Botulinum Neurotoxin A-Induced Muscle Morphology Changes in Children with Cerebral Palsy: A One-Year Follow-Up Study
Abstract
1. Introduction
2. Results
2.1. Participant Characteristics
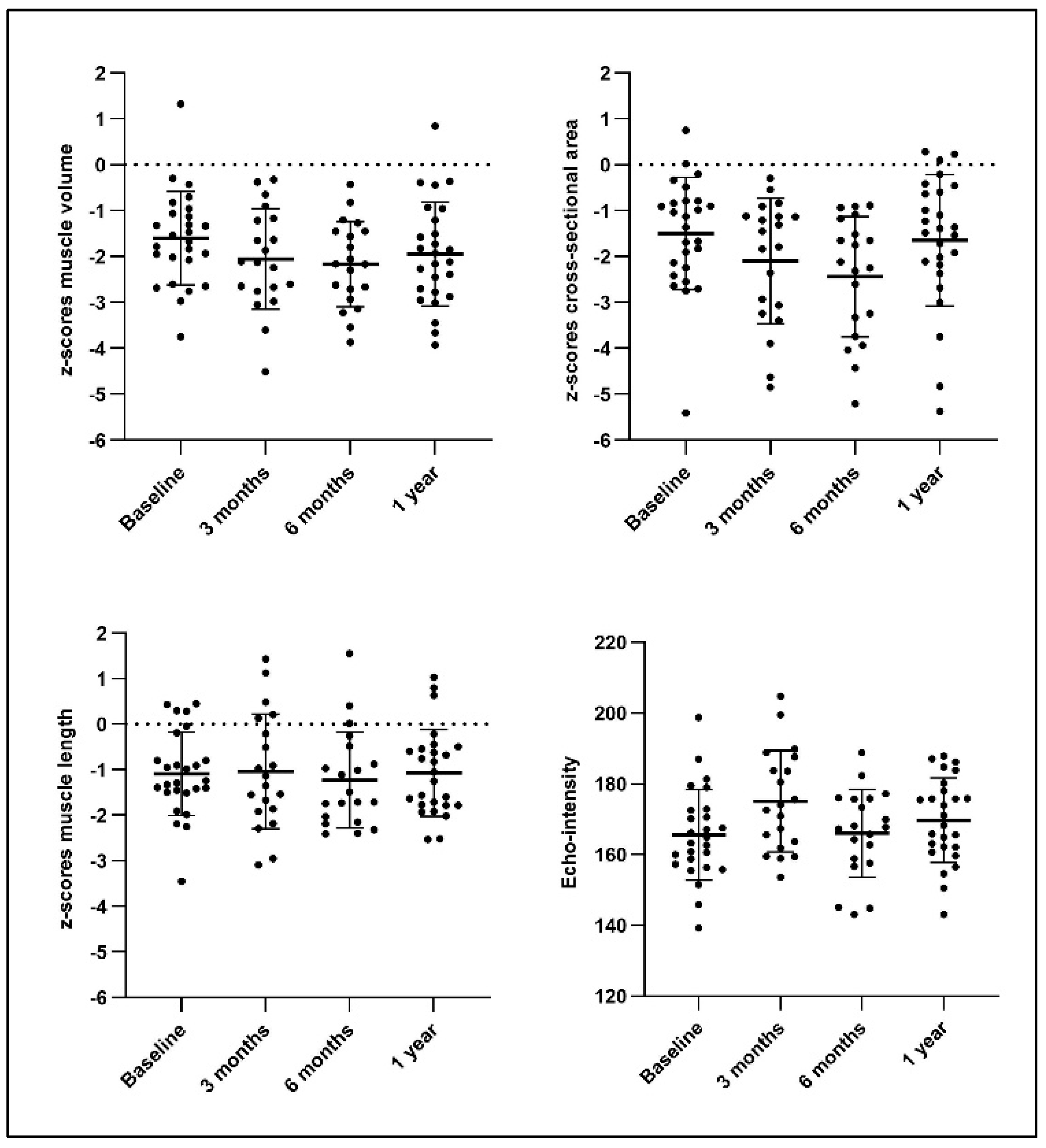
2.2. Muscle Morphology and Quality Following BoNT-A Administration
2.2.1. Effect of Disease Severity on Muscle Recovery After BoNT-A Injection
2.2.2. Effect of BoNT-A History on Muscle Recovery After BoNT-A Injection
2.3. Muscle Growth Comparison Between the Intervention and Control Groups
2.4. Muscle Size Comparison Between the Intervention Group and SCP-Reference Database
3. Discussion
3.1. Time Course of Muscle (Morphology) Parameters After BoNT-A Injection
3.1.1. Muscle Volume
3.1.2. Cross-Sectional Area
3.1.3. Muscle Length
3.1.4. Integration of Muscle Morphology Outcomes
3.1.5. Muscle Quality
3.1.6. Effect of Disease Severity and History of Previous BoNT-A Injections on Muscle Recovery
3.2. Muscle Comparison Between the Intervention and Control Groups at the One-Year Follow-Up
3.3. Qualitative Muscle Size Description Post-BoNT-A Relative to the SCP Reference Database
3.4. Summary
4. Conclusions
5. Materials and Methods
5.1. Participants
5.2. BoNT-A Administration
5.3. Three-Dimensional Freehand Ultrasound Assessment
5.4. Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SCP | Spastic cerebral palsy |
| BoNT-A | Botulinum neurotoxin type A |
| MG | Medial gastrocnemius |
| MV | Muscle volume |
| nMV | Normalized muscle volume |
| EI | Echo intensity |
| CSA | Anatomical cross-sectional area |
| nCSA | Normalized anatomical cross-sectional area |
| ML | Muscle belly length |
| nML | Normalized muscle belly length |
| zMV | Z-scores of the muscle volume |
| zCSA | Z-scores of the anatomical cross-sectional area |
| zML | Z-scores of the muscle length |
| TD | Typically developing children |
| 3DfUS | Three-dimensional freehand ultrasound |
| SEM | Standard error of measurement |
References
- Multani, I.; Manji, J.; Tang, M.J.; Herzog, W.; Howard, J.J.; Graham, H.K. Sarcopenia, Cerebral Palsy, and Botulinum Toxin Type a. JBJS Rev. 2019, 7, e4. [Google Scholar] [CrossRef] [PubMed]
- Michael-Asalu, A.; Taylor, G.; Campbell, H.; Lelea, L.-L.; Kirby, R.S. Cerebral Palsy. Adv. Pediatr. 2019, 66, 189–208. [Google Scholar] [CrossRef]
- Love, S.C.; Novak, I.; Kentish, M.; Desloovere, K.; Heinen, F.; Molenaers, G.; O’Flaherty, S.; Graham, H.K. Botulinum Toxin Assessment, Intervention and After-care for Lower Limb Spasticity in Children with Cerebral Palsy: International Consensus Statement. Eur. J. Neurol. 2010, 17, 9–37. [Google Scholar] [CrossRef] [PubMed]
- Blumetti, F.C.; Belloti, J.C.; Tamaoki, M.J.; Pinto, J.A. Botulinum Toxin Type A in the Treatment of Lower Limb Spasticity in Children with Cerebral Palsy. Cochrane Database Syst. Rev. 2019, 2019, CD001408. [Google Scholar] [CrossRef]
- Molenaers, G. The Effects of Quantitative Gait Assessment and Botulinum Toxin A on Musculoskeletal Surgery in Children with Cerebral Palsy. J. Bone Jt. Surg. 2006, 88, 161. [Google Scholar] [CrossRef]
- Aktaş, E. Botulinum Toxin Type A Injection Increases Range of Motion in Hip, Knee and Ankle Joint Contractures of Children with Cerebral Palsy. Jt. Dis. Relat. Surg. 2019, 30, 155–162. [Google Scholar] [CrossRef]
- Koog, Y.H.; Min, B.-I. Effects of Botulinum Toxin A on Calf Muscles in Children with Cerebral Palsy: A Systematic Review. Clin. Rehabil. 2010, 24, 685–700. [Google Scholar] [CrossRef]
- Fonseca, P.R., Jr.; Calhes Franco de Moura, R.; Galli, M.; Santos Oliveira, C. Effect of Physiotherapeutic Intervention on the Gait after the Application of Botulinum Toxin in Children with Cerebral Palsy: Systematic Review. Eur. J. Phys. Rehabil. Med. 2018, 54, 757–765. [Google Scholar] [CrossRef]
- Choi, J.Y.; Kim, S.K.; Park, E.S. The Effect of Botulinum Toxin Injections on Gross Motor Function for Lower Limb Spasticity in Children with Cerebral Palsy. Toxins 2019, 11, 651. [Google Scholar] [CrossRef]
- Fortuna, R.; Vaz, M.A.; Sawatsky, A.; Hart, D.A.; Herzog, W. A Clinically Relevant BTX-A Injection Protocol Leads to Persistent Weakness, Contractile Material Loss, and an Altered MRNA Expression Phenotype in Rabbit Quadriceps Muscles. J. Biomech. 2015, 48, 1700–1706. [Google Scholar] [CrossRef]
- Fortuna, R.; Aurélio Vaz, M.; Rehan Youssef, A.; Longino, D.; Herzog, W. Changes in Contractile Properties of Muscles Receiving Repeat Injections of Botulinum Toxin (Botox). J. Biomech. 2011, 44, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Ward, S.R.; Minamoto, V.B.; Suzuki, K.P.; Hulst, J.B.; Bremner, S.N.; Lieber, R.L. Recovery of Rat Muscle Size but Not Function More than 1 Year after a Single Botulinum Toxin Injection. Muscle Nerve 2018, 57, 435–441. [Google Scholar] [CrossRef] [PubMed]
- De Beukelaer, N.; Vandekerckhove, I.; Huyghe, E.; Molenberghs, G.; Peeters, N.; Hanssen, B.; Ortibus, E.; Van Campenhout, A.; Desloovere, K. Morphological Medial Gastrocnemius Muscle Growth in Ambulant Children with Spastic Cerebral Palsy: A Prospective Longitudinal Study. J. Clin. Med. 2023, 12, 1564. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.A.; Reid, S.; Elliott, C.; Shipman, P.; Valentine, J. Muscle Volume Alterations in Spastic Muscles Immediately Following Botulinum Toxin Type-A Treatment in Children with Cerebral Palsy. Dev. Med. Child. Neurol. 2013, 55, 813–820. [Google Scholar] [CrossRef]
- Alexander, C.; Elliott, C.; Valentine, J.; Stannage, K.; Bear, N.; Donnelly, C.J.; Shipman, P.; Reid, S. Muscle Volume Alterations after First Botulinum Neurotoxin A Treatment in Children with Cerebral Palsy: A 6-month Prospective Cohort Study. Dev. Med. Child. Neurol. 2018, 60, 1165–1171. [Google Scholar] [CrossRef]
- Peeters, N.; Papageorgiou, E.; Hanssen, B.; De Beukelaer, N.; Staut, L.; Degelaen, M.; Van den Broeck, C.; Calders, P.; Feys, H.; Van Campenhout, A.; et al. The Short-Term Impact of Botulinum Neurotoxin-A on Muscle Morphology and Gait in Children with Spastic Cerebral Palsy. Toxins 2022, 14, 676. [Google Scholar] [CrossRef]
- De Beukelaer, N.; Weide, G.; Huyghe, E.; Vandekerckhove, I.; Hanssen, B.; Peeters, N.; Uytterhoeven, J.; Deschrevel, J.; Maes, K.; Corvelyn, M.; et al. Reduced Cross-Sectional Muscle Growth Six Months after Botulinum Toxin Type-A Injection in Children with Spastic Cerebral Palsy. Toxins 2022, 14, 139. [Google Scholar] [CrossRef] [PubMed]
- Park, E.S.; Sim, E.; Rha, D.-W.; Jung, S. Architectural Changes of the Gastrocnemius Muscle after Botulinum Toxin Type A Injection in Children with Cerebral Palsy. Yonsei Med. J. 2014, 55, 1406. [Google Scholar] [CrossRef][Green Version]
- Multani, I.; Manji, J.; Hastings-Ison, T.; Khot, A.; Graham, K. Botulinum Toxin in the Management of Children with Cerebral Palsy. Pediatr. Drugs 2019, 21, 261–281. [Google Scholar] [CrossRef]
- Barber, L.; Hastings-Ison, T.; Baker, R.; Kerr Graham, H.; Barrett, R.; Lichtwark, G. The Effects of Botulinum Toxin Injection Frequency on Calf Muscle Growth in Young Children with Spastic Cerebral Palsy: A 12-Month Prospective Study. J. Child. Orthop. 2013, 7, 425–433. [Google Scholar] [CrossRef]
- Vandekerckhove, I.; Hanssen, B.; Peeters, N.; Dewit, T.; De Beukelaer, N.; Van den Hauwe, M.; De Waele, L.; Van Campenhout, A.; De Groote, F.; Desloovere, K. Anthropometric-related Percentile Curves for Muscle Size and Strength of Lower Limb Muscles of Typically Developing Children. J. Anat. 2025. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Hanssen, B.; Peeters, N.; Dewit, T.; Huyghe, E.; Dan, B.; Molenaers, G.; Van Campenhout, A.; Bar-On, L.; Van den Broeck, C.; Calders, P.; et al. Reliability of 3D Freehand Ultrasound to Assess Lower Limb Muscles in Children with Spastic Cerebral Palsy and Typical Development. J. Anat. 2023, 242, 986–1002. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, A.S.; Ertl-Wagner, B.; Britsch, S.; Schröder, J.M.; Nikolin, S.; Weis, J.; Müller-Felber, W.; Koerte, I.; Stehr, M.; Berweck, S.; et al. Muscle Biopsy Substantiates Long-term MRI Alterations One Year after a Single Dose of Botulinum Toxin Injected into the Lateral Gastrocnemius Muscle of Healthy Volunteers. Mov. Disord. 2009, 24, 1494–1503. [Google Scholar] [CrossRef]
- Williams, S.A.; Elliott, C.; Valentine, J.; Gubbay, A.; Shipman, P.; Reid, S. Combining Strength Training and Botulinum Neurotoxin Intervention in Children with Cerebral Palsy: The Impact on Muscle Morphology and Strength. Disabil. Rehabil. 2013, 35, 596–605. [Google Scholar] [CrossRef]
- Deschrevel, J.; Andries, A.; Maes, K.; De Beukelaer, N.; Corvelyn, M.; Staut, L.; De Houwer, H.; Costamagna, D.; Desloovere, K.; Van Campenhout, A.; et al. Short-Term Effects of Botulinum Toxin-A Injection on the Medial Gastrocnemius Histological Features in Ambulant Children with Cerebral Palsy: A Longitudinal Pilot Study. Toxins 2024, 16, 69. [Google Scholar] [CrossRef]
- Lambrechts, C.; Deschrevel, J.; Maes, K.; Andries, A.; De Beukelaer, N.; Hanssen, B.; Vandekerckhove, I.; Van Campenhout, A.; Gayan-Ramirez, G.; Desloovere, K. The Relation between Macro- and Microscopic Muscular Alterations of the Medial Gastrocnemius in Children with Spastic Cerebral Palsy. J. Anat. 2025. [Google Scholar] [CrossRef]
- Scholtes, V.A.; Dallmeijer, A.J.; Knol, D.L.; Speth, L.A.; Maathuis, C.G.; Jongerius, P.H.; Becher, J.G. Effect of Multilevel Botulinum Toxin A and Comprehensive Rehabilitation on Gait in Cerebral Palsy. Pediatr. Neurol. 2007, 36, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Peeters, N.; Van Campenhout, A.; Hanssen, B.; Cenni, F.; Schless, S.-H.; Van den Broeck, C.; Desloovere, K.; Bar-On, L. Joint and Muscle Assessments of the Separate Effects of Botulinum NeuroToxin-A and Lower-Leg Casting in Children with Cerebral Palsy. Front. Neurol. 2020, 11, 210. [Google Scholar] [CrossRef]
- Hareb, F.; Bertoncelli, C.M.; Rosello, O.; Rampal, V.; Solla, F. Botulinum Toxin in Children with Cerebral Palsy: An Update. Neuropediatrics 2020, 51, 001–005. [Google Scholar] [CrossRef]
- Strobl, W.; Theologis, T.; Brunner, R.; Kocer, S.; Viehweger, E.; Pascual-Pascual, I.; Placzek, R. Best Clinical Practice in Botulinum Toxin Treatment for Children with Cerebral Palsy. Toxins 2015, 7, 1629–1648. [Google Scholar] [CrossRef]
- Schless, S.; Hanssen, B.; Cenni, F.; Bar-On, L.; Aertbeliën, E.; Molenaers, G.; Desloovere, K. Estimating Medial Gastrocnemius Muscle Volume in Children with Spastic Cerebral Palsy: A Cross-sectional Investigation. Dev. Med. Child. Neurol. 2018, 60, 81–87. [Google Scholar] [CrossRef]
- Gough, M.; Shortland, A.P. Could Muscle Deformity in Children with Spastic Cerebral Palsy Be Related to an Impairment of Muscle Growth and Altered Adaptation? Dev. Med. Child. Neurol. 2012, 54, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Barrett, R.S.; Lichtwark, G.A. Gross Muscle Morphology and Structure in Spastic Cerebral Palsy: A Systematic Review. Dev. Med. Child. Neurol. 2010, 52, 794–804. [Google Scholar] [CrossRef] [PubMed]
- Weidensteiner, C.; Madoerin, P.; Deligianni, X.; Haas, T.; Bieri, O.; Akinci D’Antonoli, T.; Bracht-Schweizer, K.; Romkes, J.; De Pieri, E.; Santini, F.; et al. Quantification and Monitoring of the Effect of Botulinum Toxin A on Paretic Calf Muscles of Children with Cerebral Palsy with MRI: A Preliminary Study. Front. Neurol. 2021, 12, 630435. [Google Scholar] [CrossRef] [PubMed]
- Minamoto, V.B.; Suzuki, K.P.; Bremner, S.N.; Lieber, R.L.; Ward, S.R. Dramatic Changes in Muscle Contractile and Structural Properties after 2 Botulinum Toxin Injections. Muscle Nerve 2015, 52, 649–657. [Google Scholar] [CrossRef]
- Kaya Keles, C.S.; Ates, F. Botulinum Toxin Intervention in Cerebral Palsy-Induced Spasticity Management: Projected and Contradictory Effects on Skeletal Muscles. Toxins 2022, 14, 772. [Google Scholar] [CrossRef]
- Yang, X.; Tang, H.; He, L.; Peng, T.; Li, J.; Zhang, J.; Liu, L.; Zhou, H.; Chen, Z.; Zhao, J.; et al. Proteomic Changes of Botulinum Neurotoxin Injection on Muscle Growth in Children with Spastic Cerebral Palsy. Proteom. Clin. Appl. 2024, 18, e2300070. [Google Scholar] [CrossRef]
- Costamagna, D.; Bastianini, V.; Corvelyn, M.; Duelen, R.; Deschrevel, J.; De Beukelaer, N.; De Houwer, H.; Sampaolesi, M.; Gayan-Ramirez, G.; Van Campenhout, A.; et al. Botulinum Toxin Treatment of Adult Muscle Stem Cells from Children with Cerebral Palsy and HiPSC-Derived Neuromuscular Junctions. Cells 2023, 12, 2072. [Google Scholar] [CrossRef]
- Verreydt, I.; Hanssen, B.; Molenaers, G.; De Beukelaer, N.; Vandekerckhove, I.; Papageorgiou, E.; Huenaerts, C.; Ortibus, E.; Van Campenhout, A.; Desloovere, K. Effect of Selective Dorsal Rhizotomy on Neuromuscular Symptoms, Muscle Morphology, and Motor Function in Children with Spastic Cerebral Palsy. Dev. Med. Child. Neurol. 2024, 67, 812–820. [Google Scholar] [CrossRef]
- Fortuna, R.; Sawatsky, A.; Fuller, J.J.; Herzog, W. Effects of β-Hydroxy-β-Methylbutyrate Supplementation on Muscle Mass and Strength in Onabotulinumtoxin Type-A-Injected and Contralateral Quadriceps Femoris in Rabbits. J. Rehabil. Med. 2021, 53, jrm00229. [Google Scholar] [CrossRef]
- Cermak, N.M.; Res, P.T.; de Groot, L.C.; Saris, W.H.; van Loon, L.J. Protein Supplementation Augments the Adaptive Response of Skeletal Muscle to Resistance-Type Exercise Training: A Meta-Analysis. Am. J. Clin. Nutr. 2012, 96, 1454–1464. [Google Scholar] [CrossRef] [PubMed]
- Theis, N.; Brown, M.A.; Wood, P.; Waldron, M. Leucine Supplementation Increases Muscle Strength and Volume, Reduces Inflammation, and Affects Wellbeing in Adults and Adolescents with Cerebral Palsy. J. Nutr. 2021, 151, 59–64. [Google Scholar] [CrossRef]
- Popescu, M.N.; Căpeț, C.; Beiu, C.; Berteanu, M. The Elias University Hospital Approach: A Visual Guide to Ultrasound-Guided Botulinum Toxin Injection in Spasticity: Part I—Distal Upper Limb Muscles. Toxins 2025, 17, 107. [Google Scholar] [CrossRef]
- Molenaers, G.; Desloovere, K.; Eyssen, M.; Decaf, J.; Jonkers, I.; Cock, P. De Botulinum Toxin Type A Treatment of Cerebral Palsy: An Integrated Approach. Eur. J. Neurol. 1999, 6, s51–s57. [Google Scholar] [CrossRef]
- Cenni, F.; Monari, D.; Desloovere, K.; Aertbeliën, E.; Schless, S.-H.; Bruyninckx, H. The Reliability and Validity of a Clinical 3D Freehand Ultrasound System. Comput. Methods Programs Biomed. 2016, 136, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Cenni, F.; Schless, S.-H.; Monari, D.; Bar-On, L.; Aertbeliën, E.; Bruyninckx, H.; Hanssen, B.; Desloovere, K. An Innovative Solution to Reduce Muscle Deformation during Ultrasonography Data Collection. J. Biomech. 2018, 77, 194–200. [Google Scholar] [CrossRef]
- Treece, G.M.; Prager, R.W.; Gee, A.H.; Berman, L. Fast Surface and Volume Estimation from Non-Parallel Cross-Sections, for Freehand Three-Dimensional Ultrasound. Med. Image Anal. 1999, 3, 141–173. [Google Scholar] [CrossRef]




| Intervention Group | Control Group | SCP reference Group | |
|---|---|---|---|
| Sample size | n = 26 | n = 26 | n = 87 |
| Gender | female n = 10/male n = 16 | female n = 14/male n = 12 | female n = 31/male n = 56 |
| Age (years) | 5.19 (3.26) | 4.98 (2.15) | 6.96 (2.61) |
| Height (m) | 1.05 (0.13) | 1.06 (0.15) | 1.19 (0.17) |
| Body weight (kg) | 17.97 (5.70) | 18.11 (5.35) | 23.61 (7.99) |
| GMFCS level | I n = 11/II n = 7/III n = 8 | I n = 11/II n = 7/III n = 8 | I n = 55/II n = 21/III n = 11 |
| Topographical involvement | unilateral n = 10 bilateral n = 16 | unilateral n = 11 bilateral n = 15 | unilateral n = 46 bilateral n = 41 |
| BoNT-A-naive/history | naive n = 17 / history n = 9 | naive n = 20 / history n = 6 | naive n = 53 / history n = 34 |
| Other injected muscles | hamstrings, n = 21 soleus, n = 6 psoas, n = 8 adductors, n = 6 rectus femoris, n = 2 | NA | NA |
| AFO use post-BoNT-A | n = 26 | NA | NA |
| Serial casting post-BoNT-A | n = 24 | NA | NA |
| Differences | zMV | zCSA | zML | EI | ||||
|---|---|---|---|---|---|---|---|---|
| MD | p-Value | MD | p-Value | MD | p-Value | MD | p-Value | |
| 3 months−baseline | −0.50 | 0.005 | −0.59 | 0.020 | 0.07 | 0.60 | 9.29 | <0.0001 * |
| 6 months−baseline | −0.42 | 0.003 | −0.82 | 0.0005 * | 0.01 | 0.95 | −0.76 | 0.74 |
| 1 year−baseline | −0.34 | 0.008 | −0.15 | 0.45 | 0.02 | 0.86 | 4.14 | 0.12 |
| 6 months−3 months | 0.79 | 0.60 | −0.22 | 0.27 | −0.06 | 0.71 | −10.05 | 0.004 |
| 1 year−3 months | 0.16 | 0.36 | 0.43 | 0.047 | −0.04 | 0.76 | −5.15 | 0.12 |
| 1 year−6 months | 0.08 | 0.44 | 0.655 | 0.003 | 0.02 | 0.86 | 4.90 | 0.032 |
| Differences | zMV | zCSA | zML | EI | ||||
|---|---|---|---|---|---|---|---|---|
| MD | p-Value | MD | p-Value | MD | p-Value | MD | p-Value | |
| GMFCS I (n = 11) | ||||||||
| 3 months−baseline | −0.62 | 0.042 | −0.64 | 0.056 | 0.20 | 0.35 | 6.82 | 0.06 |
| 6 months−baseline | −0.28 | 0.14 | −0.88 | 0.004 | 0.22 | 0.43 | −0.98 | 0.81 |
| 1 year−baseline | −0.12 | 0.44 | 0.09 | 0.55 | 0.25 | 0.26 | 3.82 | 0.33 |
| 6 months−3 months | 0.34 | 0.087 | −0.24 | 0.48 | 0.02 | 0.91 | −7.80 | 0.14 |
| 1 year−3 months | 0.50 | 0.076 | 0.73 | 0.053 | 0.06 | 0.78 | −3.00 | 0.51 |
| 1 year−6 months | 0.16 | 0.29 | 0.97 | 0.002 * | 0.04 | 0.76 | 4.80 | 0.22 |
| GMFCS II + III (n = 15) | ||||||||
| 3 months−baseline | −0.37 | 0.043 | −0.54 | 0.13 | 0.13 | 0.36 | 11.56 | 0.0001 * |
| 6 months−baseline | −0.55 | 0.007 | −0.76 | 0.024 | −0.29 | 0.16 | −0.67 | 0.79 |
| 1 year−baseline | −0.51 | 0.006 | −0.32 | 0.31 | −0.14 | 0.46 | 4.36 | 0.22 |
| 6 months−3 months | −0.19 | 0.38 | −0.21 | 0.24 | −0.41 | 0.16 | −12.23 | 0.008 |
| 1 year−3 months | −0.14 | 0.42 | 0.22 | 0.33 | −0.27 | 0.21 | −7.20 | 0.12 |
| 1 year−6 months | 0.04 | 0.76 | 0.43 | 0.14 | 0.14 | 0.31 | 5.03 | 0.066 |
| Differences | zMV | zCSA | zML | EI | ||||
|---|---|---|---|---|---|---|---|---|
| MD | p-Value | MD | p-Value | MD | p-Value | MD | p-Value | |
| BoNT-A-naive (n = 17) | ||||||||
| 3 months−baseline | −0.65 | 0.004 | −0.66 | 0.031 | 0.17 | 0.29 | 10.09 | 0.002 * |
| 6 months−baseline | −0.63 | <0.0001 * | −0.69 | 0.0009 * | −0.11 | 0.59 | −2.55 | 0.28 |
| 1 year−baseline | −0.49 | 0.002 * | −0.35 | 0.10 | 0.02 | 0.90 | 5.70 | 0.098 |
| 6 months−3 months | 0.02 | 0.93 | −0.02 | 0.93 | −0.27 | 0.092 | −12.64 | 0.001 * |
| 1 year−3 months | 0.17 | 0.52 | 0.31 | 0.33 | −0.15 | 0.46 | −4.39 | 0.28 |
| 1 year−6 months | 0.15 | 0.28 | 0.33 | 0.19 | 0.13 | 0.13 | 8.25 | 0.002 * |
| BoNT-A history (n = 9) | ||||||||
| 3 months−baseline | −0.22 | 0.43 | −0.44 | 0.33 | −0.07 | 0.72 | 8.40 | 0.012 |
| 6 months−baseline | −0.01 | 0.96 | −0.99 | 0.052 | 0.35 | 0.25 | 4.24 | 0.42 |
| 1 year−baseline | −0.07 | 0.74 | 0.24 | 0.51 | 0.02 | 0.90 | 1.17 | 0.76 |
| 6 months−3 months | 0.21 | 0.28 | −0.55 | 0.037 | 0.42 | 0.16 | −4.16 | 0.49 |
| 1 year−3 months | 0.15 | 0.41 | 0.68 | 0.011 | 0.09 | 0.58 | −7.23 | 0.19 |
| 1 year−6 months | −0.06 | 0.70 | 1.22 | 0.0002 * | −0.32 | 0.034 | −3.07 | 0.43 |
| Intervention group | Control group | |||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Follow-Up | MD/% | p-Value | Baseline | Follow-Up | MD/% | p-Value | |
| zMV | −1.61 (−2.050, −1.16) | −1.95 (−2.46, −1.43) | −0.34 | 0.007 | −1.83 (−2.27, −1.38) | −1.75 (−2.27, −1.24) | 0.08 | 0.55 |
| zCSA | −1.50 (−2.052, −0.94) | −1.65 (−2.28, −1.010) | −0.15 | 0.44 | −1.61 (−2.17, −1.057) | −1.54 (−2.17, −0.90) | 0.07 | 0.69 |
| zML | −1.09 (−1.51, −0.68) | −1.07 (−1.48, −0.66) | 0.02 | 0.87 | −1.01 (−1.43, −0.60) | −1.02 (−1.43, −0.61) | −0.01 | 0.98 |
| EI | 165.61 (160.36, 170.86) | 169.75 (165.060, 174.43) | 4.14 | 0.12 | 158.86 (153.61, 149.15) | 153.84 (149.15, 158.52) | −5.02 | 0.063 |
| Growth rate (mL/mo) | 0.44 (0.39, 0.49) | 0.31 (0.18, 0.44) | −0.13/ −30% | 0.035 | 0.44 (0.39, 0.49) | 0.58 (0.45, 0.71) | 0.14/ +32% | 0.029 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lambrechts, C.; De Beukelaer, N.; Vandekerckhove, I.; Verreydt, I.; Andries, A.; Cenni, F.; Gayan-Ramirez, G.; Desloovere, K.; Van Campenhout, A. Botulinum Neurotoxin A-Induced Muscle Morphology Changes in Children with Cerebral Palsy: A One-Year Follow-Up Study. Toxins 2025, 17, 327. https://doi.org/10.3390/toxins17070327
Lambrechts C, De Beukelaer N, Vandekerckhove I, Verreydt I, Andries A, Cenni F, Gayan-Ramirez G, Desloovere K, Van Campenhout A. Botulinum Neurotoxin A-Induced Muscle Morphology Changes in Children with Cerebral Palsy: A One-Year Follow-Up Study. Toxins. 2025; 17(7):327. https://doi.org/10.3390/toxins17070327
Chicago/Turabian StyleLambrechts, Charlotte, Nathalie De Beukelaer, Ines Vandekerckhove, Ineke Verreydt, Anke Andries, Francesco Cenni, Ghislaine Gayan-Ramirez, Kaat Desloovere, and Anja Van Campenhout. 2025. "Botulinum Neurotoxin A-Induced Muscle Morphology Changes in Children with Cerebral Palsy: A One-Year Follow-Up Study" Toxins 17, no. 7: 327. https://doi.org/10.3390/toxins17070327
APA StyleLambrechts, C., De Beukelaer, N., Vandekerckhove, I., Verreydt, I., Andries, A., Cenni, F., Gayan-Ramirez, G., Desloovere, K., & Van Campenhout, A. (2025). Botulinum Neurotoxin A-Induced Muscle Morphology Changes in Children with Cerebral Palsy: A One-Year Follow-Up Study. Toxins, 17(7), 327. https://doi.org/10.3390/toxins17070327







