR), DON + T-2 + HT-2 (97.7%-complete feed, 97.2%-TMR), DON + T-2 + HT-2 + ZEN (89.3%-complete feed, 97.2%-TMR), ZEN + T-2 + HT-2 (89.4%-complete feed, 97.2%-TMR) | [75] | |
| Thailand | Dairy feed samples | 115 | 96.6% (≥2) | 69 metabolites, including major mycotoxins | ZEN + FB1-65.9%, ZEN + DON-56.8% | [76] |
| Pakistan | Poultry feeds | 150 | 100% | AFs, DON, NIV, ZEN, NEO, OTA T-2, HT-2, 3-ADON, DAS, 15-ADON, STC, DOM-1, F-X | AFs + FBs-100% | [77] |
| Spain | Compounded feed for cattle, pigs, poultry, and sheep | 400 | 63.5% (≥2); 37.8% (=2); 16.8% (=3); 7.3% (=4); 1.5% (=5) | AFs (B1, B2, G1, G2), OT (A, B), ZEN, DON, STER | ZEN + DON (23.8%); AFG2 + ZEN + DON (13%) and AFB1 + ZEN + DON (11%); AFG2 + AFG1 + ZEN + DON (2%); AFB2 + AFB1 + ZEN + DON + STER (3%) | [66] |
| Kenya | Fish feed | 78 | 87% (≥2); 13% (=8); 1% (=17) | Regulated (DON, ZEN, FUM, OTA, AF, T-2/HT-2) and non-regulated mycotoxins | Not stated | [78] |
| Dairy and poultry feed | 67 (47 finished feed; 24 feed ingredients) | 96% (≥2); 75% (≥5); 13% (≥8) | Regulated (DON, ZEN, FUM, OTA, AF, T-2/HT-2) and non-regulated mycotoxins | Not stated | [64] | |
| Taiwan | Swine feed | 820 | 91.3% (≥2) | (DON, ZEN, FUM, OTA, AF) | ZEN + DON (13.87%); ZEN + FUM + DON (12.47%); AF + ZEN + DON (16.16%); AF + ZEN + FUM + DON (23.54%) | [79] |
| Livestock | Mycotoxin Combination | Type of Interaction | Effect Compared with Individual Toxin | References |
|---|---|---|---|---|
| Pigs | DON + ZEN | Synergistic | Greater reduction in weight gain and feed intake than DON or ZEN alone | [120] |
| Poultry | FB1 + DAS/OTA; FB1 + AF | Additive | Reduction in body weight, carcass and organ weights | [130,131] |
| AF + DON | [113] | |||
| AF +T-2 | Synergism | [134] | ||
| AF + DAS | [135] | |||
| OTA + DON | Antagonistic | [134] | ||
| Ruminants | AFB1 + OTA + ZEN | Additive/Synergistic | Significant drop in DMI and milk yield; individual mycotoxins alone showed minimal effects | [120,125] |
| Rabbits | AFB1 + OTA | Synergistic | 40% reduction in weight gain vs. 12% individually; 25% mortality vs. 12.5% | [137] |
| Species | Mycotoxin Combo | Interaction Type | Affected System | Effect of Severity vs. Individual Toxin | References |
|---|---|---|---|---|---|
| Pig | AFB1 + DON | Synergistic | Immune | Stronger immunosuppression; ↓ lymphocyte proliferation, ↓ IgG, IgA, ↓ IL-2, IFN-γ | [138,139] |
| ZEN + DON | Synergistic | Reproductive | Severe ovarian dysfunction; ↓ aromatase, ↓ LH receptor expression, aneuploidy | [140] | |
| Broilers | DON + OTA | Synergistic/ Additive | Immune | ↓ Macrophage activity, ↓ phagocytosis, ↑ susceptibility to infection | [141,142] |
| AFB1 + FB1 | Synergistic/ Additive | Immune and Liver Pathology | Severe liver damage, ↓ NDV antibody titers, complex immune responses | [122,143,144,147] | |
| OTA + AFB1 | Synergistic | Histopathological changes and apoptosis in the kidney and liver | [110] | ||
| Dairy goats | AFB1 + OTA + ZEN (or FB1) | Synergistic | Immune and Antioxidant | Elevated liver enzymes (ALT, ALP), ↑ MDA, ↓ SOD, ↓ GSH-Px | [122] |
| Dairy cows and calves | AFB1 + DON + ZEN | Additive/Synergistic | Immune | ↑ Somatic cell count, impaired immune function | [149] |
| DON + ZEN | Less than additive | Immune | ↓ WBC count, mild neutrophil suppression | [148] |
| Species | Mycotoxin Combo | Interaction Type | Major Production Impacts | References |
|---|---|---|---|---|
| Dairy cattle | AFB1 + DON; AFB1 + DON + ZEN | Synergistic | ↓ Milk yield (14%), ↑ somatic cell count, ↓ milk fat and protein, reduced DMI, altered rumen fermentation | [125,156] |
| Dairy goats, cows | AFB1 + DON + ZEN; AFB1 + OTA; AFB1 + OTA + ZEN | Synergistic | ↓ Milk yield (18%), ↓ milk protein (12%), synergistic effects between toxins | [112,155] |
| Sheep | AFB1 + OTA | synergistic | ↓ Fertility rate, fecundity rate, lambing rate, litter size and number, ↑ still birth | [154] |
| Broilers/pullets | FUM + DON + ZEN; AF + OTA | Synergistic/Additive/less than additive | ↓ Average daily gain, ↑ feed conversion ratio (20%), ↓ egg production and hatchability, egg mass and egg weight | [157,158,160,161] |
| Pigs (sows) | ZEN + DON | Synergistic | ↑ Mean weight of uterus, concentration of FSH | [162] |
| Pigs (grower–finishers) | DON + ZEN + FB1 | Synergistic | ↑ Backfat thickness, ↓ loin eye area, darker meat colour, ↓ water holding capacity | [104] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akinmoladun, O.F.; Fon, F.N.; Nji, Q.; Adeniji, O.O.; Tangni, E.K.; Njobeh, P.B. Multiple Mycotoxin Contamination in Livestock Feed: Implications for Animal Health, Productivity, and Food Safety. Toxins 2025, 17, 365. https://doi.org/10.3390/toxins17080365
Akinmoladun OF, Fon FN, Nji Q, Adeniji OO, Tangni EK, Njobeh PB. Multiple Mycotoxin Contamination in Livestock Feed: Implications for Animal Health, Productivity, and Food Safety. Toxins. 2025; 17(8):365. https://doi.org/10.3390/toxins17080365
Chicago/Turabian StyleAkinmoladun, Oluwakamisi F., Fabia N. Fon, Queenta Nji, Oluwaseun O. Adeniji, Emmanuel K. Tangni, and Patrick B. Njobeh. 2025. "Multiple Mycotoxin Contamination in Livestock Feed: Implications for Animal Health, Productivity, and Food Safety" Toxins 17, no. 8: 365. https://doi.org/10.3390/toxins17080365
APA StyleAkinmoladun, O. F., Fon, F. N., Nji, Q., Adeniji, O. O., Tangni, E. K., & Njobeh, P. B. (2025). Multiple Mycotoxin Contamination in Livestock Feed: Implications for Animal Health, Productivity, and Food Safety. Toxins, 17(8), 365. https://doi.org/10.3390/toxins17080365





