Isolation of a Novel Streptomyces sp. TH05 with Potent Cyanocidal Effects on Microcystis aeruginosa
Abstract
1. Introduction
2. Results
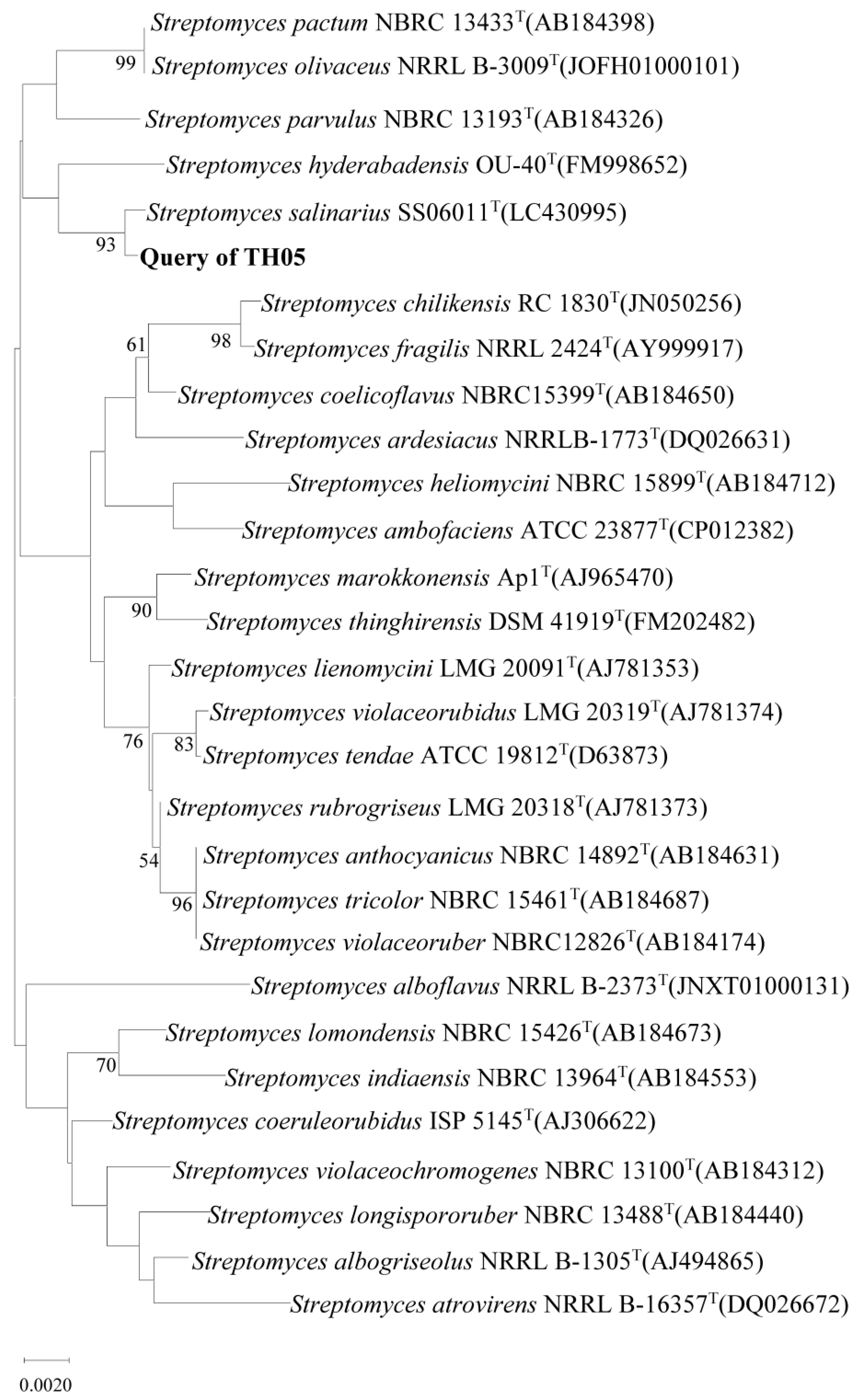
2.1. Isolation and Identification of Cyanocidal Bacteria
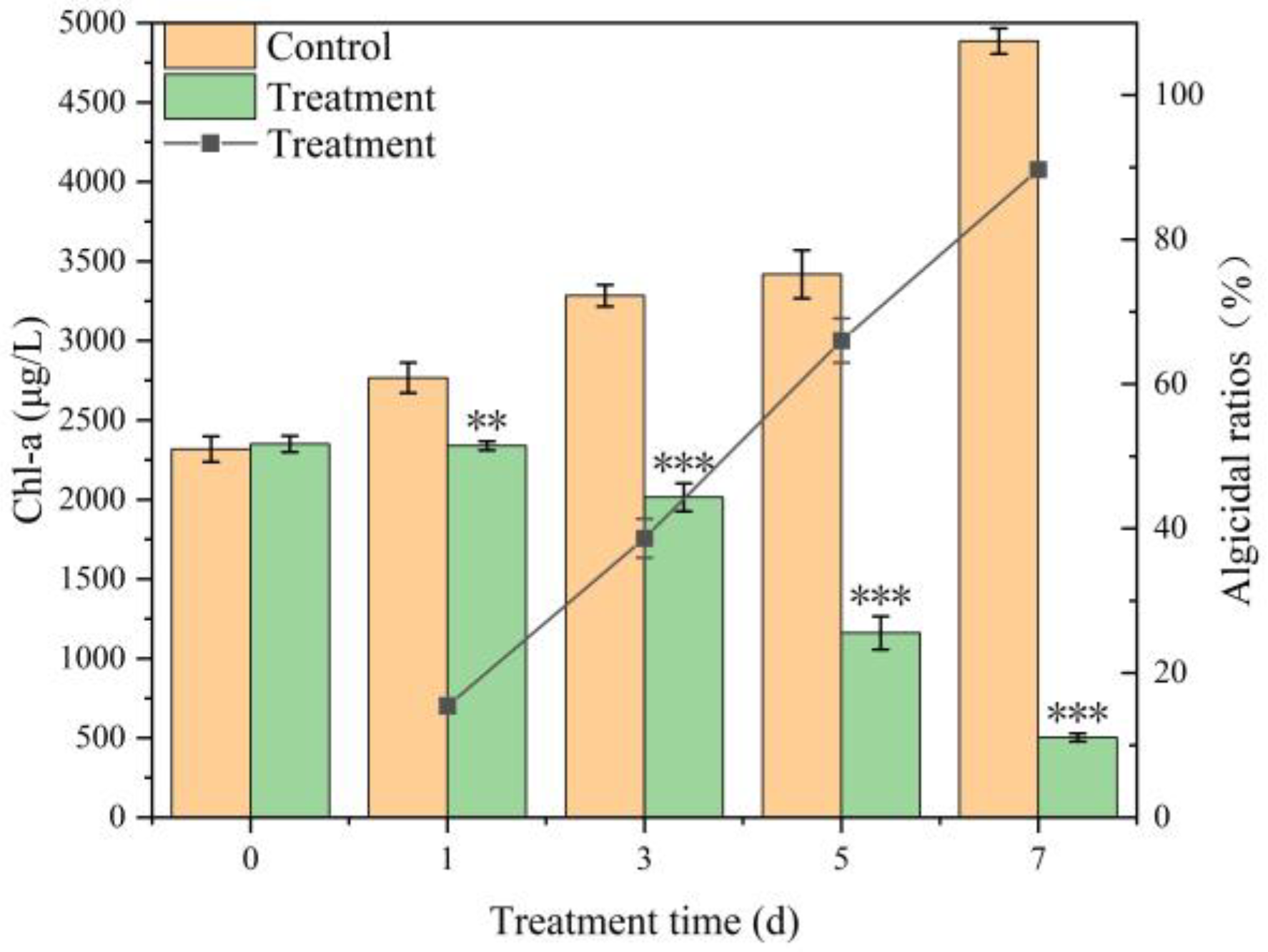
2.2. Cyanocidal Activity of Streptomyces sp. TH05
2.3. Cyanocidal Mode of Strain TH05
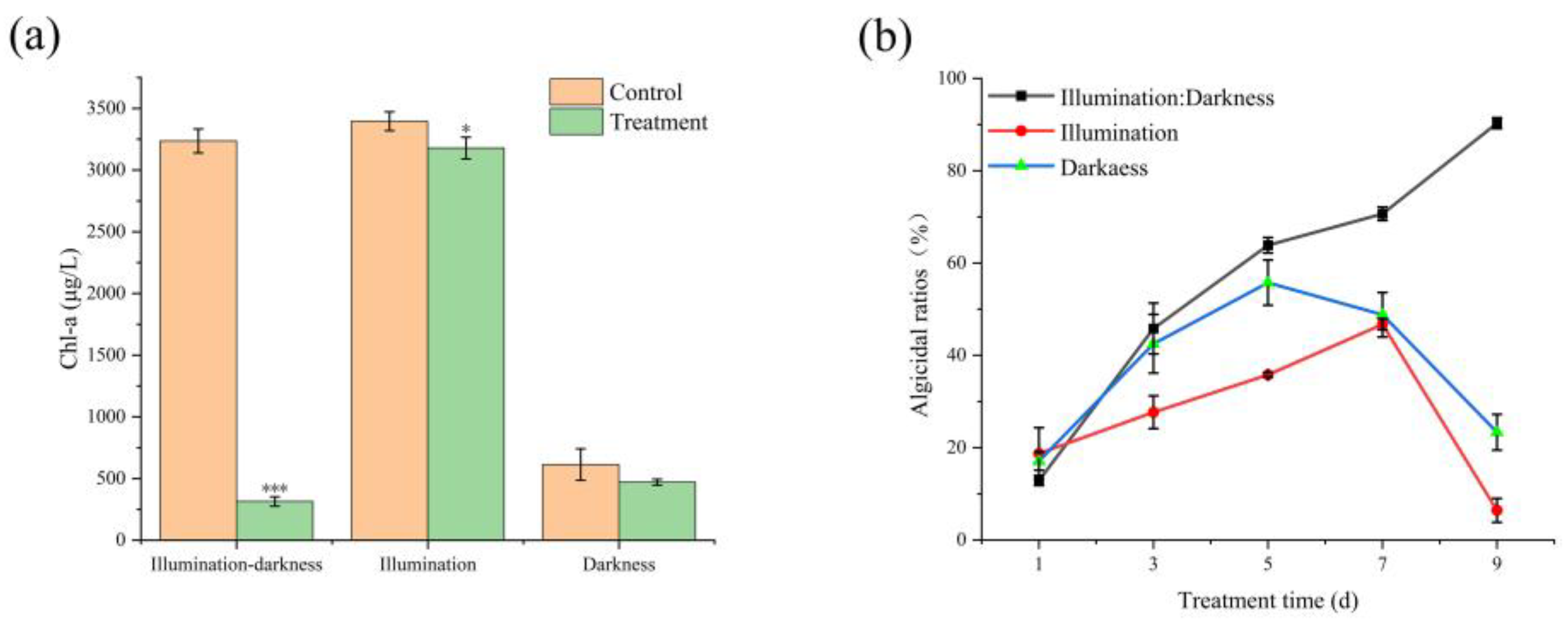
2.4. Effect of Illumination on Cyanocidal Activity
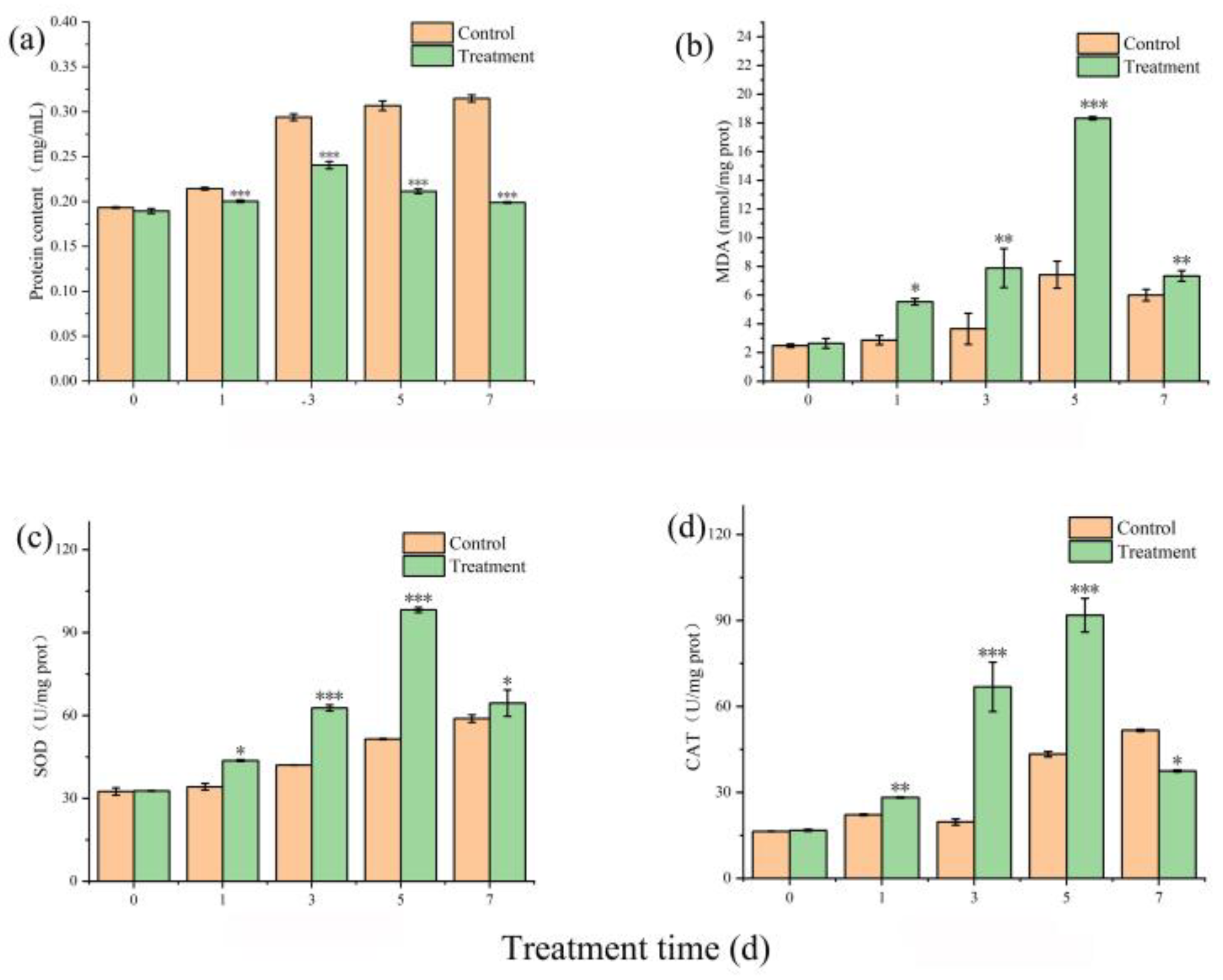
2.5. Assay of Antioxidants
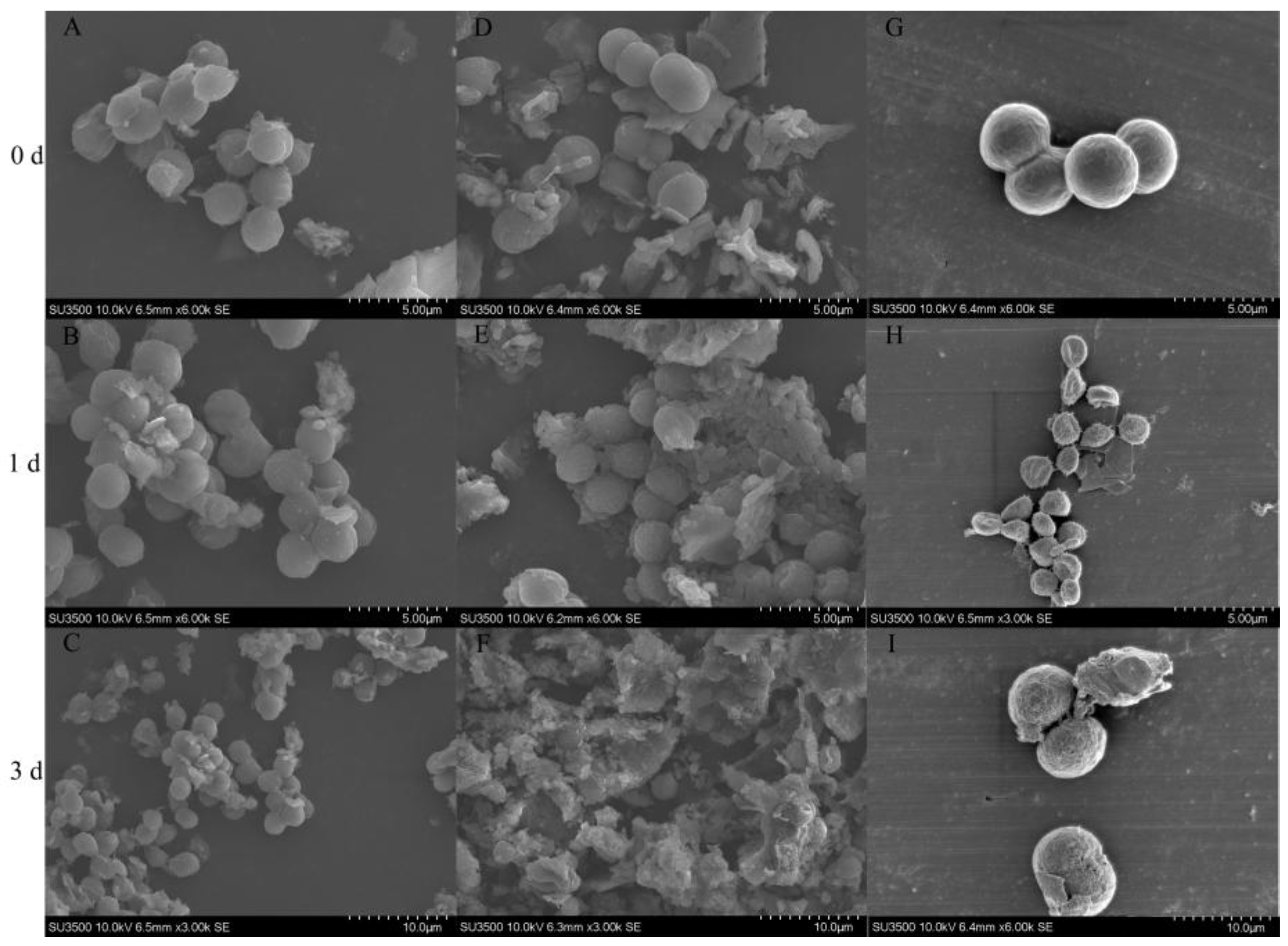
2.6. Effect of Strain TH05 on the Cell Morphology of FACHB 905
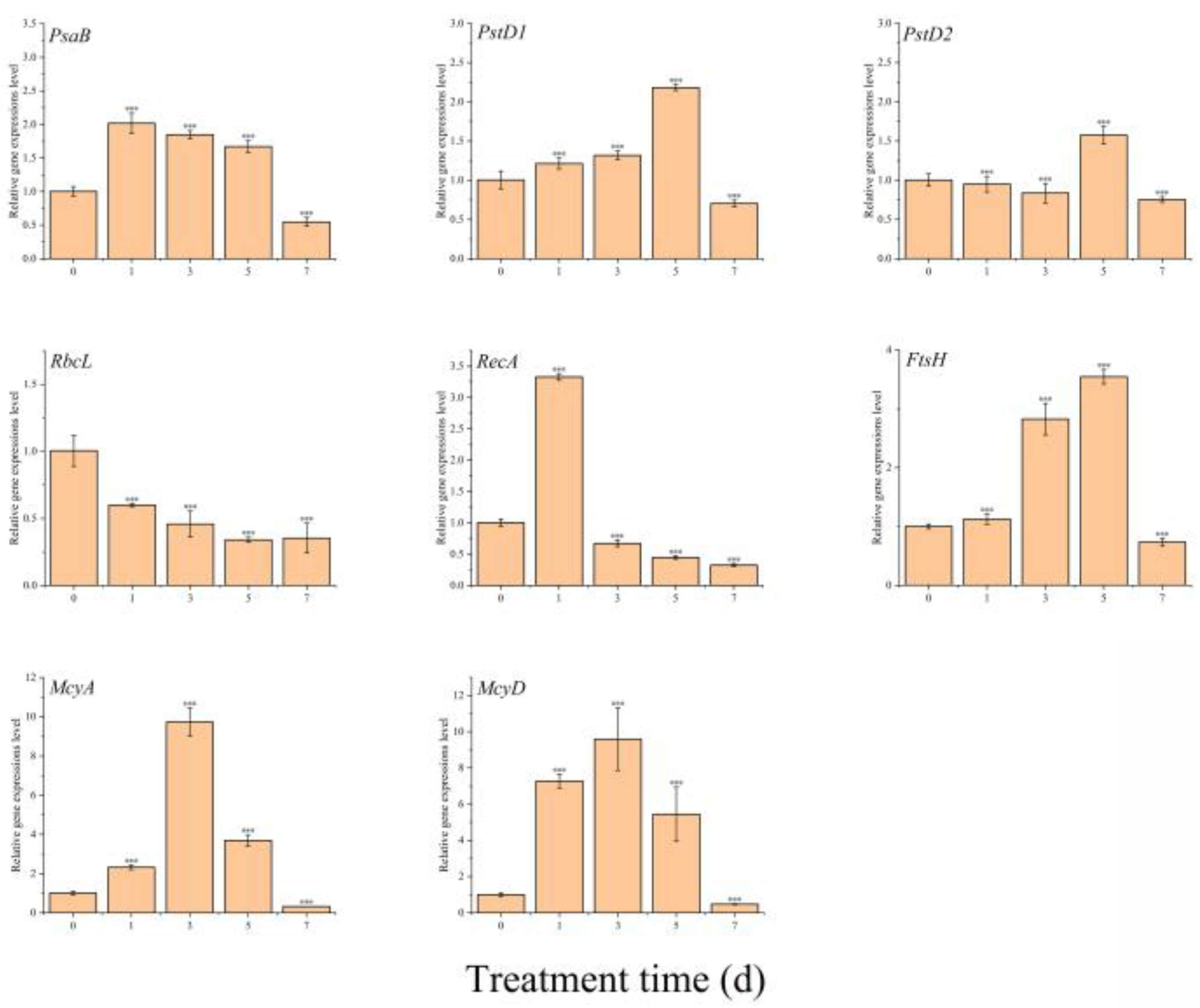
2.7. Effect of TH05 on the Transcription of Key Genes in Microcystis aeruginosa
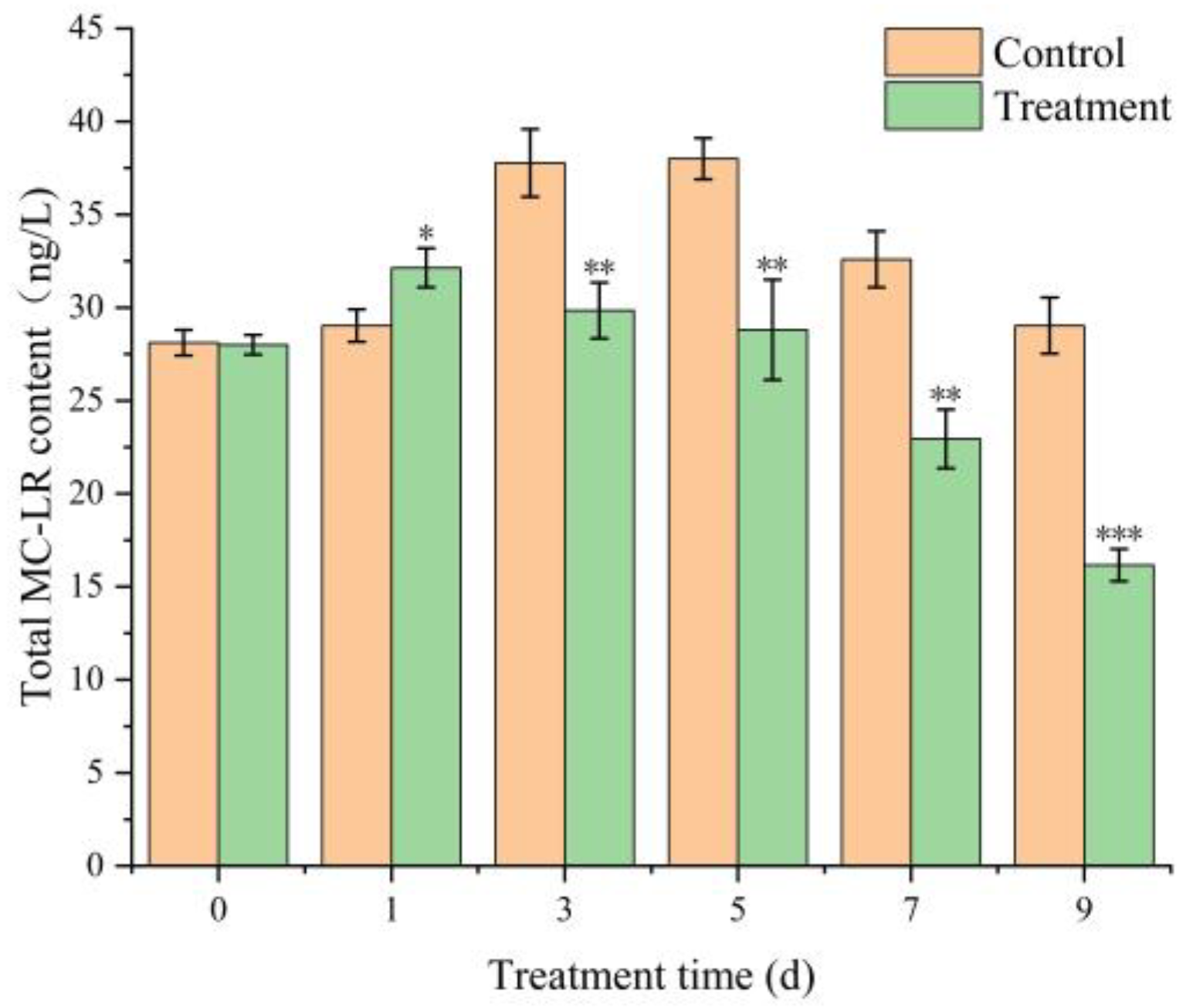
2.8. Effect of TH05 on Microcystin Levels in Algae Cells
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Microorganisms and Culture Conditions
5.2. Screening and Identification of Cyanocidal Bacteria
5.3. Morphology and Molecular Identification of Cyanocidal Bacteria Streptomyces sp. TH05
5.4. Effect of Illumination on the of TH05
5.5. Cyanocidal Mode of TH05
5.6. Method for Extracting Crude Enzyme Solution of Algae
5.7. Determination of Soluble Protein in Algae Solution
5.8. Antioxidant Measurements
5.9. Cyanobacterial Cell Morphological Observation by Scanning Electron Microscopy (SEM)
5.10. Effect of TH05 on Key Gene Expression in Microcystis aeruginosa
5.11. Microcystin Extraction and Quantification by ELISA
5.12. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pham, T.-L.; Utsumi, M. An overview of the accumulation of microcystins in aquatic ecosystems. J. Environ. Manag. 2018, 213, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Preece, E.P.; Hardy, F.J.; Moore, B.C.; Bryan, M. A review of microcystin detections in estuarine and marine waters: Environmental implications and human health risk. Harmful Algae 2017, 61, 31–45. [Google Scholar] [CrossRef]
- Souza, N.R.; Metcalf, J.S. Microcystins. In Handbook of Algal Science, Technology and Medicine; Elsevier: Amsterdam, The Netherlands, 2020; pp. 561–574. [Google Scholar]
- Sellner, K.G.; Rensel, J.E. Prevention, Control, and Mitigation of Harmful Algal Bloom Impacts on Fish, Shellfish, and Human Consumers. In Harmful Algal Blooms: A Compendium; Wiley: Hoboken, NJ, USA, 2018; pp. 435–492. [Google Scholar]
- Kim, W.; Park, Y.; Jung, J.; Jeon, C.O.; Toyofuku, M.; Lee, J.; Park, W. Biological and chemical approaches for controlling harmful Microcystis blooms. J. Microbiol. 2024, 62, 249–260. [Google Scholar] [CrossRef]
- Wang, L.; Yi, Z.; Zhang, P.; Xiong, Z.; Zhang, G.; Zhang, W. Comprehensive strategies for microcystin degradation: A review of the physical, chemical, and biological methods and genetic engineering. J. Environ. Manag. 2024, 365, 121707. [Google Scholar] [CrossRef]
- Ibrahim, N.H.; Iqbal, A.; Mohammad-Noor, N.; Roziawati, M.R. A review on the biological, physical and chemical mitigation of harmful algal bloom. Squalen Bull. Mar. Fish. Postharvest Biotechnol. 2022, 17, 95–110. [Google Scholar]
- Sun, R.; Sun, P.; Zhang, J.; Esquivel-Elizondo, S.; Wu, Y. Microorganisms-based methods for harmful algal blooms control: A review. Bioresour. Technol. 2018, 248, 12–20. [Google Scholar] [CrossRef]
- Hwang, S.O.; Cho, I.H.; Kim, H.K.; Hwang, E.A.; Han, B.H.; Kim, B.H. Toward a brighter future: Enhanced sustainable methods for preventing algal blooms and improving water quality. Hydrobiology 2024, 3, 100–118. [Google Scholar] [CrossRef]
- Voser, T.M.; Campbell, M.D.; Carroll, A.R. How different are marine microbial natural products compared to their terrestrial counterparts? Nat. Prod. Rep. 2022, 39, 7–19. [Google Scholar] [CrossRef]
- Jagannathan, S.V.; Manemann, E.M.; Rowe, S.E.; Callender, M.C.; Soto, W. Marine actinomycetes, new sources of biotechnological products. Mar. Drugs 2021, 19, 365. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-H.; Lee, J.-H.; Kim, C.-J.; Lee, J.-C.; Cho, M.-H.; Lee, J. Extracellular protease in Actinomycetes culture supernatants inhibits and detaches Staphylococcus aureus biofilm formation. Biotechnol. Lett. 2012, 34, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Jakubiec-Krzesniak, K.; Rajnisz-Mateusiak, A.; Guspiel, A.; Ziemska, J.; Solecka, J. Secondary metabolites of actinomycetes and their antibacterial, antifungal and antiviral properties. Pol. J. Microbiol. 2018, 67, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xie, Y.; Zhang, R.; Zhang, Z.; Hu, X.; Cheng, Y.; Geng, R.; Ma, Z.; Li, R. Discovery of a high-efficient algicidal bacterium against Microcystis aeruginosa based on examinations toward culture strains and natural bloom samples. Toxins 2023, 15, 220. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zeng, Y.; Li, J.; Yang, C.; Zhang, X.; Luo, F.; Dai, X. An algicidal Streptomyces amritsarensis strain against Microcystis aeruginosa strongly inhibits microcystin synthesis simultaneously. Sci. Total Environ. 2019, 650, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Wang, J.; Yang, C.; Ding, M.; Hamilton, P.B.; Zhang, X.; Yang, C.; Zhang, L.; Dai, X. A Streptomyces globisporus strain kills Microcystis aeruginosa via cell-to-cell contact. Sci. Total Environ. 2021, 769, 144489. [Google Scholar] [CrossRef]
- Zhang, B.-H.; Chen, W.; Li, H.-Q.; Yang, J.-Y.; Zha, D.-M.; Duan, Y.-Q.; Hozzein, W.N.; Xiao, M.; Gao, R.; Li, W.-J. L-valine, an antialgal amino acid from Streptomyces jiujiangensis JXJ 0074T. Appl. Microbiol. Biotechnol. 2016, 100, 4627–4636. [Google Scholar] [CrossRef]
- Hamed, S.M.; Selim, S.; Klöck, G.; AbdElgawad, H. Sensitivity of two green microalgae to copper stress: Growth, oxidative and antioxidants analyses. Ecotoxicol. Environ. Saf. 2017, 144, 19–25. [Google Scholar] [CrossRef]
- Jafari, M.; Shahsavar, A.R.; Talebi, M.; Hesami, M. Exogenous melatonin protects lime plants from drought stress-induced damage by maintaining cell membrane structure, detoxifying ROS and regulating antioxidant systems. Horticulturae 2022, 8, 257. [Google Scholar] [CrossRef]
- Ni, L.; Yue, F.; Zhang, J.; Chen, H.; Wang, Z. Cell membrane damage induced by continuous stress of artemisinin sustained-release microspheres (ASMs) on Microcystis aeruginosa at different physiological stages. Environ. Sci. Pollut. Res. 2020, 27, 12624–12634. [Google Scholar] [CrossRef]
- Ali, S.S.; Ahsan, H.; Zia, M.K.; Siddiqui, T.; Khan, F.H. Understanding oxidants and antioxidants: Classical team with new players. J. Food Biochem. 2020, 44, e13145. [Google Scholar] [CrossRef]
- Zhou, S.; Cai, H.; He, X.; Tang, Z.; Lu, S. Enzyme-mimetic antioxidant nanomaterials for ROS scavenging: Design, classification, and biological applications. Coord. Chem. Rev. 2024, 500, 215536. [Google Scholar] [CrossRef]
- Hong, Y.; Hu, H.Y.; Xie, X.; Li, F.; Liu, W. Gramine-induced growth inhibition, oxidative damage and antioxidant responses in freshwater cyanobacterium Microcystis aeruginosa. Aquat. Toxicol. 2009, 91, 262–269. [Google Scholar] [CrossRef]
- García-Caparrós, P.; De Filippis, L.; Gul, A.; Hasanuzzaman, M.; Ozturk, M.; Altay, V.; Lao, M.T. Oxidative stress and antioxidant metabolism under adverse environmental conditions: A review. Bot. Rev. 2021, 87, 421–466. [Google Scholar] [CrossRef]
- Hernández-Prieto, M.A.; Semeniuk, T.A.; Giner-Lamia, J.; Futschik, M.E. The transcriptional landscape of the photosynthetic model cyanobacterium Synechocystis sp. PCC 68. Sci. Rep. 2016, 6, 22168. [Google Scholar] [CrossRef]
- Haraguchi, N.; Kaseda, J.; Nakayama, Y.; Nishiyama, Y.; Kojima, H. Characterization of mutants expressing thermostable D1 and D2 polypeptides of photosystem II in the cyanobacterium Synechococcus elongatus PCC 79. J. Biosci. Bioeng. 2018, 126, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, M.R.; Senhorinho, G.N.A.; Scott, J.A. Microalgae under environmental stress as a source of antioxidants. Algal Res. 2020, 52, 102104. [Google Scholar] [CrossRef]
- Liu, C.; Shi, N.; Wu, H.; An, X.; Zheng, J.; Duan, Y.; Sun, D.; Feng, Y.; Zhang, L. Cytogenetic analyses of PSL1 mutant, a novel low-temperature-sensitive purple-striped leaf color mutant in wheat. Crop Sci. 2018, 58, 1919–1931. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, T.; Xing, Y.; Zeng, X.; Wang, L.; Liu, Z.; Shi, J.; Zhu, X.; Ma, L.; Li, Y.; et al. YGL9, encoding the putative chloroplast signal recognition particle 43 kDa protein in rice, is involved in chloroplast development. J. Integr. Agric. 2016, 15, 944–953. [Google Scholar] [CrossRef]
- Liu, L.; Zhu, B.; Wang, G.X. Azoxystrobin-induced excessive reactive oxygen species (ROS) production and inhibition of photosynthesis in the unicellular green algae Chlorella vulgaris. Environ. Sci. Pollut. Res. 2015, 22, 7766–7775. [Google Scholar] [CrossRef]
- Bolay, P.; Schlüter, S.; Grimm, S.; Knübel, G.; Wilde, A. The transcriptional regulator RbcR controls ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisCO) genes in the cyanobacterium Synechocystis sp. PCC 68. New Phytol. 2022, 235, 432–445. [Google Scholar] [CrossRef]
- Xue, X.; Gao, N.; Xu, F. Toxicity of perfluorooctane sulfonate (PFOS) and perfluorobutane sulfonate (PFBS) to Scenedesmus obliquus: Photosynthetic characteristics, oxidative damage and transcriptome analysis. Environ. Pollut. 2022, 315, 120397. [Google Scholar] [CrossRef]
- Chen, Y.-D.; Zhu, Y.; Xin, J.-P.; Li, L.; Liu, J.; Zhang, H.; Zhou, Y. Succinic acid inhibits photosynthesis of Microcystis aeruginosa via damaging PSII oxygen-evolving complex and reaction center. Environ. Sci. Pollut. Res. 2021, 28, 58470–58479. [Google Scholar] [CrossRef] [PubMed]
- Sijil, P.V.; Adki, V.R.; Sarada, R.; Chauhan, V.S. Stress induced modifications in photosystem II electron transport, oxidative status, and expression pattern of accD and rbcL genes in an oleaginous microalga Desmodesmus sp. Bioresour. Technol. 2020, 318, 124036. [Google Scholar]
- Pham, P.; Wood, E.A.; Cox, M.M.; Sivaramakrishnan, P.; Chitteni-Pattu, S. RecA and SSB genome-wide distribution in ssDNA gaps and ends in Escherichia coli. Nucleic Acids Res. 2023, 51, 5527–5543. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Ko, S.R.; Ahn, C.Y.; Oh, H.M.; Kim, H.S. Microcystin biosynthesis and mcyA expression in geographically distinct Microcystis strains under different nitrogen, phosphorus, and boron regimes. Biomed. Res. Int. 2016, 2016, 5985910. [Google Scholar] [CrossRef]
- Ma, Y.; Yan, F.; An, L.; Wu, Q.; Liu, Y.; Zhao, J. Transcriptome analysis of changes in Microcystis aeruginosa growth and microcystin production under low concentrations of ethinyl estradiol. Sci. Total Environ. 2023, 859, 160253. [Google Scholar] [CrossRef]
- Yang, C.; Xiang, W.; Ng, W.L.; Zhao, J.; Lin, X. Multi-algicidal mechanism and potential application of Streptomyces sp. strain P-10 against the bloom-forming Prorocentrum donghaiense. J. Appl. Microbiol. 2025, 136, lxaf109. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Kouchiwa, T.; Hodoki, Y.; Hotta, K.; Uchida, H.; Harada, K.-I. Distribution and identification of actinomycetes lysing cyanobacteria in a eutrophic lake. J. Appl. Phycol. 1998, 10, 391–397. [Google Scholar] [CrossRef]
- Choi, H.; Kim, B.; Kim, J.; Han, M. Streptomyces neyagawaensis as a control for the hazardous biomass of Microcystis aeruginosa (Cyanobacteria) in eutrophic freshwaters. Biol. Control 2005, 33, 335–343. [Google Scholar] [CrossRef]
- Pokrzywinski, K.L.; Place, A.R.; Warner, M.E.; Coyne, K.J. Investigation of the algicidal exudate produced by Shewa sp. Harmful Algae 2012, 19, 23–29. [Google Scholar] [CrossRef]
- Shi, J.; Wang, W.; Wang, F.; Zhang, Y.; Zhang, M.; Liu, Y. Efficient inactivation of harmful algae K. mikimotoi by a novel algicidal bacterium via a rare direct contact pathway: Performances and mechanisms. Sci. Total Environ. 2023, 892, 164441. [Google Scholar] [CrossRef]
- Hu, Y.; Huang, X.; Cheng, X.; Zhang, Y.; Liu, Y.; Chen, W. Bacillus cereus strain L7 lyses Cylindrospermopsis raciborskii through intercellular contact. Algal Res. 2023, 71, 103015. [Google Scholar] [CrossRef]
- Fuentes, J.L.; Garbayo, I.; Cuaresma, M.; Montero, Z.; González-Del-Valle, M.; Vílchez, C. Impact of microalgae–bacteria interactions on the production of algal biomass and associated compounds. Mar. Drugs 2016, 14, 100. [Google Scholar] [CrossRef] [PubMed]
- Imai, I. Interactions between harmful algae and algicidal and growth-inhibiting bacteria associated with seaweeds and seagrasses. In Marine Protists: Diversity and Dynamics; Ohtsuka, S., Suzaki, T., Horiguchi, T., Suzuki, N., Not, F., Eds.; Springer: Tokyo, Japan, 2015; pp. 1–14. [Google Scholar]
- Kong, Y.; Wang, Q.; Chen, Y.; Yang, Y.; Shen, H.; Lu, C. Anticyanobacterial process and action mechanism of Streptomyces sp. HJC-D1 on Microcystis aeruginosa. Environ. Prog. Sustain. Energy 2020, 39, e13389. [Google Scholar] [CrossRef]
- Boukaew, S.; Yossan, S.; Cheirsilp, B.; Petlamul, W.; Prasertsan, P. Influences of culture media, temperature and light/dark conditions on growth and antifungal activity of Streptomyces spp. against Botrytis cinerea, in vitro and on tomato leaf. Preprints 2021, 2021040385. [Google Scholar] [CrossRef]
- Wang, Q.; Wangjin, X.; Zhang, Y.; Sun, Q.; Wang, Y.; Duan, L. The toxicity of virgin and UV-aged PVC microplastics on the growth of freshwater algae Chlamydomonas reinhardtii. Sci. Total Environ. 2020, 749, 141603. [Google Scholar] [CrossRef]
- Wang, J.; Yin, X.; Xu, M.; Zhang, D.; Sun, P.; Xie, X.; Zhang, X.; Han, X. Isolation and characterization of a high-efficiency algicidal bacterium Pseudoalteromonas sp. LD-B6 against the harmful dinoflagellate Noctiluca scintillans. Front. Microbiol. 2022, 13, 1026936. [Google Scholar] [CrossRef]
- Zeng, G.; Liang, D.; Tang, C.; Liu, Y.; Zhang, Y.; He, Y.; Wu, H. The algicidal potential of a floating-bed system against Microcystis aeruginosa in laboratory conditions. Water 2023, 15, 36. [Google Scholar] [CrossRef]
- Akhter, K.; Kiani, H.A.; Ghous, T.; Ahmad, A.; Rehman, R.; Bano, K. Production, partial purification and optimization of oilseed-based protease and its application as an efficient eco-friendly alternative for destaining and dehairing process. Waste Biomass Valorization 2024, 15, 3929–3939. [Google Scholar] [CrossRef]
- Tao, C.; Niu, X.; Zhang, D.; Liu, Y.; Zhou, L.; Han, Y.; Mo, Y.; Wu, K.; Lin, Y.; Lin, Z. Inhibitory effect of malonic acid on Microcystis aeruginosa: Cell morphology, oxidative stress and gene expression. Algal Res. 2024, 80, 103513. [Google Scholar] [CrossRef]
- Li, W.; Guo, J.; Han, X.; Da, X.; Wang, K.; Zhao, H.; Huang, S.-T.; Li, B.; He, H.; Jiang, R.; et al. A novel protein domain is important for photosystem II complex assembly and photoautotrophic growth in angiosperms. Mol. Plant 2023, 16, 374–392. [Google Scholar] [CrossRef]
- Inagaki, N. Processing of D1 protein: A mysterious process carried out in thylakoid lumen. Int. J. Mol. Sci. 2022, 23, 2520. [Google Scholar] [CrossRef] [PubMed]
- Rantala, M.; Rantala, S.; Aro, E.M. Composition, phosphorylation and dynamic organization of photosynthetic protein complexes in plant thylakoid membrane. Photochem. Photobiol. Sci. 2020, 19, 604–619. [Google Scholar] [CrossRef] [PubMed]
- Berry, J.O.; Yerramsetty, P.; Zielinski, A.M.; Mure, C.M. Photosynthetic gene expression in higher plants. Photosynth. Res. 2013, 117, 91–120. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Sui, X.; Wang, S.; Wei, Y.; Huang, H.; Hu, L.; Zhang, Z. The response of rbcL, rbcS and rca genes in cucumber (Cucumis sativus L.) to growth and induction light intensity. Acta Physiol. Plant. 2014, 36, 2779–2791. [Google Scholar] [CrossRef]
- Del Val, E.; Nasser, W.; Abaibou, H.; Reverchon, S. Design and comparative characterization of RecA variants. Sci. Rep. 2021, 11, 21106. [Google Scholar] [CrossRef]
- Bečková, M.; Yu, J.; Krynická, V.; Kozlo, A.; Shao, S.; Koník, P.; Komenda, J.; Murray, J.W.; Nixon, P.J. Structure of Psb29/Thf1 and its association with the FtsH protease complex involved in photosystem II repair in cyanobacteria. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2017, 372, 20160394. [Google Scholar] [CrossRef]
- Lee, J.; Hong, S.; An, S.A.; Khim, J.S. Methodological advances and future directions of microalgal bioassays for evaluation of potential toxicity in environmental samples: A review. Environ. Int. 2023, 173, 107869. [Google Scholar] [CrossRef]
- Coyne, K.J.; Wang, Y.; Johnson, G. Algicidal bacteria: A review of current knowledge and applications to control harmful algal blooms. Front. Microbiol. 2022, 13, 871177. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, Q.; Chen, C.; Xie, B.; Tang, B.-G.; Fan, M.-H.; Hu, Q.-J.; Liao, Z.; Yan, X.-J. The growth inhibitory effects and non-targeted metabolomic profiling of Microcystis aeruginosa treated by Scenedesmus sp. Chemosphere 2023, 338, 139446. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, H.; Zheng, W.; Liu, Y.; Liu, H.; Sun, J.; Cai, H.; Zhang, R. Toxic effects of prodigiosin secreted by Hahella sp. KA22 on harmful alga Phaeocystis globosa. Front. Microbiol. 2017, 8, 12. [Google Scholar] [CrossRef]
- Mukai, K.; Shimasaki, Y.; Qiu, X.; Oshima, Y.; Nishikawa, T.; Honjo, T. Gene expression stability of candidate reference genes under different culture conditions for quantitative PCR in the raphidophyte Chattonella marina. Phycologia 2020, 59, 556–563. [Google Scholar] [CrossRef]
- Peng, C.; An, D.; Ding, W.X.; Zhu, Y.-X.; Ye, L.; Li, J. Fungichromin production by Streptomyces sp. WP-1, an endophyte from Pinus dabeshanensis, and its antifungal activity against Fusarium oxysporum. Appl. Microbiol. Biotechnol. 2020, 104, 10437–10449. [Google Scholar] [CrossRef]
- Zhou, S.; Yin, H.; Tang, S.; Peng, H.; Yin, D.; Yang, Y.; Liu, Z.; Dang, Z. Physiological responses of Microcystis aeruginosa against the algicidal bacterium Pseudomonas aeruginosa. Ecotoxicol. Environ. Saf. 2016, 127, 214–221. [Google Scholar] [CrossRef]
- Mao, L.; Huang, J.; Mao, H.; Xu, L.; Xu, X. Self-floating capsule of algicidal bacteria Bacillus sp. HL and its performance in the dissolution of Microcystis aeruginosa. J. Environ. Manag. 2022, 320, 115832. [Google Scholar] [CrossRef]
- Zhang, X.; Shi, H.T.; Feng, X.C.; Jiang, C.-Y.; Wang, W.-Q.; Xiao, Z.-J.; Xu, Y.-J.; Zeng, Q.-Y.; Ren, N.-Q. Efficient aerobic denitrification without nitrite accumulation by Pseudomonas mendocina HITSZ-D1 isolated from sewage sludge. Bioresour. Technol. 2023, 379, 129039. [Google Scholar] [CrossRef]
- Petukhov, D.V.; Tovstik, E.V.; Bakulina, A.V.; Sazanova, M.L.; Burkov, A.A. Soil Streptomyces sp. strain 2K1: Phylogenetic position, effect on Fusarium proliferatum growth. Theor. Appl. Ecol. 2020, 111–116. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, S.W.; Li, K.T. Antagonistic activity and mechanism of an isolated Streptomyces corchorusii strain AUH-1 against phytopathogenic fungi. World J. Microbiol. Biotechnol. 2019, 35, 1–9. [Google Scholar] [CrossRef]
- Kim, M.; Shin, B.; Lee, J.; Park, J.; Kim, Y. Culture-independent and culture-dependent analyses of the bacterial community in the phycosphere of cyanobloom-forming Microcystis aeruginosa. Sci. Rep. 2019, 9, 20422. [Google Scholar] [CrossRef]
- Ouyang, P.; Wang, C.; Wang, P.; Liu, Y.; Zhang, H. Effects of mixed allelochemicals on the growth of Microcystis aeruginosa, microcystin production, extracellular polymeric substances, and water quality. Water 2020, 12, 18. [Google Scholar] [CrossRef]
- Wei, N.; Hu, L.; Song, L.; Gan, N.; Gao, Y. Microcystin-bound protein patterns in different cultures of Microcystis aeruginosa and field samples. Toxins 2016, 8, 2. [Google Scholar] [CrossRef]
- Song, Q.; Niu, X.; Zhang, D.; Zhang, Y.; Li, A. The behaviors of Microcystis aeruginosa and microcystins during the Fe2+/persulfate (PS) preoxidation–coagulation and flocs storage period. Environ. Res. 2020, 186, 109524. [Google Scholar] [CrossRef]
- Peng, G.; Lin, S.; Fan, Z.; Wang, Z.; Gan, N. Transcriptional and physiological responses to nutrient loading on toxin formation and photosynthesis in Microcystis aeruginosa FACHB-9. Toxins 2017, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Feng, H.; Clara, T.; Huang, F.; Liu, Y.; Zhang, Q. Identification and characterization of the dominant Microcystis sp. cyanobacteria detected in Lake Dong Ting, China. Toxicol. Environ. Health A 2019, 82, 1143–1152. [Google Scholar] [CrossRef]
- Zhang, H.; Li, B.; Liu, Y.; Chuan, H.; Liu, Y.; Xie, P. Immunoassay technology: Research progress in microcystin-LR detection in water samples. J. Hazard. Mater. 2022, 424, 127406. [Google Scholar] [CrossRef]
- Zhao, L.; Xu, K.; Juneau, P.; Huang, P.; Lian, Y.; Zheng, X.; Zhong, Q.; Zhang, W.; Xiao, F.; Wu, B.; et al. Light modulates the effect of antibiotic norfloxacin on photosynthetic processes of Microcystis aeruginosa. Aquat. Toxicol. 2021, 235, 105826. [Google Scholar] [CrossRef]


| Characteristic | TH05 | Streptomyces salinarius SS06011T |
|---|---|---|
| NaCl (%) | 0–10% | 0–10% |
| Temperature (°C) | 23–40 | 25–45 |
| pH | 6.0–11.0 | 6.0–11.0 |
| H2S production | − | + |
| Oxidase | − | + |
| Amylase | − | + |
| Degradation Substrate | ||
| Starch | − | + |
| Gelatin | − | + |
| Tyrosine | + | + |
| API ZYM Tests | ||
| Esterase (C4) | + | + |
| Esterase lipase (C8) | + | + |
| Lipase (C14) | + | − |
| Carbon source utilization | + | − |
| API 50CH Tests | ||
| D-Glucose | + | + |
| Xylose | + | − |
| Mannitol | + | + |
| Mannose | + | + |
| Sucrose | − | − |
| Galactose | + | + |
| Utilized as Sole Carbon Sources | ||
| D-Mannose | + | + |
| Citric acid | + | + |
| Cellobiose | + | + |
| Gene | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| psaB | TCTCGCCTGAACCACCACCTC | GTCCCAACCAACGTGCTGACC |
| pstD1 | GTGCAGTTGTTCCCTCCTCCAA | GCTACACAGATCCAAGGACGCA |
| pstD2 | CCGAGCTTTTGAACCCACCCA | TCCCACTACACCCACAGCACT |
| rbcL | AGATATCCGTTTCCCCGTCGCT | GGCCGAGTTTGGGTTTGATGGT |
| recA | GCCTCGGTGCGTTTAGATATCC | ACCACGTTAGTTTGTTCGGCG |
| FtsH | GGAGTTATTTGTCGGCACTGGGG | GTTGGTTTAAAGTCTGCTCGCGC |
| mcyA | CGGAGGAAGTGGAGGATGCTT | CTGGATGCCGCTGGACAATCT |
| mcyD | GCGTGGCTGGTATCTTCCGTT | TGGGTGGGTTCAAAAGCTCGG |
| elf-p | GGTAGAATTGCCCACCTCGGT | TCCCGTCCCAGATAAGAACCA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Zhu, S.; Tao, S.; Zhang, S.; Wang, R.; Zhang, L. Isolation of a Novel Streptomyces sp. TH05 with Potent Cyanocidal Effects on Microcystis aeruginosa. Toxins 2025, 17, 354. https://doi.org/10.3390/toxins17070354
Wang X, Zhu S, Tao S, Zhang S, Wang R, Zhang L. Isolation of a Novel Streptomyces sp. TH05 with Potent Cyanocidal Effects on Microcystis aeruginosa. Toxins. 2025; 17(7):354. https://doi.org/10.3390/toxins17070354
Chicago/Turabian StyleWang, Xuhan, Siqi Zhu, Shenchen Tao, Shaoyong Zhang, Ruijun Wang, and Liqin Zhang. 2025. "Isolation of a Novel Streptomyces sp. TH05 with Potent Cyanocidal Effects on Microcystis aeruginosa" Toxins 17, no. 7: 354. https://doi.org/10.3390/toxins17070354
APA StyleWang, X., Zhu, S., Tao, S., Zhang, S., Wang, R., & Zhang, L. (2025). Isolation of a Novel Streptomyces sp. TH05 with Potent Cyanocidal Effects on Microcystis aeruginosa. Toxins, 17(7), 354. https://doi.org/10.3390/toxins17070354





