Translational Impact of Genetics and Epigenetics of CGRP System on Chronic Migraine Treatment with Onabotulinumtoxin A and Other Biotech Drugs
Abstract
1. Introduction
2. Epigenetic Mechanisms and Migraine
3. Influence of Environment on Migraine Susceptibility and Features
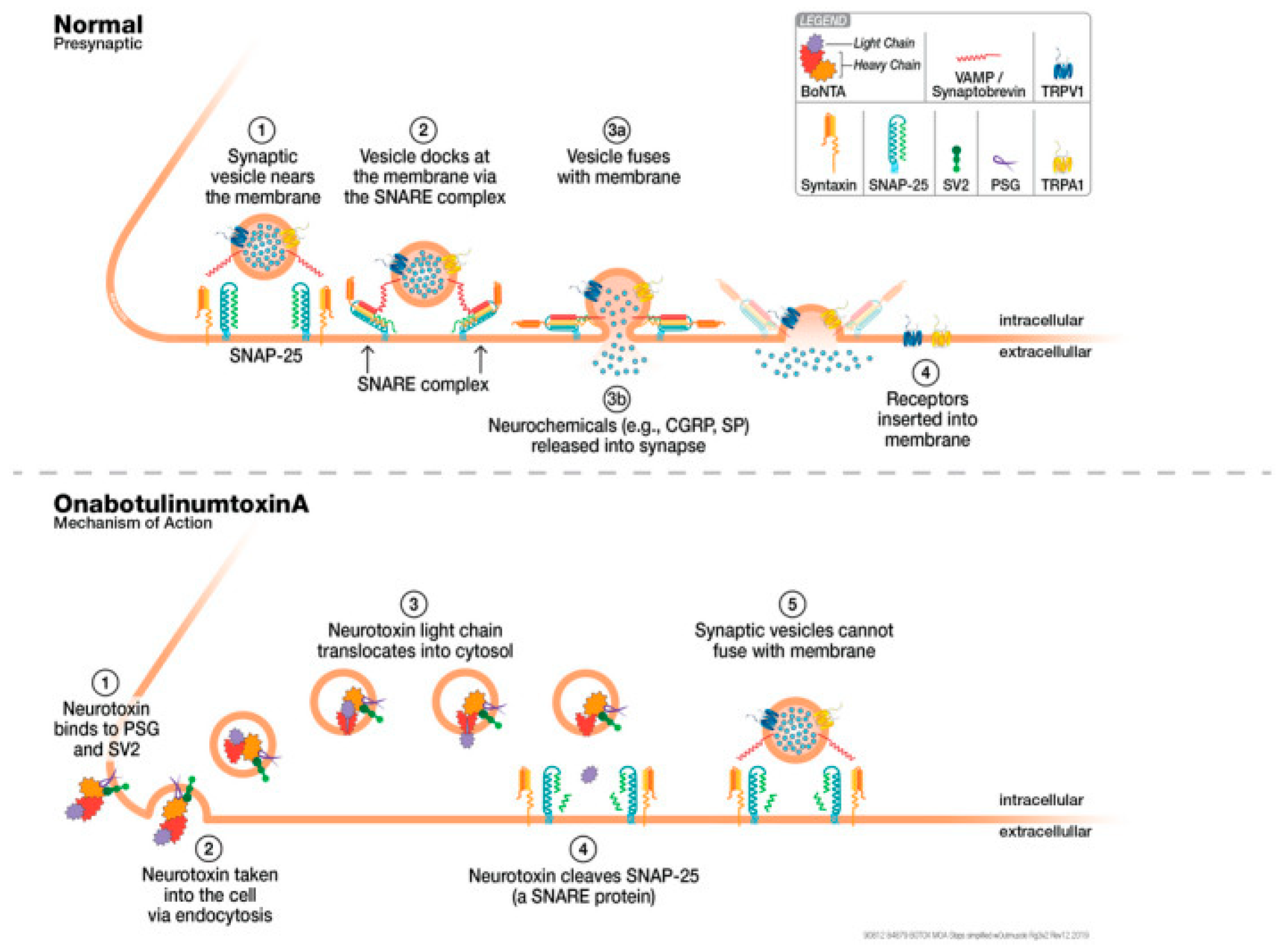
4. CGRP Pathway Modifications
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Raggi, A.; Leonardi, M.; Arruda, M.; Caponnetto, V.; Castaldo, M.; Coppola, G.; Della Pietra, A.; Fan, X.; Garcia-Azorin, D.; Gazerani, P.; et al. Hallmarks of primary headache: Part 1—Migraine. J. Headache Pain 2024, 25, 189. [Google Scholar] [CrossRef] [PubMed]
- Steiner, T.J.; Stovner, L.J.; Vos, T.; Jensen, R.; Katsarava, Z. Migraine is first cause of disability in under 50s: Will health politicians now take notice? J. Headache Pain 2018, 19, 17. [Google Scholar] [CrossRef] [PubMed]
- Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd ed.; International Headache Society: London, UK, 2018; Volume 38, pp. 1–211. [Google Scholar] [CrossRef]
- Bigal, M.E.; Liberman, J.N.; Lipton, R.B. Age-dependent prevalence and clinical features of migraine. Neurology 2006, 67, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Corasaniti, M.T.; Lawrence, G.W.; Bagetta, G.; Iannacchero, R.; Tarsitano, A.; Monteleone, A.; Pagliaro, M.; Tonin, P.; Sandrini, G.; Nicotera, P.; et al. Combination of anti-CGRP/CGRP-R mAbs with onabotulinumtoxin A as a novel therapeutic approach for refractory chronic migraine: A retrospective study of real-world clinical evidence and a protocol for a double-blind, randomized clinical trial to establish the efficacy and safety. Front. Pharmacol. 2023, 14, 1296577. [Google Scholar] [CrossRef]
- Scuteri, D.; Tonin, P.; Nicotera, P.; Vulnera, M.; Altieri, G.C.; Tarsitano, A.; Bagetta, G.; Corasaniti, M.T. Pooled Analysis of Real-World Evidence Supports Anti-CGRP mAbs and OnabotulinumtoxinA Combined Trial in Chronic Migraine. Toxins 2022, 14, 529. [Google Scholar] [CrossRef] [PubMed]
- Haut, S.R.; Bigal, M.E.; Lipton, R.B. Chronic disorders with episodic manifestations: Focus on epilepsy and migraine. Lancet Neurol. 2006, 5, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Dodick, D.W. Migraine. Lancet 2018, 391, 1315–1330. [Google Scholar] [CrossRef] [PubMed]
- Bigal, M.E.; Lipton, R.B. Clinical course in migraine: Conceptualizing migraine transformation. Neurology 2008, 71, 848–855. [Google Scholar] [CrossRef] [PubMed]
- Scher, A.I.; Stewart, W.F.; Ricci, J.A.; Lipton, R.B. Factors associated with the onset and remission of chronic daily headache in a population-based study. Pain 2003, 106, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Zobdeh, F.; Eremenko, I.I.; Akan, M.A.; Tarasov, V.V.; Chubarev, V.N.; Schiöth, H.B.; Mwinyi, J. The Epigenetics of Migraine. Int. J. Mol. Sci. 2023, 24, 9127. [Google Scholar] [CrossRef] [PubMed]
- Sandrini, G.; De Icco, R.; Tassorelli, C.; Smania, N.; Tamburin, S. Botulinum neurotoxin type A for the treatment of pain: Not just in migraine and trigeminal neuralgia. J. Headache Pain 2017, 18, 38. [Google Scholar] [CrossRef] [PubMed]
- De Icco, R.; Perrotta, A.; Berra, E.; Allena, M.; Alfonsi, E.; Tamburin, S.; Serrao, M.; Sandrini, G.; Tassorelli, C. OnabotulinumtoxinA Reduces Temporal Pain Processing at Spinal Level in Patients with Lower Limb Spasticity. Toxins 2019, 11, 359. [Google Scholar] [CrossRef] [PubMed]
- Jianghui, M.; Saak, V.O.; Jiafu, W.; Mark, P.; Astrid, S.; Aoki, K.R.; Gary, W.L.; Dolly, J.O. Activation of TRPV1 Mediates Calcitonin Gene-Related Peptide Release, Which Excites Trigeminal Sensory Neurons and Is Attenuated by a Retargeted Botulinum Toxin with Anti-Nociceptive Potential. J. Neurosci. 2009, 29, 4981. [Google Scholar] [CrossRef]
- Welch, M.J.; Purkiss, J.R.; Foster, K.A. Sensitivity of embryonic rat dorsal root ganglia neurons to Clostridium botulinum neurotoxins. Toxicon 2000, 38, 245–258. [Google Scholar] [CrossRef] [PubMed]
- David, M.S.; Mark, H.; Eric, J.A.; Cynthia, L.C.; Mark, W.G.; Gary, S.G.; Melissa, J.A.; David, G.; Sonja, P.; Joseph, J.; et al. Practice guideline update summary: Botulinum neurotoxin for the treatment of blepharospasm, cervical dystonia, adult spasticity, and headache. Neurology 2016, 86, 1818. [Google Scholar] [CrossRef] [PubMed]
- Dodick, D.W.; Turkel, C.C.; DeGryse, R.E.; Aurora, S.K.; Silberstein, S.D.; Lipton, R.B.; Diener, H.-C.; Brin, M.F. OnabotulinumtoxinA for Treatment of Chronic Migraine: Pooled Results from the Double-Blind, Randomized, Placebo-Controlled Phases of the PREEMPT Clinical Program. Headache J. Head. Face Pain 2010, 50, 921–936. [Google Scholar] [CrossRef] [PubMed]
- Aurora, S.; Dodick, D.; Turkel, C.; DeGryse, R.; Silberstein, S.; Lipton, R.; Diener, H.; Brin, M. OnabotulinumtoxinA for treatment of chronic migraine: Results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 1 trial. Cephalalgia 2010, 30, 793–803. [Google Scholar] [CrossRef] [PubMed]
- Diener, H.; Dodick, D.; Aurora, S.; Turkel, C.; DeGryse, R.; Lipton, R.; Silberstein, S.; Brin, M. OnabotulinumtoxinA for treatment of chronic migraine: Results from the double-blind, randomized, placebo-controlled phase of the PREEMPT 2 trial. Cephalalgia 2010, 30, 804–814. [Google Scholar] [CrossRef] [PubMed]
- Martelletti, P.; Edvinsson, L.; Ashina, M. Shaping the future of migraine targeting Calcitonin-Gene-Related-Peptide with the Disease-Modifying Migraine Drugs (DMMDs). J. Headache Pain 2019, 20, 60. [Google Scholar] [CrossRef] [PubMed]
- Andreou, A.P.; Edvinsson, L. Mechanisms of migraine as a chronic evolutive condition. J. Headache Pain 2019, 20, 117. [Google Scholar] [CrossRef] [PubMed]
- Berger, S.L.; Kouzarides, T.; Shiekhattar, R.; Shilatifard, A. An operational definition of epigenetics. Genes Dev. 2009, 23, 781–783. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Zhou, H.; Feng, Z.; Xu, Z.; Tang, Y.; Wu, M. Epigenetics in Neurodevelopment: Emerging Role of Circular RNA. Front. Cell. Neurosci. 2019, 13, 327. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, Y.; Wang, C.; Wang, X. TET (Ten-eleven translocation) family proteins: Structure, biological functions and applications. Signal Transduct. Target. Ther. 2023, 8, 297. [Google Scholar] [CrossRef] [PubMed]
- Kordacka, J.; Gruszka, R.; Zakrzewska, M. Serum microRNA qPCR profiling and validation indicate upregulation of circulating miR-145-5p and miR-26a-5p in migraineurs. J. Headache Pain 2024, 25, 198. [Google Scholar] [CrossRef] [PubMed]
- Ludhiadch, A.; Bhardwaj, N.; Gotra, P.; Kumar, R.; Munshi, A. Common microRNAs in Epilepsy and Migraine: Their Possibility as Candidates for Biomarkers and Therapeutic Targets during Comorbid Onset of Both Conditions. CNS Neurol. Disord. Drug Targets 2023, 22, 698–710. [Google Scholar] [CrossRef] [PubMed]
- Greco, R.; Bighiani, F.; Demartini, C.; Zanaboni, A.; Francavilla, M.; Facchetti, S.; Vaghi, G.; Allena, M.; Martinelli, D.; Guaschino, E.; et al. Expression of miR-155 in monocytes of people with migraine: Association with phenotype, disease severity and inflammatory profile. J. Headache Pain 2024, 25, 138. [Google Scholar] [CrossRef] [PubMed]
- Ha, W.-S.; Chu, M.K. Altered immunity in migraine: A comprehensive scoping review. J. Headache Pain 2024, 25, 95. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, V.J.; Gómez-Galván, J.B.; Asskour, L.; Torres-Ferrús, M.; Alpuente, A.; Caronna, E.; Pozo-Rosich, P. A study of differential microRNA expression profile in migraine: The microMIG exploratory study. J. Headache Pain 2023, 24, 11. [Google Scholar] [CrossRef] [PubMed]
- Eising, E.; A Datson, N.; van den Maagdenberg, A.M.J.M.; Ferrari, M.D. Epigenetic mechanisms in migraine: A promising avenue? BMC Med. 2013, 11, 26. [Google Scholar] [CrossRef] [PubMed]
- Mehta, D.; de Boer, I.; Sutherland, H.G.; Pijpers, J.A.; Bron, C.; Bainomugisa, C.; Haupt, L.M.; van den Maagdenberg, A.; Griffiths, L.R.; Nyholt, D.R.; et al. Alterations in DNA methylation associate with reduced migraine and headache days after medication withdrawal treatment in chronic migraine patients: A longitudinal study. Clin. Epigenetics 2023, 15, 190. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Mayordomo, R.; Ruiz, M.; Pascual, J.; Gallego de la Sacristana, M.; Vidriales, I.; Sobrado, M.; Cernuda-Morollon, E.; Gago-Veiga, A.B.; Garcia-Azorin, D.; Telleria, J.J.; et al. CALCA and TRPV1 genes polymorphisms are related to a good outcome in female chronic migraine patients treated with OnabotulinumtoxinA. J. Headache Pain 2019, 20, 39. [Google Scholar] [CrossRef] [PubMed]
- Iyengar, S.; Johnson, K.W.; Ossipov, M.H.; Aurora, S.K. CGRP and the Trigeminal System in Migraine. Headache 2019, 59, 659–681. [Google Scholar] [CrossRef] [PubMed]
- Barbanti, P.; Egeo, G.; Aurilia, C.; Altamura, C.; d’Onofrio, F.; Finocchi, C.; Albanese, M.; Aguggia, M.; Rao, R.; Zucco, M.; et al. Predictors of response to anti-CGRP monoclonal antibodies: A 24-week, multicenter, prospective study on 864 migraine patients. J. Headache Pain 2022, 23, 138. [Google Scholar] [CrossRef] [PubMed]
- Waliszewska-Prosół, M.; Vuralli, D.; Martelletti, P. What to do with non-responders to CGRP(r) monoclonal antibodies: Switch to another or move to gepants? J. Headache Pain 2023, 24, 163. [Google Scholar] [CrossRef] [PubMed]
- Ornello, R.; Zelli, V.; Compagnoni, C.; Caponnetto, V.; De Matteis, E.; Tiseo, C.; Tessitore, A.; Sacco, S. MicroRNA profiling in women with migraine: Effects of CGRP-targeting treatment. J. Headache Pain 2024, 25, 80. [Google Scholar] [CrossRef] [PubMed]
- Menon, S.; Buteri, J.; Roy, B.; Murrell, M.; Quinlan, S.; MacMillan, J.C.; Lea, R.A.; Haupt, L.M.; Griffiths, L.R. Association study of calcitonin gene-related polypeptide-alpha (CALCA) gene polymorphism with migraine. Brain Res. 2011, 1378, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, H.G.; Buteri, J.; Menon, S.; Haupt, L.M.; Macgregor, E.A.; Lea, R.A.; Griffiths, L.R. Association study of the calcitonin gene-related polypeptide-alpha (CALCA) and the receptor activity modifying 1 (RAMP1) genes with migraine. Gene 2013, 515, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Cox, H.C.; Lea, R.A.; Bellis, C.; Carless, M.; Dyer, T.D.; Curran, J.; Charlesworth, J.; Macgregor, S.; Nyholt, D.; Chasman, D.; et al. A genome-wide analysis of ‘Bounty’ descendants implicates several novel variants in migraine susceptibility. Neurogenetics 2012, 13, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Rubino, E.; Boschi, S.; Giorgio, E.; Pozzi, E.; Marcinnò, A.; Gallo, E.; Roveta, F.; Grassini, A.; Brusco, A.; Rainero, I. Analysis of the DNA methylation pattern of the promoter region of calcitonin gene-related peptide 1 gene in patients with episodic migraine: An exploratory case-control study. Neurobiol. Pain 2022, 11, 100089. [Google Scholar] [CrossRef] [PubMed]
- Scuteri, D.; Corasaniti, M.T.; Tonin, P.; Nicotera, P.; Bagetta, G. Role of CGRP pathway polymorphisms in migraine: A systematic review and impact on CGRP mAbs migraine therapy. J. Headache Pain 2021, 22, 87. [Google Scholar] [CrossRef] [PubMed]
- Belinskaia, M.; Wang, J.; Kaza, S.K.; Antoniazzi, C.; Zurawski, T.; Dolly, J.O.; Lawrence, G.W. Bipartite Activation of Sensory Neurons by a TRPA1 Agonist Allyl Isothiocyanate Is Reflected by Complex Ca(2+) Influx and CGRP Release Patterns: Enhancement by NGF and Inhibition with VAMP and SNAP-25 Cleaving Botulinum Neurotoxins. Int. J. Mol. Sci. 2023, 24, 1338. [Google Scholar] [CrossRef] [PubMed]
- Burstein, R.; Blumenfeld, A.M.; Silberstein, S.D.; Manack Adams, A.; Brin, M.F. Mechanism of Action of OnabotulinumtoxinA in Chronic Migraine: A Narrative Review. Headache 2020, 60, 1259–1272. [Google Scholar] [CrossRef] [PubMed]
- Pellesi, L.; Garcia-Azorin, D.; Rubio-Beltrán, E.; Ha, W.-S.; Messina, R.; Ornello, R.; Petrusic, I.; Raffaelli, B.; Labastida-Ramirez, A.; Ruscheweyh, R.; et al. Combining treatments for migraine prophylaxis: The state-of-the-art. J. Headache Pain 2024, 25, 214. [Google Scholar] [CrossRef] [PubMed]
- Waliszewska-Prosół, M.; Montisano, D.A.; Antolak, M.; Bighiani, F.; Cammarota, F.; Cetta, I.; Corrado, M.; Ihara, K.; Kartamysheva, R.; Petrušić, I.; et al. The impact of primary headaches on disability outcomes: A literature review and meta-analysis to inform future iterations of the Global Burden of Disease study. J. Headache Pain 2024, 25, 27. [Google Scholar] [CrossRef] [PubMed]
- Dolcetti, R. Predictive Value of FcR Polymorphisms: A Further Step on the Long and Winding Road to Application. JAMA Oncol. 2017, 3, 342–343. [Google Scholar] [CrossRef] [PubMed]
- Fila, M.; Sobczuk, A.; Pawlowska, E.; Blasiak, J. Epigenetic Connection of the Calcitonin Gene-Related Peptide and Its Potential in Migraine. Int. J. Mol. Sci. 2022, 23, 6151. [Google Scholar] [CrossRef] [PubMed]
- Fila, M.; Pawlowska, E.; Szczepanska, J.; Blasiak, J. Epigenetic Connections of the TRPA1 Ion Channel in Pain Transmission and Neurogenic Inflammation—A Therapeutic Perspective in Migraine? Mol. Neurobiol. 2023, 60, 5578–5591. [Google Scholar] [CrossRef] [PubMed]
- Mennini, F.S.; Fioravanti, L.; Piasini, L.; Palazzo, F.; Coloprisco, G.; Martelletti, P. A one-year retrospective economic evaluation of botulinum toxin type A treatment of chronic tension headache. J. Headache Pain 2004, 5, 188–191. [Google Scholar] [CrossRef]
- Corasaniti, M.T.; Bagetta, G.; Nicotera, P.; Tarsitano, A.; Tonin, P.; Sandrini, G.; Lawrence, G.W.; Scuteri, D. Safety of Onabotulinumtoxin A in Chronic Migraine: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Toxins 2023, 15, 332. [Google Scholar] [CrossRef] [PubMed]
- Ailani, J.; McAllister, P.; Winner, P.K.; Chakhava, G.; Krog Josiassen, M.; Lindsten, A.; Sperling, B.; Ettrup, A.; Cady, R. Rapid resolution of migraine symptoms after initiating the preventive treatment eptinezumab during a migraine attack: Results from the randomized RELIEF trial. BMC Neurol. 2022, 22, 205. [Google Scholar] [CrossRef] [PubMed]
- Winner, P.K.; McAllister, P.; Chakhava, G.; Ailani, J.; Ettrup, A.; Krog Josiassen, M.; Lindsten, A.; Mehta, L.; Cady, R. Effects of Intravenous Eptinezumab vs Placebo on Headache Pain and Most Bothersome Symptom When Initiated During a Migraine Attack: A Randomized Clinical Trial. JAMA 2021, 325, 2348–2356. [Google Scholar] [CrossRef] [PubMed]
- Ceccardi, G.; Schiano di Cola, F.; Caratozzolo, S.; Di Pasquale, M.; Bolchini, M.; Padovani, A.; Rao, R. Onabotulinumtoxin-A: Previous Prophylactic Treatment Might Improve Subsequent Anti-CGRP Monoclonal Antibodies Response in Patients with Chronic Migraine. Toxins 2023, 15, 677. [Google Scholar] [CrossRef] [PubMed]
- Shahid, S.; Iqbal, M. Is Eptinezumab the Key to Reducing Chronic Migraine Disability? SN Compr. Clin. Med. 2024, 6, 116. [Google Scholar] [CrossRef]
- Scuteri, D.; Lawrence, G.W.; Iannacchero, R.; Trimboli, M.; Nicotera, P.; Corasaniti, M.T.; Bagetta, G. Efficacy and safety of mAbs anti-CGRP/CGRP R (eptinezumab and erenumab) or atogepant in combination with onabotulinumtoxinA in refractory chronic migraine: A clinical trial protocol. Pain Manag. 2025, 15, 177–181. [Google Scholar] [CrossRef] [PubMed]

| Gene | Polymorphism | Effect | Reference |
|---|---|---|---|
| CALC A gene | rs3781719 | allele C present at 26.9% in responders and at 40.9% in non-responders (p = 0.007; OR = 3.11 (1.33–7.26)) | [32] |
| TRPV1 gene | rs222749 | allele A representing 4.17% in responders and 12.5% in non-responders (p = 0.013; OR = 3.29 (1.28–8.43)) | [32] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scuteri, D.; Martelletti, P. Translational Impact of Genetics and Epigenetics of CGRP System on Chronic Migraine Treatment with Onabotulinumtoxin A and Other Biotech Drugs. Toxins 2025, 17, 355. https://doi.org/10.3390/toxins17070355
Scuteri D, Martelletti P. Translational Impact of Genetics and Epigenetics of CGRP System on Chronic Migraine Treatment with Onabotulinumtoxin A and Other Biotech Drugs. Toxins. 2025; 17(7):355. https://doi.org/10.3390/toxins17070355
Chicago/Turabian StyleScuteri, Damiana, and Paolo Martelletti. 2025. "Translational Impact of Genetics and Epigenetics of CGRP System on Chronic Migraine Treatment with Onabotulinumtoxin A and Other Biotech Drugs" Toxins 17, no. 7: 355. https://doi.org/10.3390/toxins17070355
APA StyleScuteri, D., & Martelletti, P. (2025). Translational Impact of Genetics and Epigenetics of CGRP System on Chronic Migraine Treatment with Onabotulinumtoxin A and Other Biotech Drugs. Toxins, 17(7), 355. https://doi.org/10.3390/toxins17070355






