The Impact of Strength Changes on Active Function Following Botulinum Neurotoxin-A (BoNT-A): A Systematic Review
Abstract
1. Introduction
2. Results
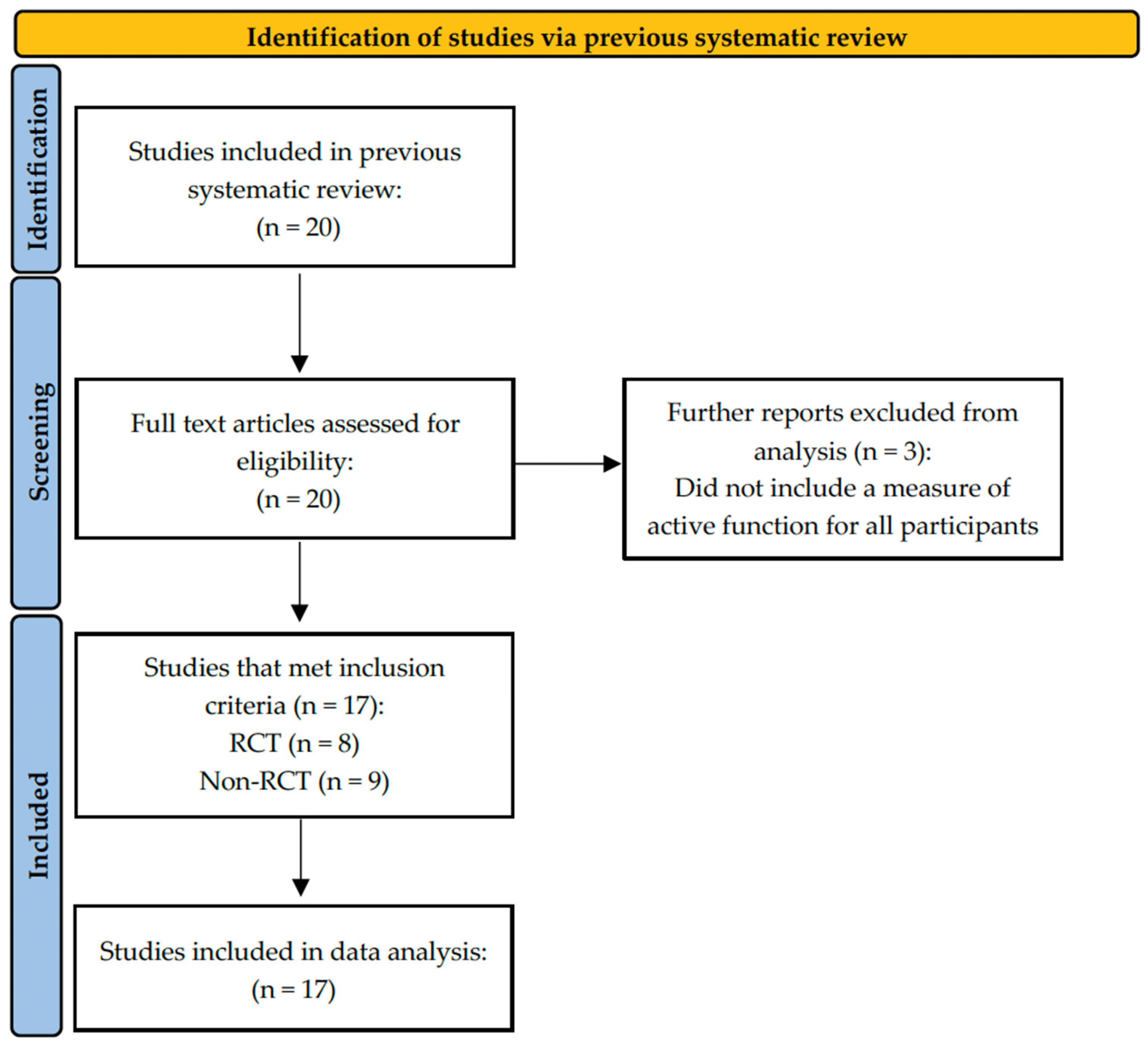
2.1. Studies Included
2.2. Study Characteristics
2.3. Outcomes: WHO-ICF Framework
2.4. Overall Outcome
2.4.1. Strength and Active Function Outcomes of the Upper Limb (n = 7)
2.4.2. Strength and Active Function Outcomes of the Lower Limb (n = 10)
2.4.3. Strength, QoL (n = 4) and Participation (n = 1) Outcomes
2.5. Meta-Analysis
2.6. Quality Assessment
3. Discussion
4. Conclusions
5. Future Directions
6. Methods
6.1. Study Design
6.2. Search Strategy
6.3. Eligibility Criteria
- A measure of active function, participation, or quality of life was used to assess all participants.
- The measure of active function was relevant to the muscle injected (agonist) or the opposing muscle (antagonist).
- The measure of active function demonstrated established clinometric properties (i.e., data related to validity, reliability, etc. have been reported).
6.4. Study Selection
6.5. Data Extraction
6.6. Methodological Assessment of Quality
6.7. Data Analysis and Synthesis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 10MWT | Ten-meter walk test |
| 2MWT | Two-minute walk test |
| 6MWT | Six-minute walk test |
| ABCA | Activities-specific Balance Confidence Scale |
| ARAT | Action Research Arm Test |
| BBS | Berg Balance Scale |
| BBT | Box and Block Test |
| Bf | Barefoot, no aids |
| BoNT-A | Botulinum neurotoxin-A |
| C | Control |
| Ca | Casting |
| Ch | Chronic |
| DCD | Dynamic Computerised Dynamometry |
| DF | Dorsiflexors |
| E | Experimental |
| EE | Elbow Extensors |
| EF | Elbow Flexors |
| EQ-5D | EuroQual-5D |
| ES | E-stims |
| FAC | Functional Ambulation Category |
| FE | Finger Extensors |
| FF | Finger Flexors |
| FS | Fast Speed |
| FIM-UL | Functional Independence Measure-Upper Limb |
| FWC | Functional Walking Category |
| GAS | Goal Attainment Scale |
| GSM | Grip Strength Meter |
| HAb | Hip Abductors |
| HAd | Hip Adductors |
| HHD | Handheld Dynamometry |
| HSP | Hereditary Spastic Paraplegia |
| inj | injection |
| ISCI | Incomplete Spinal Cord Injury |
| ISQ | In status quo |
| KE | Knee Extensors |
| KF | Knee Flexors |
| M | Median |
| MeSH | Medical subject headings |
| MBI | Modified Barthel Index |
| mD&B | Modified Downs and Black scale |
| MI | Motricity Index |
| MRC | Medical Research Council scale |
| MS | Multiple Sclerosis |
| MVC | Maximal Voluntary Contraction |
| MVP | Maximal Voluntary Power |
| n | Sample |
| Nm | Newton Metres |
| NHPT | Nine Hole Peg Test |
| Nm | Newton Meters |
| PEDro | Physiotherapy Evidence Database scale |
| PF | Plantarflexors |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PROSPERO | Prospective Register of Systematic Reviews |
| QDASH | Quick Disabilities of Arm, Shoulder, and Hand |
| QMA | Quantitative Muscle Assessment |
| QoL | Quality of Life |
| QOL SF 36-PH | Quality of Life Short Form 36-Physical Health |
| QOL SF 36-MH | Quality of Life Short Form 36-Mental Health |
| RCT | Randomised Controlled Trial |
| RMA-UL | Rivermead Motor Assessment Scale–Upper Limb |
| RMA-LL | Rivermead Motor Assessment Scale–Lower Limb |
| s | seconds |
| Sa | Subacute |
| SS | Self-Selected |
| St | Stretching |
| SIS | Stroke Impact Scale |
| SATISPART | Instrument for the assessment of activity and participation following a stroke |
| SPRS | Spastic Paraplegia Rating Scale |
| Ta | Taping |
| TUG | Timed Up and Go test |
| UMNS | Upper Motor Neuron Syndrome |
| WHO-ICF | World Health Organisation International Classification of Functioning, Disability and Health |
| WE | Wrist Extensors |
| WF | Wrist Flexors |
| y | Years |
References
- Menken, M.; Munsat, T.L.; Toole, J.F. The global burden of disease study: Implications for neurology. Arch. Neurol. 2000, 57, 418–420. [Google Scholar] [CrossRef]
- Cadilhac, D.A.; Mahal, A. Costs of neurological disorders. Neuroepidemiology 2024, 58, 409–411. [Google Scholar] [CrossRef] [PubMed]
- Lance, J.W. Pathophysiology of spasticity and clinical experience with baclofen. In Spasticity: Disordered Motor Control; Medical Publishers: Chicago, IL, USA, 1980; pp. 185–204. [Google Scholar]
- Tardieu, G.; Shentoub, S.; Delarue, R. Research on a technic for measurement of spasticity. Rev. Neurol. 1954, 91, 143–144. [Google Scholar] [PubMed]
- Jethwa, A.; Mink, J.; Macarthur, C.; Knights, S.; Fehlings, T.; Fehlings, D. Development of the Hypertonia Assessment Tool (HAT): A discriminative tool for hypertonia in children. Dev. Med. Child. Neurol. 2010, 52, e83–e87. [Google Scholar] [CrossRef]
- Love, S.; Gibson, N.; Smith, N.; Bear, N.; Blair, E. Interobserver reliability of the Australian spasticity assessment scale (ASAS). Dev. Med. Child. Neurol. 2016, 58, 18–24. [Google Scholar] [CrossRef]
- Katoozian, L.; Tahan, N.; Zoghi, M.; Bakhshayesh, B. The onset and frequency of spasticity after first ever stroke. J. Natl. Med. Assoc. 2018, 110, 547–552. [Google Scholar] [CrossRef]
- Zeng, H.; Chen, J.; Guo, Y.; Tan, S. Prevalence and risk factors for spasticity after stroke: A systematic review and meta-analysis. Front. Neurol. 2021, 11, 616097. [Google Scholar] [CrossRef]
- Williams, G.; Banky, M.; Olver, J. Distribution of lower limb spasticity does not influence mobility outcome following traumatic brain injury: An observational study. J. Head Trauma Rehabil. 2015, 30, E49–E57. [Google Scholar] [CrossRef]
- Hugos, C.L.; Cameron, M.H. Assessment and Measurement of Spasticity in MS: State of the Evidence. Curr. Neurol. Neurosci. Rep. 2019, 19, 79. [Google Scholar] [CrossRef]
- Farrell, I.I.I.J.W.; Motl, R.W.; Learmonth, Y.C.; Pilutti, L.A. Persons with Multiple Sclerosis Exhibit Strength Asymmetries in both Upper and Lower Extremities. Physiotherapy 2021, 111, 83–91. [Google Scholar] [CrossRef]
- Scivoletto, G.; Tamburella, F.; Laurenza, L.; Torre, M.; Molinari, M. Who is going to walk? A review of the factors influencing walking recovery after spinal cord injury. Front. Hum. Neurosci. 2014, 8, 141. [Google Scholar] [CrossRef]
- Hardy, S.E.; Gill, T.M. Factors associated with recovery of independence among newly disabled older persons. Arch. Intern. Med. 2005, 165, 106–112. [Google Scholar] [CrossRef]
- Chacon-Barba, J.C.; Moral-Munoz, J.A.; Miguel-Rubio, D.; Lucena-Anton, D. Effects of resistance training on spasticity in people with stroke: A systematic review. Brain Sci. 2024, 14, 57. [Google Scholar] [CrossRef]
- del Blanco, J.A.; Taboada-Iglesias, Y. Effects of resistance exercise in patients with spasticity: Systematic review. Apunt. Sports Med. 2021, 56, 100356. [Google Scholar] [CrossRef]
- Li, S.; Francisco, G.E.; Rymer, W.Z. A new definition of poststroke spasticity and the interference of spasticity with motor recovery from acute to chronic stages. Neurorehabil. Neural Repair. 2021, 35, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Elia, A.E.; Filippini, G.; Calandrella, D.; Albanese, A. Botulinum neurotoxins for post-stroke spasticity in adults: A systematic review. J. Mov. Disord. 2009, 24, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.A.; Pereira, G. The efficacy of Botulinum Toxin A for limb spasticity on improving activity restriction and quality of life: A systematic review and meta-analysis using the GRADE approach. Clin. Rehabil. 2016, 30, 549–558. [Google Scholar] [CrossRef]
- Gupta, A.D.; Chu, W.H.; Howell, S.; Chakraborty, S.; Koblar, S.; Visvanathan, R.; Cameron, I.; Wilson, D. A systematic review: Efficacy of botulinum toxin in walking and quality of life in post-stroke lower limb spasticity. Syst. Rev. 2018, 7, 1–9. [Google Scholar] [CrossRef]
- Ada, L.; Dorsch, S.; Canning, C.G. Strengthening Interventions Increase Strength and Improve Activity after Stroke: A Systematic Review. Aust. J. Physiother. 2006, 52, 241–248. [Google Scholar] [CrossRef]
- Noguchi, K.S.; Moncion, K.; Wiley, E.; Morgan, A.; Huynh, E.; Balbim, G.M.; Elliott, B.; Harris-Blake, C.; Krysa, B.; Koetsier, B. Prescribing strength training for stroke recovery: A systematic review and meta-analysis of randomised controlled trials. Br. J. Sports Med. 2025, 59, 185–197. [Google Scholar] [CrossRef]
- Stroke Foundation. Australian and New Zealand Living Clinical Guidelines for Stroke Management. Available online: https://informme.org.au/en/Guidelines/Clinical-Guidelines-for-Stroke-Management (accessed on 6 May 2025).
- Doan, T.-N.; Kuo, M.-Y.; Chou, L.-W. Efficacy and optimal dose of botulinum toxin a in post-stroke lower extremity spasticity: A systematic review and meta-analysis. Toxins 2021, 13, 428. [Google Scholar] [CrossRef]
- Fortuna, R.; Vaz, M.A.; Sawatsky, A.; Hart, D.A.; Herzog, W. A clinically relevant BTX-A injection protocol leads to persistent weakness, contractile material loss, and an altered mRNA expression phenotype in rabbit quadriceps muscles. J. Biomech. 2015, 48, 1700–1706. [Google Scholar] [CrossRef]
- Williams, S.A.; Elliott, C.; Valentine, J.; Gubbay, A.; Shipman, P.; Reid, S. Combining strength training and botulinum neurotoxin intervention in children with cerebral palsy: The impact on muscle morphology and strength. Disabil. Rehabil. 2013, 35, 596–605. [Google Scholar] [CrossRef]
- Wissel, J. Towards flexible and tailored botulinum neurotoxin dosing regimens for focal dystonia and spasticity–Insights from recent studies. Toxicon 2018, 147, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Gill, R.; Banky, M.; Yang, Z.; Medina Mena, P.; Woo, C.C.A.; Bryant, A.; Olver, J.; Moore, E.; Williams, G. The Effect of Botulinum Neurotoxin-A (BoNT-A) on Muscle Strength in Adult-Onset Neurological Conditions with Focal Muscle Spasticity: A Systematic Review. Toxins 2024, 16, 347. [Google Scholar] [CrossRef] [PubMed]
- Olver, J.; Esquenazi, A.; Fung, V.S.; Singer, B.J.; Ward, A.B. Botulinum toxin assessment, intervention and aftercare for lower limb disorders of movement and muscle tone in adults: International consensus statement. Eur. J. Neurol. 2010, 17 (Suppl. 2), 57–73. [Google Scholar] [CrossRef] [PubMed]
- Hoare, B.J.; Wallen, M.A.; Imms, C.; Villanueva, E.; Rawicki, H.B.; Carey, L. Botulinum toxin A as an adjunct to treatment in the management of the upper limb in children with spastic cerebral palsy (UPDATE). Cochrane Database Syst. Rev. 2010, 1, CD003469. [Google Scholar] [CrossRef]
- Esquenazi, A.; Novak, I.; Sheean, G.; Singer, B.J.; Ward, A.B. International consensus statement for the use of botulinum toxin treatment in adults and children with neurological impairments–introduction. Eur. Neurol. 2010, 17, 1–8. [Google Scholar] [CrossRef]
- Koskinen, S. Functional Outcome and Health-Related Quality of Life After Traumatic Brain Injury in the Framework of the International Classification of Functioning, Disability and Health (ICF). University of Helsinki, Helsinki, Finland, November 2011. Available online: https://helda.helsinki.fi/server/api/core/bitstreams/9857c838-cb5e-4f00-b2a5-3011ec1df6d8/content (accessed on 16 July 2025).
- Wilde, E.A.; Whiteneck, G.G.; Bogner, J.; Bushnik, T.; Cifu, D.X.; Dikmen, S.; French, L.; Giacino, J.T.; Hart, T.; Malec, J.F. Recommendations for the use of common outcome measures in traumatic brain injury research. Arch. Phys. Med. Rehabil. 2010, 91, 1650–1660.e1617. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gotzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef]
- Baricich, A.; Picelli, A.; Carda, S.; Smania, N.; Cisari, C.; Santamato, A.; de Sire, A.; Invernizzi, M. Electrical stimulation of antagonist muscles after botulinum toxin type A for post-stroke spastic equinus foot. A randomized single-blind pilot study. Ann. Phys. Rehabil. Med. 2019, 62, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Bernuz, B.; Genet, F.; Terrat, P.; Pradon, D.; Barbot, F.; Bussel, B.; Bensmail, D. Botulinum toxin effect on voluntary and stretch reflex-related torque produced by the quadriceps: An isokinetic pilot study. Neurorehabil. Neural Repair. 2012, 26, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Bollens, B.; Gustin, T.; Stoquart, G.; Detrembleur, C.; Lejeune, T.; Deltombe, T. A randomized controlled trial of selective neurotomy versus botulinum toxin for spastic equinovarus foot after stroke. Neurorehabil. Neural Repair. 2013, 27, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Carda, S.; Invernizzi, M.; Baricich, A.; Cisari, C. Casting, taping or stretching after botulinum toxin type A for spastic equinus foot: A single-blind randomized trial on adult stroke patients. Clin. Rehabil. 2011, 25, 1119–1127. [Google Scholar] [CrossRef]
- Cinone, N.; Letizia, S.; Santoro, L.; Facciorusso, S.; Armiento, R.; Picelli, A.; Ranieri, M.; Santamato, A. Combined Effects of Isokinetic Training and Botulinum Toxin Type A on Spastic Equinus Foot in Patients with Chronic Stroke: A Pilot, Single-blind, Randomized Controlled Trial. Toxins 2019, 11, 210. [Google Scholar] [CrossRef]
- de Niet, M.; de Bot, S.T.; van de Warrenburg, B.P.; Weerdesteyn, V.; Geurts, A.C. Functional effects of botulinum toxin type-A treatment and subsequent stretching of spastic calf muscles: A study in patients with hereditary spastic paraplegia. J. Rehabil. Med. 2015, 47, 147–153. [Google Scholar] [CrossRef]
- Diniz de Lima, F.; Faber, I.; Servelhere, K.R.; Bittar, M.F.R.; Martinez, A.R.M.; Piovesana, L.G.; Martins, M.P.; Martins, C.R., Jr.; Benaglia, T.; de Sa Carvalho, B.; et al. Randomized Trial of Botulinum Toxin Type A in Hereditary Spastic Paraplegia-The SPASTOX Trial. J. Mov. Disord. 2021, 36, 1654–1663. [Google Scholar] [CrossRef]
- Giray, E.; Gencer Atalay, K.; Eren, N.; Gunduz, O.H.; Karadag-Saygi, E. Effects of dynamic lycra orthosis as an adjunct to rehabilitation after botulinum toxin-A injection of the upper-limb in adults following stroke: A single-blinded randomized controlled pilot study. Top. Stroke Rehabil. 2020, 27, 473–481. [Google Scholar] [CrossRef]
- Hameau, S.; Bensmail, D.; Robertson, J.; Boudarham, J.; Roche, N.; Zory, R. Isokinetic assessment of the effects of botulinum toxin injection on spasticity and voluntary strength in patients with spastic hemiparesis. Eur. J. Phys. Rehabil. Med. 2014, 50, 515–523. Available online: https://www.ncbi.nlm.nih.gov/pubmed/24651151 (accessed on 16 July 2025).
- Lannin, N.A.; Ada, L.; English, C.; Ratcliffe, J.; Faux, S.; Palit, M.; Gonzalez, S.; Olver, J.; Schneider, E.; Crotty, M.; et al. Long-term effect of additional rehabilitation following botulinum toxin-A on upper limb activity in chronic stroke: The InTENSE randomised trial. BMC Neurol. 2022, 22, 154. [Google Scholar] [CrossRef]
- Lannin, N.A.; Ada, L.; English, C.; Ratcliffe, J.; Faux, S.G.; Palit, M.; Gonzalez, S.; Olver, J.; Cameron, I.; Crotty, M.; et al. Effect of Additional Rehabilitation After Botulinum Toxin-A on Upper Limb Activity in Chronic Stroke: The InTENSE Trial. Stroke 2020, 51, 556–562. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Gracies, J.M.; Park, S.B.; Lee, K.H.; Lee, J.Y.; Shin, J.H. Botulinum Toxin Injections and Electrical Stimulation for Spastic Paresis Improve Active Hand Function Following Stroke. Toxins 2018, 10, 426. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.H.; Choi, E.H.; Lim, J.Y. Comparison of Effects of Botulinum Toxin Injection Between Subacute and Chronic Stroke Patients: A Pilot Study. Medicine 2016, 95, e2851. [Google Scholar] [CrossRef]
- Pandyan, A.D.; Vuadens, P.; van Wijck, F.M.; Stark, S.; Johnson, G.R.; Barnes, M.P. Are we underestimating the clinical efficacy of botulinum toxin (type A)? Quantifying changes in spasticity, strength and upper limb function after injections of Botox to the elbow flexors in a unilateral stroke population. Clin. Rehabil. 2002, 16, 654–660. [Google Scholar] [CrossRef]
- Rousseaux, M.; Compere, S.; Launay, M.J.; Kozlowski, O. Variability and predictability of functional efficacy of botulinum toxin injection in leg spastic muscles. J. Neurol. Sci. 2005, 232, 51–57. [Google Scholar] [CrossRef]
- Rousseaux, M.; Kozlowski, O.; Froger, J. Efficacy of botulinum toxin A in upper limb function of hemiplegic patients. J. Neurol. 2002, 249, 76–84. [Google Scholar] [CrossRef]
- Rousseaux, M.; Launay, M.J.; Kozlowski, O.; Daveluy, W. Botulinum toxin injection in patients with hereditary spastic paraparesis. Eur. J. Neurol. 2007, 14, 206–212. [Google Scholar] [CrossRef]
- Leung, J.; King, C.; Fereday, S. Effectiveness of a programme comprising serial casting, botulinum toxin, splinting and motor training for contracture management: A randomized controlled trial. Clin. Rehabil. 2019, 33, 1035–1044. [Google Scholar] [CrossRef]
- Chen, Y.T.; Liu, Y.; Zhang, C.; Magat, E.; Zhou, P.; Zhang, Y.; Li, S. Comprehensive Assessment of the Time Course of Biomechanical, Electrophysiological and Neuro-Motor Effects after Botulinum Toxin Injections in Elbow Flexors of Chronic Stroke Survivors with Spastic Hemiplegia: A Cross Sectional Observation Study. Toxins 2022, 14, 104. [Google Scholar] [CrossRef]
- Chen, Y.T.; Zhang, C.; Liu, Y.; Magat, E.; Verduzco-Gutierrez, M.; Francisco, G.E.; Zhou, P.; Zhang, Y.; Li, S. The Effects of Botulinum Toxin Injections on Spasticity and Motor Performance in Chronic Stroke with Spastic Hemiplegia. Toxins 2020, 12, 492. [Google Scholar] [CrossRef]
- Shah, S.; Vanclay, F.; Cooper, B. Improving the sensitivity of the Barthel Index for stroke rehabilitation. J. Clin. Epidemiol. 1989, 42, 703–709. [Google Scholar] [CrossRef]
- O’Connor, S.R.; Tully, M.A.; Ryan, B.; Bradley, J.M.; Baxter, G.D.; McDonough, S.M. Failure of a numerical quality assessment scale to identify potential risk of bias in a systematic review: A comparison study. BMC Res. Notes 2015, 8, 224. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.Y.; Koh, J.H.; Paik, N.J.; Lim, J.Y.; Koh, J.H.; Paik, N.J. Intramuscular botulinum toxin-A reduces hemiplegic shoulder pain: A randomized, double-blind, comparative study versus intraarticular triamcinolone acetonide. Stroke 2008, 39, 126–131. [Google Scholar] [CrossRef]
- Sutton, A.J. Publication Bias. In The Handbook of Research Synthesis and Meta-Analysis; Cooper, H., Hedges, L.V., Valentine, J.C., Eds.; Sage: New York, NY, USA, 2009; Volume 2, pp. 435–452. [Google Scholar]
- Higgins, J. Commentary: Heterogeneity in meta-analysis should be expected and appropriately quantified. Int. J. Epidemiol. 2008, 37, 1158–1160. [Google Scholar] [CrossRef] [PubMed]
- de Yébenes Prous, M.J.G.; Salvanés, F.R.; Ortells, L.C. Responsiveness of outcome measures. Reumatol. Clínica (Engl. Ed.). 2008, 4, 240–247. [Google Scholar] [CrossRef]
- Johansson, G.M.; Häger, C.K. A modified standardized nine hole peg test for valid and reliable kinematic assessment of dexterity post-stroke. J. Neuroeng. Rehabil. 2019, 16, 8. [Google Scholar] [CrossRef]
- Chen, H.M.; Chen, C.C.; Hsueh, I.P.; Huang, S.L.; Hsieh, C.L. Test-retest reproducibility and smallest real difference of 5 hand function tests in patients with stroke. Neurorehabil. Neural Repair 2009, 23, 435–440. [Google Scholar] [CrossRef]
- Nightingale, E.J.; Pourkazemi, F.; Hiller, C.E. Systematic review of timed stair tests. J. Rehabil. Res. Dev. 2014, 51, 335–350. [Google Scholar] [CrossRef]
- Scheuringer, M.; Grill, E.; Boldt, C.; Stucki, G. Examination of the Validity of the FIM in Patients with Neurological Conditions in Early Post-acute Rehabilitation Facilities. Phys. Med. Rehab. Kuror. 2005, 15, A54. [Google Scholar] [CrossRef]
- Sheean, G.; Lannin, N.A.; Turner-Stokes, L.; Rawicki, B.; Snow, B. Botulinum toxin assessment, intervention and after-care for upper limb hypertonicity in adults: International consensus statement. Eur. J. Neurol. 2010, 17, 74–93. [Google Scholar] [CrossRef]
- Lanig, I.S.; New, P.W.; Burns, A.S.; Bilsky, G.; Benito-Penalva, J.; Bensmail, D.; Yochelson, M. Optimizing the Management of Spasticity in people with spinal cord damage: A clinical care pathway for assessment and treatment decision making from the ability network, an international initiative. Arch. Phys. Med. Rehabil. 2018, 99, 1681–1687. [Google Scholar] [CrossRef] [PubMed]
- Wissel, J.; Hernandez Franco, J. Changing the view on spastic movement disorder management to improve active movement competence in the upper motor neuron syndrome: A clinical perspective. Front. Neurol. 2024, 15, 1463292. [Google Scholar] [CrossRef] [PubMed]
- Cusick, A.; Lannin, N.; Kinnear, B.Z. Upper limb spasticity management for patients who have received Botulinum Toxin A injection: Australian therapy practice. Aust. Occup. Ther. J. 2015, 62, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Esquenazi, A.; Zorowitz, R.D.; Ashford, S.; Maisonobe, P.; Page, S.J.; Jacinto, J. Clinical presentation of patients with lower limb spasticity undergoing routine treatment with botulinum toxin: Baseline findings from an international observational study. J. Rehabil. Med. 2023, 55, 4257. [Google Scholar] [CrossRef]
- Dorsch, S.; Ada, L.; Alloggia, D. Progressive resistance training increases strength after stroke but this may not carry over to activity: A systematic review. J. Physiother. 2018, 64, 84–90. [Google Scholar] [CrossRef]
- Harpster, K.; Sheehan, A.; Foster, E.A.; Leffler, E.; Schwab, S.M.; Angeli, J.M. The methodological application of goal attainment scaling in pediatric rehabilitation research: A systematic review. Disabil. Rehabil. 2019, 41, 2855–2864. [Google Scholar] [CrossRef]
- Ashford, S.; Nair, A.; Williams, H.; Esdon, J.; Steed, A.; Nyein, K.; Turner-Stokes, L. Spasticity management with botulinum toxin: A comparison of UK physiotherapy and rehabilitation medicine injectors. J. Ther. Rehabil. 2018, 25, 215–222. [Google Scholar] [CrossRef]
- Turner-Stokes, L.; Ashford, S.; Esquenazi, A.; Wissel, J.; Ward, A.B.; Francisco, G.; Lains, J.; Suputtitada, A.; Serrano, S.; Baguley, I.J. A comprehensive person-centered approach to adult spastic paresis: A consensus-based framework. Eur. J. Phys. Rehabil. Med. 2017, 54, 605–617. [Google Scholar] [CrossRef]
- Turner-Stokes, L. Goal attainment scaling (GAS) in rehabilitation: A practical guide. Clin. Rehabil. 2009, 23, 362–370. [Google Scholar] [CrossRef]
- Jankovic, J. Botulinum toxin in clinical practice. J. Neurol. Neurosurg. Psychiatry 2004, 75, 951–957. [Google Scholar] [CrossRef]
- Colosimo, C.; Charles, D.; Misra, V.P.; Maisonobe, P.; Om, S. Cumulative effects of long-term treatment with abobotulinumtoxinA in cervical dystonia: Findings from a prospective, observational study. J. Neurol. Sci. 2020, 416, 117015. [Google Scholar] [CrossRef]
- Baricich, A.; Wein, T.; Cinone, N.; Bertoni, M.; Picelli, A.; Chisari, C.; Molteni, F.; Santamato, A. BoNT-A for post-stroke spasticity: Guidance on unmet clinical needs from a Delphi panel approach. Toxins 2021, 13, 236. [Google Scholar] [CrossRef]
- Hughes, D.C.; Ellefsen, S.; Baar, K. Adaptations to endurance and strength training. Cold Spring Harb. Perspect. Med. 2018, 8, a029769. [Google Scholar] [CrossRef]
- Levy, C.E.; Giuffrida, C.; Richards, L.; Wu, S.; Davis, S.; Nadeau, S.E. Botulinum toxin A, evidence-based exercise therapy, and constraint-induced movement therapy for upper-limb hemiparesis attributable to stroke: A preliminary study. Am. J. Phys. Med. Rehabil. 2007, 86, 696–706. [Google Scholar] [CrossRef]
- Facciorusso, S.; Spina, S.; Picelli, A.; Baricich, A.; Molteni, F.; Santamato, A. May Spasticity-Related Unpleasant Sensations Interfere with Daily Activities in People with Stroke and Traumatic Brain Injury? Secondary Analysis from the CORTOX Study. J. Clin. Med. 2024, 13, 1720. [Google Scholar] [CrossRef] [PubMed]
- MacAvoy, M.C.; Green, D.P. Critical reappraisal of Medical Research Council muscle testing for elbow flexion. J. Hand. Surg. Am. 2007, 32, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Popescu, M.N.; Căpeț, C.; Beiu, C.; Berteanu, M. The Elias University Hospital Approach: A Visual Guide to Ultrasound-Guided Botulinum Toxin Injection in Spasticity: Part I—Distal Upper Limb Muscles. Toxins 2025, 17, 107. [Google Scholar] [CrossRef] [PubMed]
- Cieza, A.; Stucki, G.; Weigl, M.; Kullmann, L.; Stoll, T.; Kamen, L.; Kostanjsek, N.; Walsh, N. ICF Core Sets for chronic widespread pain. J. Rehabil. Med. 2004, 36, 63–68. [Google Scholar] [CrossRef]
- Bernhardt, J.; Borschmann, K.N.; Kwakkel, G.; Burridge, J.H.; Eng, J.J.; Walker, M.F.; Bird, M.-L.; Cramer, S.C.; Hayward, K.S.; O’Sullivan, M.J. Setting the scene for the second stroke recovery and rehabilitation roundtable. Int. J. Stroke 2019, 14, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Moore, E.J.; Banky, M.; Olver, J.; Bryant, A.L.; Williams, G. The effectiveness of therapy on outcome following (BoNT-A) injection for focal spasticity in adults with neurological conditions: A systematic review. Brain Inj. 2015, 29, 676–687. [Google Scholar] [CrossRef]
- Cieza, A.; Stucki, A.; Geyh, S.; Berteanu, M.; Quittan, M.; Simon, A.; Kostanjsek, N.; Stucki, G.; Walsh, N. ICF Core Sets for chronic ischaemic heart disease. J. Rehabil. Med. 2004, 36, 94–99. [Google Scholar] [CrossRef]
- Doan, Q.V.; Brashear, A.; Gillard, P.J.; Varon, S.F.; Vandenburgh, A.M.; Turkel, C.C.; Elovic, E.P. Relationship between disability and health-related quality of life and caregiver burden in patients with upper limb poststroke spasticity. PM&R 2012, 4, 4–10. [Google Scholar] [CrossRef]
- Schinwelski, M.J.; Sitek, E.J.; Wąż, P.; Sławek, J.W. Prevalence and predictors of post-stroke spasticity and its impact on daily living and quality of life. Neurol. Neurochir. Pol. 2019, 53, 449–457. [Google Scholar] [CrossRef]
- Williams, G.; Singer, B.J.; Ashford, S.; Hoare, B.; Hastings-Ison, T.; Fheodoroff, K.; Berwick, S.; Sutherland, E.; Hill, B. A synthesis and appraisal of clinical practice guidelines, consensus statements and Cochrane systematic reviews for the management of focal spasticity in adults and children. Disabil. Rehabil. 2022, 44, 509–519. [Google Scholar] [CrossRef] [PubMed]
| Author | Study Design | Population | Sample (n) | Mean Age (y) | Active Function Outcome Measures | Strength Outcome Measures | Follow-Up Time Points |
|---|---|---|---|---|---|---|---|
| Giray 2020 [41] | RCT | Stroke | 20 | 46 | BBT SIS | MI | 3/52 3/12 |
| Lannin 2020 [44] | RCT | Stroke | 139 | 61 | BBT GAS QOL-EQ5D-OH QOL-EQ5D-SC | Grip-HHD | 3/12 |
| Lannin 2022 [43] | RCT | Stroke | 140 | 61 | BBT GAS QOL-EQ5D-OH QOL-EQ5D-SC | Grip-HHD | 12/12 |
| Lee 2018 [45] | Non-RCT | Stroke | 15 | 45 | ARAT-Total ARAT-Grasp ARAT-Grip ARAT-Gross ARAT-Pinch BBT QDASH | MRC Grip-HHD | 2/52 6/52 |
| Lim 2016 [46] | Non-RCT | Stroke | 18 | Sa: 63 Ch: 52 | MBI ª | MRC | 4/52 |
| Pandyan 2002 [47] | Non-RCT | Stroke | 14 | 57 ^ | ARAT | Isometric Force Transducer Grip-GSM | 4/52 |
| Rousseaux 2002 [49] | Non-RCT | Stroke | 20 | 54 | FIM-UL NHPT 60 s NHPT 9 blocks RMA-UL | MRC | 2/52 2/12 5/12 |
| Author | Study Design | Population | Sample Size (n) | Mean Age (y) | Active Function Outcome Measure | Strength Outcome Measure | Follow-Up Time Points |
|---|---|---|---|---|---|---|---|
| Baricich 2019 [34] | RCT | Stroke | 30 | 59 | 10MWT 2MWT | MRC | 10/7 20/7 3/12 |
| Bernuz 2012 [35] | Non-RCT | ISCI | 15 | 43 | 10MWT-Gait Velocity 6MWT Timed Stair Climb | Isokinetic Peak Voluntary-Torque 60 °/s | 4–6/52 |
| Bollens 2013 [36] | RCT | Stroke | 16 | 52.3 | 10MWT FAC FWC ABILOCO QOL-SF36 PH QOL-SF36 MH SATISPART-Stroke | MRC | 2/12 6/12 |
| Carda 2011 [37] | RCT | Stroke | 69 | Ta: 62 Ca: 65 St: 60 | 10MWT 6MWT FAC | MRC | 3/52 3/12 |
| Cinone 2019 [38] | RCT | Stroke | 25 | E: 56 C: 56 | 10MWT 6MWT | MI Isokinetic Dynamometry Peak-Torque 60 °/s | 5/52 8/52 |
| de Niet 2015 [39] | Non-RCT | HSP | 25 | E: 48 C: 46 | 10MWT-SS/FS TUG ABC | MRC QMA | 4/52 18/52 |
| Diniz de Lima 2021 [40] | RCT | HSP | 55 | 43 | 10MWT-SS/FS SPRS | MRC | 8/52 |
| Hameau 2014 [42] | Non-RCT | Stroke | 14 | 54 | 10MWT-SS/FS 6MWT TUG Ascend Stairs Descend Stairs | Isokinetic Dynamometer- MVC-Peak-Torque (5 test variations) | 1/12 |
| Rousseaux 2005 [48] | Non-RCT | Stroke | 47 | 52 | 10MWT-SS/FS š 10MWT-Bf FAC š | MRC | 2–3/52 2–3/12 5/12 |
| Rousseaux 2007 [50] | Non-RCT | HSP | 15 | 48 M | 10MWT-SS/FS š FAC-Bf FAC š RMA-L+T | MRC | 2–3/52 2–3/12 5/12 |
| ACTIVE FUNCTION | ≤6/52 Weeks | >6/52 Weeks to ≤3/12 Months | >3 to ≤12/12 Months | |||||||
| Stronger | No Change | Weaker | Stronger | No Change | Weaker | Stronger | No Change | Weaker | ||
| UPPER LIMB AGONIST STRENGTH | ||||||||||
| Improved | FF/Grip [45] EF/Grip [47] EF/WF [46] | WF [49] | ||||||||
| No change | Global [41] | FF/Grip [45] FF/Grip [45] EF/WF [46] | Global [41] | Grip [44] | Grip [43] | WF [49] | ||||
| Worse | ||||||||||
| UPPER LIMB ANTAGONIST STRENGTH | ||||||||||
| Improved | EE [46] | FE [45] EE [47] WE [46] | WE/FE [49] | |||||||
| No change | FE [45] FE [45] EE/WE [46] | WE/FE [49] | ||||||||
| Worse | ||||||||||
| ACTIVE FUNCTION | ≤6/52 Weeks | >6/52 Weeks to ≤3/12 Months | >3 to ≤12/12 Months | |||||||
| Stronger | No Change | Weaker | Stronger | No Change | Weaker | Stronger | No Change | Weaker | ||
| LOWER LIMB AGONIST STRENGTH | ||||||||||
| Improved | Global [38] PF [48] | KE [35] PF [38] PF [39] | PF/Global [38] PF [48] | PF [39] PF [48] | ||||||
| No change | Global [38] PF [48] | KE [35] KE [42] PF [38] | HAd/PF [40] PF [36] PF [48] Global [38] | PF [38] | PF [48] [39] [36] | |||||
| Worse | ||||||||||
| LOWER LIMB ANTAGONIST STRENGTH | ||||||||||
| Improved | DF [38] | DF [34] DF ‡ [37] DF [48] HAb/DF [50] | DF [38] | DF [34] DF ‡ [37] DF [48] | DF [48] HAb/DF [50] | |||||
| No change | KF [42] DF [38] | KF [42] DF ‡ [37] DF [34] DF [48] HAb/DF [50] | DF ‡ [37] DF [36,38,48] HAb/DF [50] | DF [36,48] HAb/DF [50] | ||||||
| Worse | ||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gill, R.; Banky, M.; Yang, Z.; Medina Mena, P.; Woo, C.C.A.; Bryant, A.; Olver, J.; Moore, E.; Williams, G. The Impact of Strength Changes on Active Function Following Botulinum Neurotoxin-A (BoNT-A): A Systematic Review. Toxins 2025, 17, 362. https://doi.org/10.3390/toxins17080362
Gill R, Banky M, Yang Z, Medina Mena P, Woo CCA, Bryant A, Olver J, Moore E, Williams G. The Impact of Strength Changes on Active Function Following Botulinum Neurotoxin-A (BoNT-A): A Systematic Review. Toxins. 2025; 17(8):362. https://doi.org/10.3390/toxins17080362
Chicago/Turabian StyleGill, Renée, Megan Banky, Zonghan Yang, Pablo Medina Mena, Chi Ching Angie Woo, Adam Bryant, John Olver, Elizabeth Moore, and Gavin Williams. 2025. "The Impact of Strength Changes on Active Function Following Botulinum Neurotoxin-A (BoNT-A): A Systematic Review" Toxins 17, no. 8: 362. https://doi.org/10.3390/toxins17080362
APA StyleGill, R., Banky, M., Yang, Z., Medina Mena, P., Woo, C. C. A., Bryant, A., Olver, J., Moore, E., & Williams, G. (2025). The Impact of Strength Changes on Active Function Following Botulinum Neurotoxin-A (BoNT-A): A Systematic Review. Toxins, 17(8), 362. https://doi.org/10.3390/toxins17080362






