Recent Advancements in Lateral Flow Assays for Food Mycotoxin Detection: A Review of Nanoparticle-Based Methods and Innovations
Abstract
1. Introduction
2. Research Method
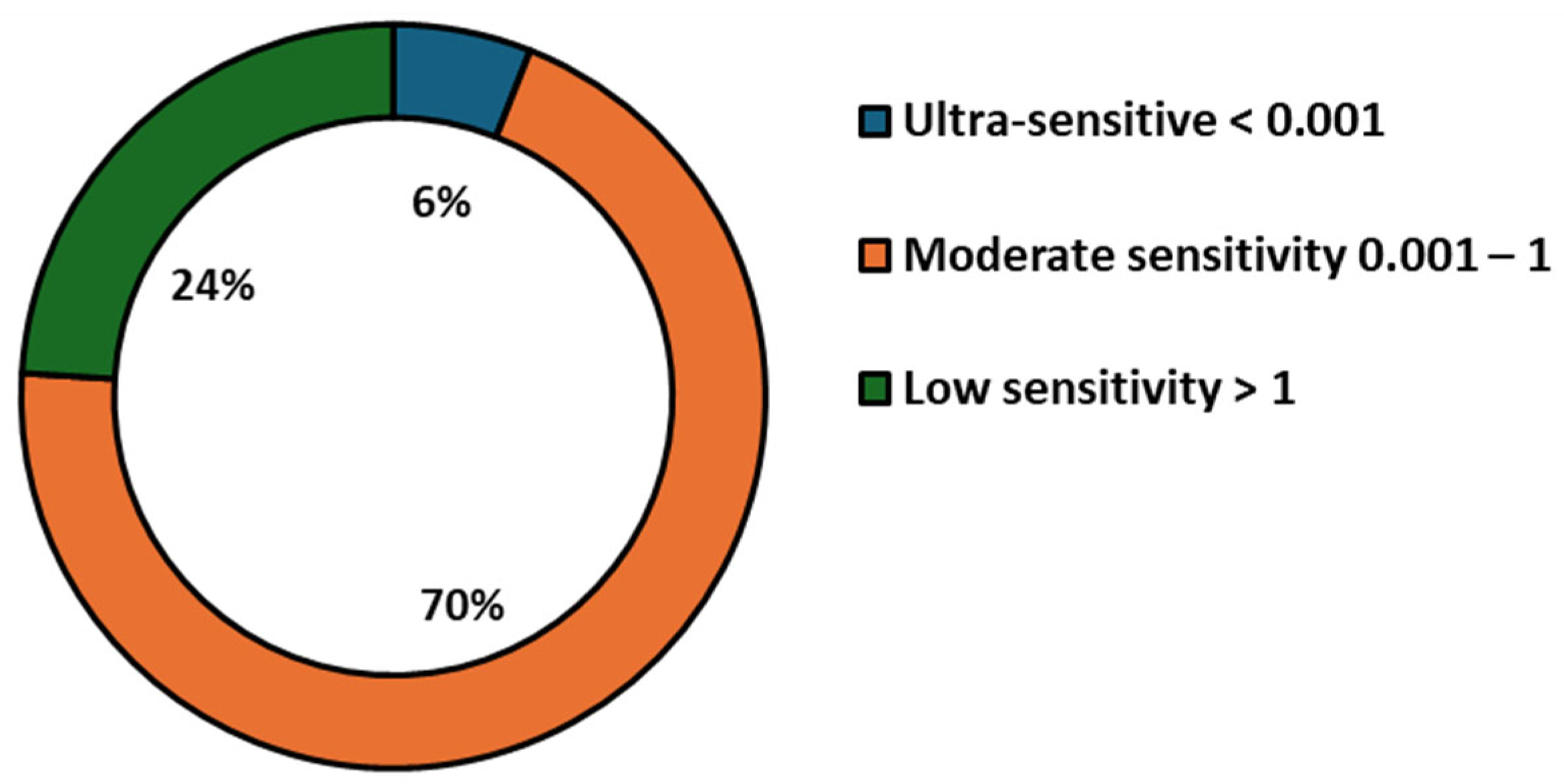
3. Results
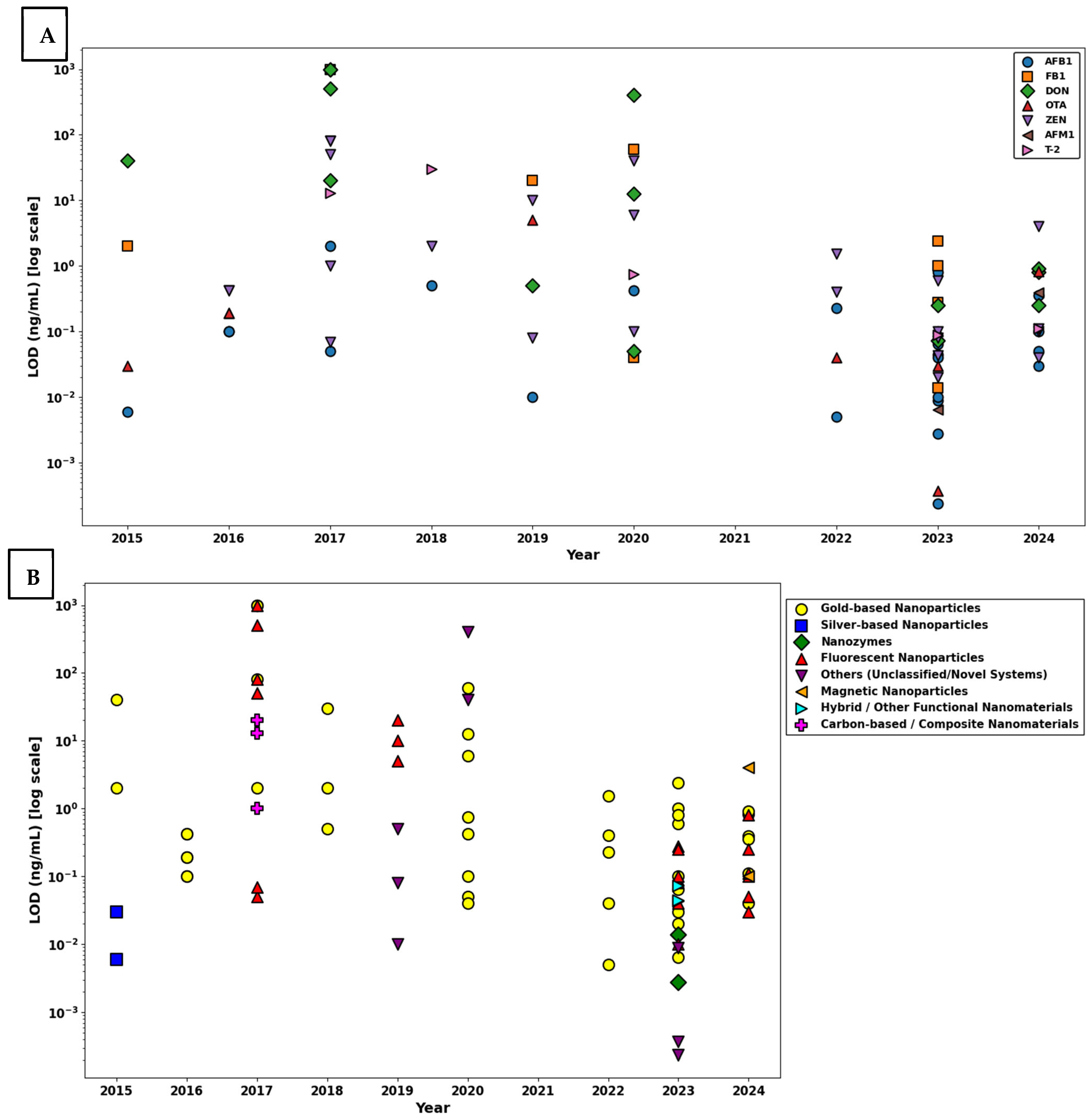
- AFB1: The LOD values exhibit substantial variability, ranging approximately from 10−4 to nearly 1 ng/mL. The median value for AFB1 is situated around 10−2 ng/mL, with the presence of several outliers positioned toward higher concentrations around 100 ng/mL, reflecting significant variability among different assay conditions or analytical platforms.
- Total Aflatoxin: Observed LOD values range narrowly between approximately 10−3 and slightly above 10−1 ng/mL, with a median concentration closely centered at about 10−2 ng/mL. The tight interquartile range indicates relatively consistent sensitivity across multiple detection methods.
- Fumonisins: This group exhibits the broadest range, with LOD values extending from slightly above 100 ng/mL up to nearly 103 ng/mL, indicating significantly lower sensitivity compared to other mycotoxins. The median is notably elevated, positioned around 102 ng/mL, emphasizing substantial analytical difficulty in achieving low detection limits.
- FB1: The LOD values are concentrated within a relatively narrow range, approximately between 101 and 102 ng/mL. The median is closely clustered at around 50 ng/mL, underscoring moderate sensitivity yet less variability compared to the general fumonisin group.
- ZEN: LOD values span approximately from below 10−2 ng/mL to above 100 ng/mL. The median LOD is situated slightly below 10−1 ng/mL. Notable outliers are observed, indicating variations possibly influenced by matrix effects or assay-specific conditions.
- T-2 Toxin: The dataset for T-2 toxin demonstrates tight clustering, ranging narrowly around 10−1 ng/mL, with limited variability and few outliers, suggesting relatively uniform assay performance across different analytical setups.
- DON: The observed range of LOD values for DON is quite wide, spanning from around 10−1 ng/mL to above 101 ng/mL. The median lies near 100 ng/mL, with noticeable outliers at higher values, indicating variability likely attributed to assay sensitivity differences and matrix interferences.
- OTA: LOD values span from below 10−2 ng/mL up to slightly above 100 ng/mL, with a median concentration close to 10−1 ng/mL. Several outliers towards the higher end suggest variable detection efficiency among reported methods.
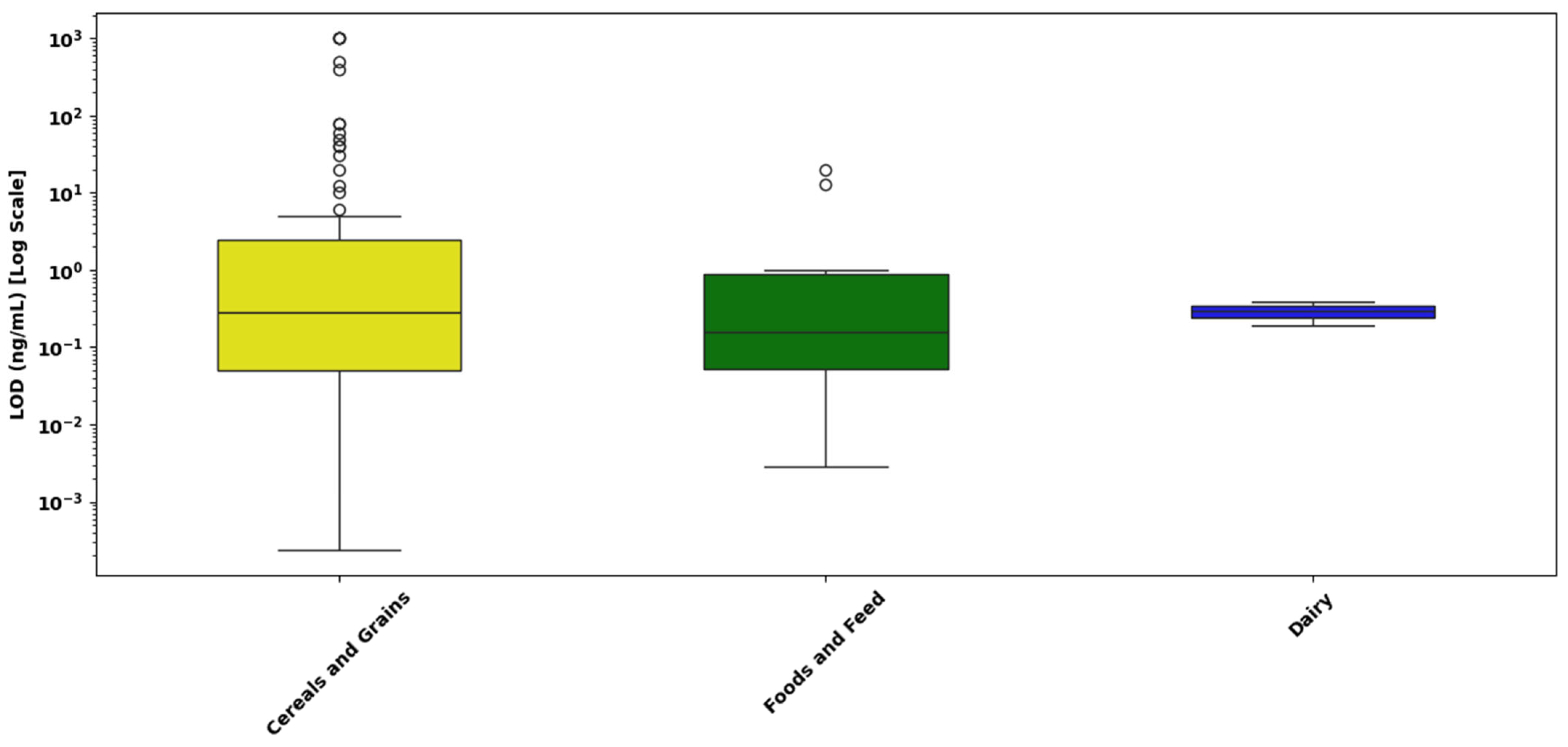
- Cereals and Grains: The LOD values for cereals and grains demonstrate substantial variability, spanning from approximately 10−4 ng/mL to above 103 ng/mL. The median is located near 10−1 ng/mL, with numerous outliers observed at higher concentrations (above 101 ng/mL). These outliers suggest notable variability due to potential differences in extraction methods, assay performance, and complex grain matrices.
- Foods and Feed: The LOD values in foods and feed range from roughly 10−3 ng/mL up to just above 1 ng/mL. The median value is close to 10−1 ng/mL, indicative of relatively consistent detection performance across these matrices. Few outliers at the higher concentration end highlight moderate variability, likely influenced by assay or matrix-specific differences.
- Dairy Products: LOD values for dairy products extend from approximately 10−3 ng/mL to around 1 ng/mL, with the median concentration positioned slightly below 10−1 ng/mL. The presence of isolated outliers at higher values suggests moderate assay variability, potentially influenced by dairy-specific interferences or methodological variations.
- Beverages and Juices: This category demonstrates the narrowest range, with LOD values closely clustered around 1 ng/mL. The median is nearly identical to this value, suggesting minimal variability and high consistency in analytical sensitivity across beverage and juice matrices.
- AFB1 demonstrated excellent assay sensitivity, with LOD values ranging from approximately 10−4 to 101 ng/mL, and a median centered around 10−1 ng/mL. The narrow IQR and the consistent clustering of values indicate robust and reproducible detection performance for this high-priority mycotoxin.
- In the case of FB1, LOD values spanned a broader range, from approximately 10−2 to over 102 ng/mL, with a median value near 100 ng/mL. The presence of several high-value outliers suggests increased variability in assay performance, potentially due to matrix effects or structural differences in the analyte affecting antibody binding efficiency.
- DON exhibited the widest variability in LODs among all the analytes, with values extending from 10−1 to nearly 103 ng/mL, and a median around 101 ng/mL. This substantial spread and elevated central tendency highlight challenges in achieving consistent sensitivity for DON using multiplex platforms, likely attributable to its hydrophilic nature and weaker immunogenic profile.
- OTA showed a highly favorable detection profile, with LODs ranging from 10−4 to 100 ng/mL and a median near 10−1 ng/mL. The narrow IQR and absence of high outliers reflect high reproducibility and minimal interference across matrices, underscoring the assay’s capacity for reliable OTA detection.
- ZEN demonstrated a moderate spread in LODs, ranging from 10−2 to 101 ng/mL, with a median near 10−1 ng/mL. While a few outliers were observed, the majority of values clustered within a consistent range, indicating satisfactory sensitivity and reliability.
- AFM1, a critical biomarker for dairy safety, exhibited the lowest LOD values, ranging from 10−3 to just below 100 ng/mL, and a median around 10−2 ng/mL. The compact distribution and lack of extreme outliers confirm high assay sensitivity and reproducibility for this analyte in milk-based matrices.
- T-2 toxin displayed a moderate range of LOD values between 10−2 and 102 ng/mL, with the median around 100 ng/mL. While the interquartile range suggests acceptable consistency, the presence of broader values indicates potential assay limitations in certain sample types or concentrations.
- Cereal and grain matrices exhibited the widest range of LOD values, with values spanning from 10−4 ng/mL to over 101 ng/mL, and a median near 10−1 ng/mL. A large number of high-value outliers were observed, some extending close to 103 ng/mL, indicating substantial variability in assay sensitivity.
- Foods and feed samples showed a narrower LOD distribution, ranging from approximately 10−3 to 100 ng/mL, with a median around 5 × 10−2 ng/mL. Although a few outliers were observed, the tighter interquartile range suggests relatively consistent assay performance across different food and feed types.
- Dairy matrices demonstrated the highest sensitivity and reproducibility, with LODs tightly clustered between 10−2 and 10−1 ng/mL, and a median around 3 × 10−2 ng/mL. No outliers were detected, and the narrow IQR reflects uniform detection performance. This may be attributed to the liquid nature of dairy samples and effective pre-treatment strategies such as centrifugation or protein precipitation, which reduce matrix interference.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khan, R.; Anwar, F.; Ghazali, F.M. A Comprehensive Review of Mycotoxins: Toxicology, Detection, and Effective Mitigation Approaches. Heliyon 2024, 10, e28361. [Google Scholar] [CrossRef] [PubMed]
- Alassane-Kpembi, I.; Schatzmayr, G.; Taranu, I.; Marin, D.; Puel, O.; Oswald, I.P. Mycotoxins Co-Contamination: Methodological Aspects and Biological Relevance of Combined Toxicity Studies. Crit. Rev. Food Sci. Nutr. 2017, 57, 3489–3507. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.A.; Wang, Y.; Shen, Z.; Li, X.; Saleemi, M.K.; He, C. Mycotoxin Contamination and Control Strategy in Human, Domestic Animal and Poultry: A Review. Microb. Pathog. 2020, 142, 104095. [Google Scholar] [CrossRef] [PubMed]
- Awuchi, C.G.; Ondari, E.N.; Nwozo, S.; Odongo, G.A.; Eseoghene, I.J.; Twinomuhwezi, H.; Ogbonna, C.U.; Upadhyay, A.K.; Adeleye, A.O.; Okpala, C.O.R. Mycotoxins’ Toxicological Mechanisms Involving Humans, Livestock and Their Associated Health Concerns: A Review. Toxins 2022, 14, 167. [Google Scholar] [CrossRef]
- Karlovsky, P.; Suman, M.; Berthiller, F.; De Meester, J.; Eisenbrand, G.; Perrin, I.; Oswald, I.P.; Speijers, G.; Chiodini, A.; Recker, T.; et al. Impact of food processing and detoxification treatments on mycotoxin contamination. Mycotoxin Res. 2016, 32, 179–205. [Google Scholar] [CrossRef]
- Pitt, J.I. Toxigenic fungi and mycotoxins. Br. Med. Bull. 2000, 56, 184–192. [Google Scholar] [CrossRef]
- Pickova, D.; Ostry, V.; Toman, J.; Malir, F. Aflatoxins: History, significant milestones, recent data on their toxicity and ways to mitigation. Toxins 2021, 13, 399. [Google Scholar] [CrossRef]
- Selvaraj, J.N.; Wang, Y.; Zhou, L.; Zhao, Y.; Xing, F.; Dai, X.; Liu, Y. Recent mycotoxin survey data and advanced mycotoxin detection techniques reported from China: A review. Food Addit. Contam. Part A 2015, 32, 440–452. [Google Scholar] [CrossRef]
- Smith, M.C.; Madec, S.; Coton, E.; Hymery, N. Natural co-occurrence of mycotoxins in foods and feeds and their in vitro combined toxicological effects. Toxins 2016, 8, 94. [Google Scholar] [CrossRef]
- Ojuri, O.T.; Ezekiel, C.N.; Sulyok, M.; Ezeokoli, O.T.; Oyedele, O.A.; Ayeni, K.I.; Eskola, M.K.; Šarkanj, B.; Hajšlová, J.; Adeleke, R.A.; et al. Assessing the mycotoxicological risk from consumption of complementary foods by infants and young children in Nigeria. Food Chem. Toxicol. 2018, 121, 37–50. [Google Scholar] [CrossRef]
- Shephard, G.S. Impact of mycotoxins on human health in developing countries. Food Addit. Contam. 2008, 25, 146–151. [Google Scholar] [CrossRef]
- Wild, C.P.; Gong, Y.Y. Mycotoxins and human disease: A largely ignored global health issue. Carcinogenesis 2010, 31, 71–82. [Google Scholar] [CrossRef]
- da Rocha, M.E.B.; Freire, F.D.C.O.; Maia, F.E.F.; Guedes, M.I.F.; Rondina, D. Mycotoxins and their effects on human and animal health. Food Control 2014, 36, 159–165. [Google Scholar] [CrossRef]
- El-Sayed, R.A.; Jebur, A.B.; Kang, W.; El-Demerdash, F.M. An overview on the major mycotoxins in food products: Characteristics, toxicity, and analysis. J. Future Foods 2022, 2, 91–102. [Google Scholar] [CrossRef]
- Martinez-Miranda, M.M.; Rosero-Moreano, M.; Taborda-Ocampo, G. Occurrence, dietary exposure, and risk assessment of aflatoxins in arepa, bread, and rice. Food Control 2019, 98, 359–366. [Google Scholar] [CrossRef]
- Oluwafemi, F.; Badmos, A.O.; Kareem, S.O.; Ademuyiwa, O.; Kolapo, A.L. Survey of aflatoxin M1 in cows’ milk from free-grazing cows in Abeokuta, Nigeria. Mycotoxin Res. 2014, 30, 207–211. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Chemical agents and related occupations. IARC Monogr. Eval. Carcinog. Risks Hum. 2012, 100, 94. [Google Scholar]
- Azziz-Baumgartner, E.; Lindblade, K.; Gieseker, K.; Rogers, H.S.; Kieszak, S.; Njapau, H.; Schleicher, R.; McCoy, L.F.; Misore, A.; DeCock, K.; et al. Case–control study of an acute aflatoxicosis outbreak, Kenya, 2004. Environ. Health Perspect. 2005, 113, 1779–1783. [Google Scholar] [CrossRef]
- Probst, C.; Njapau, H.; Cotty, P.J. Outbreak of an acute aflatoxicosis in Kenya in 2004: Identification of the causal agent. Appl. Environ. Microbiol. 2007, 73, 2762–2764. [Google Scholar] [CrossRef]
- Hamid, A.S.; Tesfamariam, I.G.; Zhang, Y.; Zhang, Z.G. Aflatoxin B1-Induced Hepatocellular Carcinoma in Developing Countries: Geographical Distribution, Mechanism of Action, and Prevention. Oncol. Lett. 2013, 5, 1087–1092. [Google Scholar] [CrossRef]
- Ben Miri, Y.; Benabdallah, A.; Chentir, I.; Djenane, D.; Luvisi, A.; De Bellis, L. Comprehensive Insights into Ochratoxin A: Occurrence, Analysis, and Control Strategies. Foods 2024, 13, 1184. [Google Scholar] [CrossRef] [PubMed]
- Reddy, L.; Bhoola, K. Ochratoxins—Food Contaminants: Impact on Human Health. Toxins 2010, 2, 771–779. [Google Scholar] [CrossRef]
- Foroud, N.A.; Baines, D.; Gagkaeva, T.Y.; Thakor, N.; Badea, A.; Steiner, B.; Bürstmayr, M.; Bürstmayr, H. Trichothecenes in Cereal Grains—An Update. Toxins 2019, 11, 634. [Google Scholar] [CrossRef]
- Krska, R.; Malachova, A.; Berthiller, F.; Egmond, H.P.V. Determination of T-2 and HT-2 Toxins in Food and Feed: An Update. World Mycotoxin J. 2014, 18, 131–142. [Google Scholar] [CrossRef]
- Polak-Śliwińska, M.; Paszczyk, B. Trichothecenes in Food and Feed, Relevance to Human and Animal Health and Methods of Detection: A Systematic Review. Molecules 2021, 26, 454. [Google Scholar] [CrossRef]
- Mahato, D.K.; Devi, S.; Pandhi, S.; Sharma, B.; Maurya, K.K.; Mishra, S.; Dhawan, K.; Selvakumar, R.; Kamle, M.; Mishra, A.K.; et al. Occurrence, Impact on Agriculture, Human Health, and Management Strategies of Zearalenone in Food and Feed: A Review. Toxins 2021, 13, 92. [Google Scholar] [CrossRef] [PubMed]
- Thenuwara, G.; Javed, B.; Singh, B.; Byrne, H.J.; Tian, F. Sex-and Gender-Specific Considerations in Mycotoxin Screening: Assessing Differential Exposure, Health Impacts, and Mitigation Strategies. Microbiol. Res. 2024, 15, 2455–2492. [Google Scholar] [CrossRef]
- Ahmed Adam, M.A.; Tabana, Y.M.; Musa, K.B.; Sandai, D.A. Effects of Different Mycotoxins on Humans, Cell Genome, and Their Involvement in Cancer. Oncol. Rep. 2017, 37, 1321–1336. [Google Scholar] [CrossRef]
- Eskola, M.; Kos, G.; Elliott, C.T.; Hajšlová, J.; Mayar, S.; Krska, R. Worldwide Contamination of Food-Crops with Mycotoxins: Validity of the Widely Cited ‘FAO Estimate’ of 25%. Crit. Rev. Food Sci. Nutr. 2020, 60, 2773–2789. [Google Scholar] [CrossRef]
- Anfossi, L.; Giovannoli, C.; Baggiani, C. Mycotoxin Detection. Curr. Opin. Biotechnol. 2016, 37, 120–126. [Google Scholar] [CrossRef]
- European Union. Commission Regulation (EU) 2023/915 of 29 May 2023 Amending Regulation (EC) No 1881/2006 as Regards Maximum Levels of Mycotoxins in Foodstuffs. Available online: https://eur-lex.europa.eu/eli/reg/2023/915/oj (accessed on 25 January 2025).
- U.S. Food and Drug Administration. Mycotoxins in Food. U.S. Food and Drug Administration. Available online: https://www.fda.gov/food/natural-toxins-food/mycotoxins (accessed on 25 January 2025).
- Codex Alimentarius Commission. Contaminants, FAO/WHO. 2024. Available online: https://www.fao.org/fao-who-codexalimentarius/thematic-areas/contaminants/en (accessed on 25 January 2025).
- Hepsag, F.; Golge, O.; Kabak, B. Quantitation of Aflatoxins in Pistachios and Groundnuts Using HPLC-FLD Method. Food Control 2014, 38, 75–81. [Google Scholar] [CrossRef]
- Mao, J.F.; Lei, S.; Liu, Y.H.; Xiao, D.; Fu, C.P.; Zhong, L.; Ouyang, H. Quantification of Aflatoxin M1 in Raw Milk by a Core-Shell Column on a Conventional HPLC with Large Volume Injection and Step Gradient Elution. Food Control 2015, 51, 156–162. [Google Scholar] [CrossRef]
- Arranz, I.; Baeyens, W.R.G.; Weken, G.; Saeger, S.D.; Peteghem, C.V. HPLC Determination of Fumonisin Mycotoxins. Crit. Rev. Food Sci. Nutr. 2004, 44, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Olsson, J.; Borjesson, T.; Lundstedt, T.; Schnurer, J. Detection and Quantification of Ochratoxin A and Deoxynivalenol in Barley Grains by GC-MS and Electronic Nose. Int. J. Food Microbiol. 2002, 72, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Scott, P.M. Gas Chromatography of Mycotoxins. J. Chromatogr. Libr. 1993, 54, 373–425. [Google Scholar] [CrossRef]
- Tanaka, T.; Yoneda, A.; Inoue, S.; Sugiura, Y.; Ueno, Y. Simultaneous Determination of Trichothecene Mycotoxins and Zearalenone in Cereals by Gas Chromatography–Mass Spectrometry. J. Chromatogr. A 2000, 882, 23–28. [Google Scholar] [CrossRef]
- Omar, S.S.; Haddad, M.A.; Parisi, S. Validation of HPLC and Enzyme-Linked Immunosorbent Assay (ELISA) Techniques for Detection and Quantification of Aflatoxins in Different Food Samples. Foods 2020, 9, 661. [Google Scholar] [CrossRef]
- Liang, Y.F.; Zhou, X.W.; Wang, F.; Shen, Y.D.; Xiao, Z.L.; Zhang, S.W.; Li, Y.J.; Wang, H. Development of a Monoclonal Antibody-Based ELISA for the Detection of Alternaria Mycotoxin Tenuazonic Acid in Food Samples. Food Anal. Methods 2020, 13, 1594–1602. [Google Scholar] [CrossRef]
- Songsermsakul, P.; Razzazi-Fazeli, E. A Review of Recent Trends in Applications of Liquid Chromatography-Mass Spectrometry for Determination of Mycotoxins. J. Liq. Chromatogr. Relat. Technol. 2008, 31, 1641–1686. [Google Scholar] [CrossRef]
- Rundberget, T.; Wilkins, A.L. Determination of Penicillium Mycotoxins in Foods and Feeds Using Liquid Chromatography–Mass Spectrometry. J. Chromatogr. A 2002, 964, 189–197. [Google Scholar] [CrossRef]
- Welke, J.E.; Hoeltz, M.; Dottori, H.A.; Noll, I.B. Quantitative Analysis of Patulin in Apple Juice by Thin-Layer Chromatography Using a Charge Coupled Device Detector. Food Addit. Contam. Part A 2009, 26, 754–758. [Google Scholar] [CrossRef] [PubMed]
- Rahman, H.U.; Yue, X.; Yu, Q.; Zhang, W.; Zhang, Q.; Li, P. Current PCR-Based Methods for the Detection of Mycotoxigenic Fungi in Complex Food and Feed Matrices. World Mycotoxin J. 2020, 13, 139–150. [Google Scholar] [CrossRef]
- Agriopoulou, S.; Stamatelopoulou, E.; Varzakas, T. Advances in Analysis and Detection of Major Mycotoxins in Foods. Foods 2020, 9, 518. [Google Scholar] [CrossRef]
- Al-Zaban, M.I.; Alrokban, A.H.; Mahmoud, M.A. Development of a Real-Time PCR and Multiplex PCR Assay for the Detection and Identification of Mycotoxigenic Fungi in Stored Maize Grains. Mycology 2023, 14, 227–238. [Google Scholar] [CrossRef]
- Szelenberger, R.; Cichoń, N.; Zajaczkowski, W.; Bijak, M. Application of Biosensors for the Detection of Mycotoxins for the Improvement of Food Safety. Toxins 2024, 16, 249. [Google Scholar] [CrossRef]
- Shrivastava, A.; Sharma, R.K. Biosensors for the Detection of Mycotoxins. Toxin Rev. 2022, 41, 618–638. [Google Scholar] [CrossRef]
- Li, R.; Wen, Y.; Wang, F.; He, P. Recent Advances in Immunoassays and Biosensors for Mycotoxins Detection in Feedstuffs and Foods. J. Anim. Sci. Biotechnol. 2021, 12, 108. [Google Scholar] [CrossRef] [PubMed]
- Dong, M. The Essence of Modern HPLC: Advantages, Limitations, Fundamentals, and Opportunities. J. Chromatogr. Sci. 2013, 51, 212–223. [Google Scholar]
- Hosseini, S.; Vázquez-Villegas, P.; Rito-Palomares, M.; Martinez-Chapa, S.O.; Hosseini, S.; Vázquez-Villegas, P.; Rito-Palomares, M.; Martinez-Chapa, S.O. Advantages, Disadvantages and Modifications of Conventional ELISA. In Enzyme-Linked Immunosorbent Assay (ELISA) from A to Z; Springer: Singapore, 2018; pp. 67–115. [Google Scholar] [CrossRef]
- Man, Y.; Liang, G.; Li, A.; Pan, L. Recent advances in mycotoxin determination for food monitoring via microchip. Toxins 2017, 9, 324. [Google Scholar] [CrossRef]
- Lakavath, K.; Kafley, C.; Sajeevan, A.; Jana, S.; Marty, J.L.; Kotagiri, Y.G. Progress on Electrochemical Biomimetic Nanosensors for the Detection and Monitoring of Mycotoxins and Pesticides. Toxins 2024, 16, 244. [Google Scholar] [CrossRef]
- Guo, L.; Feng, J.; Fang, Z.; Xu, J.; Lu, X. Application of microfluidic “lab-on-a-chip” for the detection of mycotoxins in foods. Trends Food Sci. Technol. 2015, 46, 252–263. [Google Scholar] [CrossRef]
- Thipe, V.C.; de Oliveira Asenjo Mendes, G.; Alves, V.M.; Souza, T.; Ajayi, R.F.; Lugao, A.B.; Katti, K.V. Nanodiagnostic tools for mycotoxins detection. In Nanorobotics and Nanodiagnostics in Integrative Biology and Biomedicine; Springer International Publishing: Cham, Switzerland, 2022; pp. 361–381. [Google Scholar] [CrossRef]
- Hua, M.Z.; Li, S.; Roopesh, M.S.; Lu, X. Development of a microfluidic device to enrich and detect zearalenone in food using quantum dot-embedded molecularly imprinted polymers. Lab Chip 2024, 24, 2700–2711. [Google Scholar] [CrossRef]
- Cooper, J.M. Challenges in lab-on-a-chip technology. Front. Lab Chip Technol. 2022, 1, 979398. [Google Scholar] [CrossRef]
- Cardoso, S.; Leitao, D.C.; Dias, T.M.; Valadeiro, J.; Silva, M.D.; Chicharo, A.; Silverio, V.; Gaspar, J.; Freitas, P.P. Challenges and trends in magnetic sensor integration with microfluidics for biomedical applications. J. Phys. 2017, 50, 213001. [Google Scholar] [CrossRef]
- Koczula, K.M.; Gallotta, A. Lateral flow assays. Essays Biochem. 2016, 60, 111–120. [Google Scholar] [CrossRef]
- Peng, T.; Liu, X.; Adams, L.G.; Agarwal, G.; Akey, B.; Cirillo, J.; Deckert, V.; Delfan, S.; Fry, E.; Han, Z.; et al. Enhancing sensitivity of lateral flow assay with application to SARS-CoV-2. Appl. Phys. Lett. 2020, 117, 120601. [Google Scholar] [CrossRef]
- Sohrabi, H.; Majidi, M.R.; Khaki, P.; Jahanban-Esfahlan, A.; de la Guardia, M.; Mokhtarzadeh, A. State of the art: Lateral flow assays toward the point-of-care foodborne pathogenic bacteria detection in food samples. Compr. Rev. Food Sci. Food Saf. 2022, 21, 1868–1912. [Google Scholar] [CrossRef] [PubMed]
- Sajid, M.; Kawde, A.N.; Daud, M. Designs, formats and applications of lateral flow assay: A literature review. J. Saudi Chem. Soc. 2015, 19, 689–705. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Song, S.; Park, S.; Joo, C. Recent advances in high-sensitivity detection methods for paper-based lateral-flow assay. Biosens. Bioelectron. 2020, 152, 112015. [Google Scholar] [CrossRef]
- Ge, X.; Asiri, A.M.; Du, D.; Wen, W.; Wang, S.; Lin, Y. Nanomaterial-enhanced paper-based biosensors. TrAC Trends Anal. Chem. 2014, 58, 31–39. [Google Scholar] [CrossRef]
- Quesada-González, D.; Merkoçi, A. Nanomaterial-based devices for point-of-care diagnostic applications. Chem. Soc. Rev. 2018, 47, 4697–4709. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Wang, K.; Lu, W.; Qin, W.; Cui, D.; He, J. CdSe/ZnS quantum dot-labeled lateral flow strips for rapid and quantitative detection of gastric cancer carbohydrate antigen 72-4. Nanoscale Res. Lett. 2016, 11, 138. [Google Scholar] [CrossRef] [PubMed]
- Bruno, J. Evaluation of pathogenic big 7 E. coli aptamer-quantum dot lateral flow test strips. J. Bionanoscience 2017, 11, 148–152. [Google Scholar] [CrossRef]
- Wang, J.; Meng, H.-M.; Chen, J.; Liu, J.; Zhang, L.; Qu, L.; Li, Z.; Lin, Y. Quantum dot-based lateral flow test strips for highly sensitive detection of the tetanus antibody. ACS Omega 2019, 4, 6789–6795. [Google Scholar] [CrossRef]
- Medintz, I.L.; Uyeda, H.T.; Goldman, E.R.; Mattoussi, H. Quantum dot bioconjugates for imaging, labelling and sensing. Nat. Mater. 2005, 4, 435–446. [Google Scholar] [CrossRef]
- Kim, J.; Kwon, J.H.; Jang, J.; Lee, H.; Kim, S.; Hahn, Y.K.; Kim, S.K.; Lee, K.H.; Lee, S.; Pyo, H.; et al. Rapid and background-free detection of avian influenza virus in opaque sample using NIR-to-NIR upconversion nanoparticle-based lateral flow immunoassay platform. Biosens. Bioelectron. 2018, 112, 209–215. [Google Scholar] [CrossRef]
- Ha, Y.; Ko, S.; Kim, I.; Huang, Y.; Mohanty, K.; Huh, C.; Maynard, J.A. Recent advances incorporating superparamagnetic nanoparticles into immunoassays. ACS Appl. Nano Mater. 2018, 1, 512–521. [Google Scholar] [CrossRef]
- Gowri, A.; Ashwin Kumar, N.; Suresh Anand, B.S. Recent advances in nanomaterials based biosensors for point of care (PoC) diagnosis of COVID-19—A minireview. Trends Anal. Chem. 2021, 137, 116205. [Google Scholar] [CrossRef]
- Yin, H.-Y.; Li, Y.-T.; Tsai, W.-C.; Dai, H.-Y.; Wen, H.-W. An immunochromatographic assay utilizing magnetic nanoparticles to detect major peanut allergen Ara h 1 in processed foods. Food Chem. 2022, 375, 131844. [Google Scholar] [CrossRef]
- Noguera, P.; Posthuma-Trumpie, G.A.; van Tuil, M.; van der Wal, F.J.; de Boer, A.; Moers, A.P.H.A.; van Amerongen, A. Carbon nanoparticles in lateral flow methods to detect genes encoding virulence factors of Shiga toxin-producing Escherichia coli. Anal. Bioanal. Chem. 2011, 399, 831–838. [Google Scholar] [CrossRef]
- Amerongen, V.; Barug, D.; Lauwaars, M. Rapid Methods for Biological and Chemical Contaminants in Food and Feed; Springer: Berlin/Heidelberg, Germany, 2005; p. 416. [Google Scholar] [CrossRef]
- Qiu, W.; Xu, H.; Takalkar, S.; Gurung, A.S.; Liu, B.; Zheng, Y.; Guo, Z.; Baloda, M.; Baryeh, K.; Liu, G. Carbon nanotube-based lateral flow biosensor for sensitive and rapid detection of DNA sequence. Biosens. Bioelectron. 2015, 64, 367–372. [Google Scholar] [CrossRef]
- Sánchez-Pomales, G.; Santiago-Rodríguez, L.; Cabrera, C.R. DNA-functionalized carbon nanotubes for biosensing applications. J. Nanosci. Nanotechnol. 2009, 9, 2175–2188. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhang, S.; Zhang, X.; Baloda, M.; Gurung, A.S.; Xu, H.; Zhang, X.; Liu, G. Ultrasensitive nucleic acid biosensor based on enzyme-gold nanoparticle dual label and lateral flow strip biosensor. Biosens. Bioelectron. 2011, 26, 2018–2024. [Google Scholar] [CrossRef]
- Parolo, C.; de la Escosura-Muñiz, A.; Merkoci, A. Enhanced lateral flow immunoassay using gold nanoparticles loaded with enzymes. Biosens. Bioelectron. 2013, 40, 412–416. [Google Scholar] [CrossRef]
- Jiang, D.; Ni, D.; Rosenkrans, Z.T.; Huang, P.; Yan, X.; Cai, W. Nanozyme: New horizons for responsive biomedical applications. Chem. Soc. Rev. 2019, 48, 3683–3704. [Google Scholar] [CrossRef] [PubMed]
- Loynachan, C.N.; Thomas, M.R.; Gray, E.R.; Richards, D.A.; Kim, J.; Miller, B.S.; Brookes, J.C.; Agarwal, S.; Chudasama, V.; McKendry, R.A.; et al. Platinum nanocatalyst amplification: Redefining the gold standard for lateral flow immunoassays with ultrabroad dynamic range. ACS Nano 2018, 12, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yu, Q.; Qiu, W.; Li, K.; Qian, L.; Zhang, X.; Liu, G. Gold-platinum nanoflowers as a label and as an enzyme mimic for use in highly sensitive lateral flow immunoassays: Application to detection of rabbit IgG. Microchim. Acta 2019, 186, 357. [Google Scholar] [CrossRef]
- Gao, Z.; Ye, H.; Tang, D.; Tao, J.; Habibi, S.; Minerick, A.; Tang, D.; Xia, X. Platinum-decorated gold nanoparticles with dual functionalities for ultrasensitive colorimetric in vitro diagnostics. Nano Lett. 2017, 17, 5572–5579. [Google Scholar] [CrossRef]
- Shen, Y.; Shen, G. Signal-Enhanced Lateral Flow Immunoassay with Dual Gold Nanoparticle Conjugates for the Detection of Hepatitis B Surface Antigen. ACS Omega 2019, 4, 5083–5087. [Google Scholar] [CrossRef]
- Ren, W.; Ballou, D.R.; FitzGerald, R.; Irudayaraj, J. Plasmonic enhancement in lateral flow sensors for improved sensing of E. coli O157:H7. Biosens. Bioelectron. 2019, 126, 324–331. [Google Scholar] [CrossRef]
- Ye, H.; Liu, Y.; Zhan, L.; Liu, Y.; Qin, Z. Signal Amplification and Quantification on Lateral Flow Assays by Laser Excitation of Plasmonic Nanomaterials. Theranostics 2020, 10, 4359–4373. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Zheng, Y.; Jin, B.; You, M.; Wang, J.; Li, X.J.; Lin, M.; Xu, F.; Li, F. A portable and universal upconversion nanoparticle-based lateral flow assay platform for point-of-care testing. Talanta 2019, 201, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Liu, Y.; Wu, Y.; Xia, X.; Liao, Y.; Li, Q. Fluorescent Probe-Based Lateral Flow Assay for Multiplex Nucleic Acid Detection. Anal. Chem. 2014, 86, 5611–5614. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Hwang, J.; Kim, K.; Jeon, J.; Lee, S.; Ko, J.; Lee, J.; Kang, M.; Chung, D.R.; Choo, J. Quantitative Serodiagnosis of Scrub Typhus Using Surface-Enhanced Raman Scattering-Based Lateral Flow Assay Platforms. Anal. Chem. 2019, 91, 12275–12282. [Google Scholar] [CrossRef]
- Tran, V.; Walkenfort, B.; König, M.; Salehi, M.; Schlücker, S. Rapid, Quantitative, and Ultrasensitive Point-of-Care Testing: A Portable SERS Reader for Lateral Flow Assays in Clinical Chemistry. Angew. Chem. Int. Ed. 2019, 58, 442–446. [Google Scholar] [CrossRef]
- Qin, Z.; Chan, W.C.W.; Boulware, D.R.; Akkin, T.; Butler, E.K.; Bischof, J.C. Significantly Improved Analytical Sensitivity of Lateral Flow Immunoassays by Using Thermal Contrast. Angew. Chem. Int. Ed. 2012, 51, 4358–4361. [Google Scholar] [CrossRef]
- Wang, Y.; Qin, Z.; Boulware, D.R.; Pritt, B.S.; Sloan, L.M.; Gonzalez, I.J.; Bell, D.; Rees-Channer, R.R.; Chiodini, P.; Chan, W.C.W.; et al. Thermal Contrast Amplification Reader Yielding 8-Fold Analytical Improvement for Disease Detection with Lateral Flow Assays. Anal. Chem. 2016, 88, 11774–11782. [Google Scholar] [CrossRef]
- Bhardwaj, J.; Sharma, A.; Jang, J. Vertical Flow-Based Paper Immunosensor for Rapid Electrochemical and Colorimetric Detection of Influenza Virus Using a Different Pore Size Sample Pad. Biosens. Bioelectron. 2019, 126, 36–43. [Google Scholar] [CrossRef]
- Marquina, C.; De Teresa, J.M.; Serrate, D.; Marzo, J.; Cardoso, F.A.; Saurel, D.; Cardoso, S.; Freitas, P.P.; Ibarra, M.R. GMR Sensors and Magnetic Nanoparticles for Immuno-Chromatographic Assays. J. Magn. Magn. Mater. 2012, 324, 3495–3498. [Google Scholar] [CrossRef]
- Lei, H.; Wang, K.; Ji, X.; Cui, D. Contactless Measurement of Magnetic Nanoparticles on Lateral Flow Strips Using Tunneling Magnetoresistance (TMR) Sensors in Differential Configuration. Sensors 2016, 16, 2130. [Google Scholar] [CrossRef]
- Park, J. Smartphone-Based Lateral Flow Immunoassay Quantifications. J. Immunol. Methods 2024, 533, 113745. [Google Scholar] [CrossRef] [PubMed]
- Zhan, L.; Guo, S.Z.; Song, F.; Gong, Y.; Xu, F.; Boulware, D.R.; McAlpine, M.C.; Chan, W.C.W.; Bischof, J.C. The Role of Nanoparticle Design in Determining Analytical Performance of Lateral Flow Immunoassays. Nano Lett. 2017, 17, 7207–7212. [Google Scholar] [CrossRef] [PubMed]
- Squires, T.M.; Messinger, R.J.; Manalis, S.R. Making It Stick: Convection, Reaction and Diffusion in Surface-Based Biosensors. Nat. Biotechnol. 2008, 26, 417–426. [Google Scholar] [CrossRef]
- Zhan, L.; Granade, T.; Liu, Y.; Wei, X.; Youngpairoj, A.; Sullivan, V.; Johnson, J.; Bischof, J. Development and Optimization of Thermal Contrast Amplification Lateral Flow Immunoassays for Ultrasensitive HIV P24 Protein Detection. Microsyst. Nanoeng. 2020, 6, 54. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.; Robinson, R.; Houghtaling, J.; Fridley, G.; Ramsey, S.A.; Fu, E. Investigation of Reagent Delivery Formats in a Multivalent Malaria Sandwich Immunoassay and Implications for Assay Performance. Anal. Chem. 2016, 88, 2311–2320. [Google Scholar] [CrossRef]
- Sharma, A.; Tok, A.I.Y.; Lee, C.; Ganapathy, R.; Alagappan, P.; Liedberg, B. Magnetic Field Assisted Preconcentration of Biomolecules for Lateral Flow Assaying. Sens. Actuators B 2019, 285, 431–437. [Google Scholar] [CrossRef]
- Mashayekhi, F.; Le, A.M.; Nafisi, P.M.; Wu, B.M.; Kamei, D.T. Enhancing the Lateral-Flow Immunoassay for Detection of Proteins Using an Aqueous Two-Phase Micellar System. Anal. Bioanal. Chem. 2012, 404, 2057–2066. [Google Scholar] [CrossRef]
- Lou, D.; Fan, L.; Ji, Y.; Gu, N.; Zhang, Y. A Signal Amplifying Fluorescent Nanoprobe and Lateral Flow Assay for Ultrasensitive Detection of Cardiac Biomarker Troponin I. Anal. Methods 2019, 11, 3506–3513. [Google Scholar] [CrossRef]
- Welch, N.G.; Scoble, J.A.; Muir, B.W.; Pigram, P.J. Orientation and Characterization of Immobilized Antibodies for Improved Immunoassays (Review). Biointerphases 2017, 12, 02D301. [Google Scholar] [CrossRef]
- Di Nardo, F.; Cavalera, S.; Baggiani, C.; Giovannoli, C.; Anfossi, L. Direct vs Mediated Coupling of Antibodies to Gold Nanoparticles: The Case of Salivary Cortisol Detection by Lateral Flow Immunoassay. ACS Appl. Mater. Interfaces 2019, 11, 32758–32768. [Google Scholar] [CrossRef]
- Trilling, A.K.; Beekwilder, J.; Zuilhof, H. Antibody Orientation on Biosensor Surfaces: A Minireview. Analyst 2013, 138, 1619–1627. [Google Scholar] [CrossRef] [PubMed]
- Saha, B.; Evers, T.H.; Prins, M.W.J. How Antibody Surface Coverage on Nanoparticles Determines the Activity and Kinetics of Antigen Capturing for Biosensing. Anal. Chem. 2014, 86, 8158–8166. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Seo, H.S.; Kwon, J.H.; Kim, H.T.; Kwon, K.C.; Sim, S.J.; Cha, Y.J.; Lee, J. Multiplex Diagnosis of Viral Infectious Diseases (AIDS, Hepatitis C, and Hepatitis A) Based on Point-of-Care Lateral Flow Assay Using Engineered Proteinticles. Biosens. Bioelectron. 2015, 69, 213–225. [Google Scholar] [CrossRef]
- Lin, L.K.; Uzunoglu, A.; Stanciu, L.A. Aminolated and Thiolated PEG-Covered Gold Nanoparticles with High Stability and Antiaggregation for Lateral Flow Detection of Bisphenol A. Small 2018, 14, 1702828. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Heller, A. Reduction of the Nonspecific Binding of a Target Antibody and of Its Enzyme-Labeled Detection Probe Enabling Electrochemical Immunoassay of an Antibody through the 7 Pg/ML–100 Ng/ML (40 FM–400 PM) Range. Anal. Chem. 2005, 77, 7758–7762. [Google Scholar] [CrossRef]
- Edwards, K.A.; Baeumner, A.J. Optimization of DNA-Tagged Dye-Encapsulating Liposomes for Lateral-Flow Assays Based on Sandwich Hybridization. Anal. Bioanal. Chem. 2006, 386, 1335–1343. [Google Scholar] [CrossRef]
- Choi, D.H.; Lee, S.K.; Oh, Y.K.; Bae, B.W.; Lee, S.D.; Kim, S.; Shin, Y.B.; Kim, M.G. A Dual Gold Nanoparticle Conjugate-Based Lateral Flow Assay (LFA) Method for the Analysis of Troponin I. Biosens. Bioelectron. 2010, 25, 1999–2002. [Google Scholar] [CrossRef]
- Teerinen, T.; Lappalainen, T.; Erho, T. A Paper-Based Lateral Flow Assay for Morphine. Anal. Bioanal. Chem. 2014, 406, 5955–5965. [Google Scholar] [CrossRef]
- Peri Ibáñez, E.S.; Mazzeo, A.; Silva, C.; Juncos, M.J.; Costa Navarro, G.S.; Pallarés, H.M.; Wolos, V.J.; Fiszman, G.L.; Mundo, S.L.; Caramelo, J.J.; et al. Overcoming Limited Access to Virus Infection Rapid Testing: Development of a Lateral Flow Test for SARS-CoV-2 with Locally Available Resources. Biosensors 2024, 14, 416. [Google Scholar] [CrossRef]
- Brooks, B.D.; Albertson, A.E.; Jones, J.A.; Speare, J.O.; Lewis, R.V. Efficient Screening of High-Signal and Low-Background Antibody Pairs in the Bio-Bar Code Assay Using Prion Protein as the Target. Anal. Biochem. 2008, 382, 60–62. [Google Scholar] [CrossRef]
- Wu, D.; Dumont Milutinovic, M.; Walt, D.R. Single Molecule Array (Simoa) Assay with Optimal Antibody Pairs for Cytokine Detection in Human Serum Samples. Analyst 2015, 140, 6277–6282. [Google Scholar] [CrossRef] [PubMed]
- Tombelli, S.; Minunni, M.; Mascini, M. Analytical Applications of Aptamers. Biosens. Bioelectron. 2005, 20, 2424–2434. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Yang, S. Replacing Antibodies with Aptamers in Lateral Flow Immunoassay. Biosens. Bioelectron. 2015, 71, 230–242. [Google Scholar] [CrossRef]
- Jayasena, S.D. Aptamers: An Emerging Class of Molecules That Rival Antibodies in Diagnostics. Clin. Chem. 1999, 45, 1628–1650. [Google Scholar] [CrossRef]
- Toh, S.Y.; Citartan, M.; Gopinath, S.C.B.; Tang, T.H. Aptamers as a Replacement for Antibodies in Enzyme-Linked Immunosorbent Assay. Biosens. Bioelectron. 2015, 64, 392–403. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.W.; Ren, M.L.; Li, Y.P.; Huang, Z.B.; Shu, M.; Yang, H.W.; Xiong, Y.H.; Xu, Y. Detection of Aflatoxin B1 with Immunochromatographic Test Strips: Enhanced Signal Sensitivity Using Gold Nanoflowers. Talanta 2015, 142, 206–212. [Google Scholar] [CrossRef]
- Sojinrin, T.; Liu, K.; Wang, K.; Cui, D.; Byrne, J.; Curtin, J.F.; Tian, F. Developing Gold Nanoparticles-Conjugated Aflatoxin B1 Antifungal Strips. Int. J. Mol. Sci. 2019, 20, 6260. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, W.; Zhang, Q.; Li, P.; Tang, X. Self-Assembly Multivalent Fluorescence-Nanobody Coupled Multifunctional Nanomaterial with Colorimetric, Fluorescence and Photothermal to Enhance Immunochromatographic Assay. ACS Nano 2023, 17, 19359–19371. [Google Scholar] [CrossRef]
- Tang, Y.; Ma, P.; Khan, I.M.; Zhang, Y.; Wang, Z. An Effective Strategy of Designing “Turn On” Mode-Based Lateral Flow Chromatography for Rapid Detection of Aflatoxin B1. Food Biosci. 2023, 56, 103104. [Google Scholar] [CrossRef]
- Zhu, X.; Tang, J.; Ouyang, X.; Liao, Y.; Feng, H.; Yu, J.; Chen, L.; Lu, Y.; Yi, Y.; Tang, L. A Versatile CuCo@PDA Nanozyme-Based Aptamer-Mediated Lateral Flow Assay for Highly Sensitive, On-Site and Dual-Readout Detection of Aflatoxin B1. J. Hazard. Mater. 2024, 465, 133178. [Google Scholar] [CrossRef]
- Wang, Z.; Luo, P.; Zheng, B. A Rapid and Sensitive Fluorescent Microsphere-Based Lateral Flow Immunoassay for Determination of Aflatoxin B1 in Distillers’ Grains. Foods 2021, 10, 2109. [Google Scholar] [CrossRef]
- Yan, X.; Persaud, K.C. The Optimization of a Lateral Flow Immunoassay for Detection of Aflatoxin B 1 in Potable Water Samples. IEEE Sens. J. 2018, 19, 404–412. [Google Scholar] [CrossRef]
- Rahi, S.; Lanjekar, V.; Ghormade, V. Rationally Designed Peptide Conjugated to Gold Nanoparticles for Detection of Aflatoxin B1 in Point-of-Care Dot-Blot Assay. Food Chem. 2023, 413, 135651. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhang, Z.; Li, P.; Zhang, Q.; Zhang, W. Time-Resolved Fluorescent Immunochromatography of Aflatoxin B1 in Soybean Sauce: A Rapid and Sensitive Quantitative Analysis. Sensors 2016, 16, 1094. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, S.; Zhang, Z.; He, T.; Li, P.; Zhang, Q.; Chen, X.; Wang, D.; Li, H.; Tang, X.; Zhang, W. An On-Site, Ultra-Sensitive, Quantitative Sensing Method for the Determination of Total Aflatoxin in Peanut and Rice Based on Quantum Dot Nanobeads Strip. Toxins 2017, 9, 137. [Google Scholar] [CrossRef]
- Wu, W.; Li, Y.; Xu, Q.; Long, N.; Song, P.; Wang, J.; Zhou, L.; Fu, B.; Kong, W. Polydopamine-Coated HKUST MOFs-Based Strip Lateral Flow Immunoassay for On-Site Ultrasensitive Detection of Aflatoxin B1 in Foods. Food Control 2023, 152, 109864. [Google Scholar] [CrossRef]
- Wu, W.; Li, Y.; Song, P.; Xu, Q.; Lei, D.; Wang, J.; Fu, B.; Kong, W. UiOL@AIEgens-Assisted Lateral Flow Immunosensor for the Ultrasensitive Dual-Modal Point-of-Care Detection of Aflatoxin B1. J. Hazard. Mater. 2024, 465, 133103. [Google Scholar] [CrossRef]
- Santos, V.O.; Pelegrini, P.B.; Mulinari, F.; Lacerda, A.F.; Moura, R.S.; Cardoso, L.P.; Bührer-Sékula, S.; Miller, R.N.; Grossi-de-Sa, M.F. Development and Validation of a Novel Lateral Flow Immunoassay Device for Detection of Aflatoxins in Soy-Based Foods. Anal. Methods 2017, 9, 2715–2722. [Google Scholar] [CrossRef]
- Liu, D.; Huang, Y.; Wang, S.; Liu, K.; Chen, M.; Xiong, Y.; Yang, W.; Lai, W. A Modified Lateral Flow Immunoassay for the Detection of Trace Aflatoxin M1 Based on Immunomagnetic Nanobeads with Different Antibody Concentrations. Food Control 2015, 51, 218–224. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, C.; Yuan, J.; Tong, L.; Wang, Y.; Zhuo, D.; Huang, L.; Ni, W.; Zhang, J.; Huang, M.; et al. Hue Recognition Competitive Fluorescent Lateral Flow Immunoassay for Aflatoxin M1 Detection with Improved Visual and Quantitative Performance. Anal. Chem. 2022, 94, 10865–10873. [Google Scholar] [CrossRef]
- Anfossi, L.; Di Nardo, F.; Cavalera, S.; Giovannoli, C.; Spano, G.; Speranskaya, E.S.; Goryacheva, I.Y.; Baggiani, C. A Lateral Flow Immunoassay for Straightforward Determination of Fumonisin Mycotoxins Based on the Quenching of the Fluorescence of CdSe/ZnS Quantum Dots by Gold and Silver Nanoparticles. Microchim. Acta 2018, 185, 94. [Google Scholar] [CrossRef]
- Ling, S.; Wang, R.; Gu, X.; Wen, C.; Chen, L.; Chen, Z.; Chen, Q.A.; Xiao, S.; Yang, Y.; Zhuang, Z.; et al. Rapid Detection of Fumonisin B1 Using a Colloidal Gold Immunoassay Strip Test in Corn Samples. Toxicon 2015, 108, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Huang, Z.; Xu, Y.; Li, Y.; Ji, Y.; Su, B. Urchin-Like Gold Nanoparticle-Based Immunochromatographic Strip Test for Rapid Detection of Fumonisin B1 in Grains. Anal. Bioanal. Chem. 2015, 407, 7341–7348. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Wang, Z.; Xu, X.; Xu, L.; Kuang, H.; Xiao, J.; Xu, C. Europium Nanosphere-Based Fluorescence Strip Sensor for Ultrasensitive and Quantitative Determination of Fumonisin B1. Anal. Methods 2020, 12, 5229–5235. [Google Scholar] [CrossRef]
- Tran, T.V.; Do, B.N.; Nguyen, T.P.T.; Tran, T.T.; Tran, S.C.; Van Nguyen, B.; Van Nguyen, C.; Le, H.Q. Development of an IgY-Based Lateral Flow Immunoassay for Detection of Fumonisin B in Maize. F1000Research 2019, 8, 1042. [Google Scholar] [CrossRef]
- Qiao, W.; He, B.; Yang, J.; Ren, W.; Zhao, R.; Zhang, Y.; Bai, C.; Suo, Z.; Xu, Y.; Wei, M.; et al. Pt@AuNF Nanozyme and Horseradish Peroxidase-Based Lateral Flow Immunoassay Dual Enzymes Signal Amplification Strategy for Sensitive Detection of Zearalenone. Int. J. Biol. Macromol. 2024, 254, 127746. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Z.; Qu, X.; Zhou, J.; Yang, H.; Wang, W.; Yang, C. A Photothermal Lateral Flow Immunoassay for Zearalenone with High Sensitivity and Wide Detection Range. Sens. Actuators B Chem. 2023, 390, 133909. [Google Scholar] [CrossRef]
- Hua, Q.; Liu, Z.; Wang, J.; Liang, Z.; Zhou, Z.; Shen, X.; Lei, H.; Li, X. Magnetic Immunochromatographic Assay with Smartphone-Based Readout Device for the On-Site Detection of Zearalenone in Cereals. Food Control 2022, 134, 108760. [Google Scholar] [CrossRef]
- Ji, F.; Mokoena, M.P.; Zhao, H.; Olaniran, A.O.; Shi, J. Development of an Immunochromatographic Strip Test for the Rapid Detection of Zearalenone in Wheat from Jiangsu Province, China. PLoS ONE 2017, 12, e0175282. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, H.; Shi, H.; Zhu, J.; Wang, H. An Improved Up-Conversion Nanoparticles-Based Immunochromatographic Assay for Rapid Detection of Zearalenone in Cereals. Food Chem. 2023, 412, 135555. [Google Scholar] [CrossRef]
- Liu, Z.; Hua, Q.; Wang, J.; Liang, Z.; Zhou, Z.; Shen, X.; Lei, H.; Li, X. Prussian Blue Immunochromatography with Portable Smartphone-Based Detection Device for Zearalenone in Cereals. Food Chem. 2022, 369, 131008. [Google Scholar] [CrossRef]
- Wu, S.; Liu, L.; Duan, N.; Li, Q.; Zhou, Y.; Wang, Z. Aptamer-based lateral flow test strip for rapid detection of zearalenone in corn samples. J. Agric. Food Chem. 2018, 66, 1949–1954. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Fu, Q.; Xie, J.; Wang, H.; Tang, Y. Development of a High Sensitivity Quantum Dot-Based Fluorescent Quenching Lateral Flow Assay for the Detection of Zearalenone. Anal. Bioanal. Chem. 2019, 411, 2169–2175. [Google Scholar] [CrossRef]
- Chen, X.; Gao, Z.; Long, T.; Xie, J.; Li, X.; Huang, Z. Development of Two Immunochromatographic Test Strips Based on Signal Amplification and Selenium Nanoparticles for the Rapid Detection of T-2 Mycotoxin. Food Chem. 2023, 424, 136419. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, C.; Wen, K.; Jiang, H.; Shen, J.; Zhang, S.; Wang, Z. Comparison of Fluorescent Microspheres and Colloidal Gold as Labels in Lateral Flow Immunochromatographic Assays for the Detection of T-2 Toxin. Molecules 2016, 21, 27. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, D.; Li, J.; Zhang, Q.; Li, P. Monoclonal Antibody–Europium Conjugate-Based Lateral Flow Time-Resolved Fluoroimmunoassay for Quantitative Determination of T-2 Toxin in Cereals and Feed. Anal. Methods 2015, 7, 2822–2829. [Google Scholar] [CrossRef]
- Sun, B.; Wu, H.; Jia, P.; Cao, Y.; Xuan, C.; Feng, Q.; Yan, H.; Wang, L. Dual-Modal Lateral Flow Immunoassay Based on Cauliflower-Like ReS2@Pt Core-Shell Nanospheres Mediated Ultra-Sensitive Detection of Deoxynivalenol in Food Samples. Chem. Eng. J. 2024, 497, 155533. [Google Scholar] [CrossRef]
- Shen, Y.; Zhao, X.; Zhang, Z.; Fang, K.; Chen, S.; Tian, S.; Fei, J.; Zhu, J. A Novel Core-Shell Up-Conversion Nanoparticles Immunochromatographic Assay for the Detection of Deoxynivalenol in Cereals. Talanta 2024, 272, 125806. [Google Scholar] [CrossRef] [PubMed]
- Kong, Z.; Liu, C.; Li, P.; Li, G.; Yuan, J.; Yan, W.; Zhao, X.; Zhang, X.; Xing, C. Development and Application of Lateral Flow Strip with Three Test Lines for Detection of Deoxynivalenol in Wheat. Food Chem. 2023, 421, 136114. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, Y.; Zhao, L.; Li, X.; Dong, Y. Development of Aptamer-Based Au Nanoparticle Lateral Flow Test Strips for the Detection of Deoxynivalenol in Corn. ACS Food Sci. Technol. 2022, 3, 182–190. [Google Scholar] [CrossRef]
- Zhao, Z.; Ren, M.; Zhang, W.; Chen, Z.; Zhang, L.; Qu, X.; Shi, J.; Xia, W.; Xu, X.; Yang, Y. SERS-Lateral Flow Immunoassay Based on AuNR@Ag@SiO2-AuNP Assembly for Ultra-Sensitive Detection of Deoxynivalenol in Grain. LWT–Food Sci. Technol. 2024, 212, 117015. [Google Scholar] [CrossRef]
- Chen, X.; Wei, X.; Cheng, S.; Liu, Z.; Su, Y.; Xiong, Y.; Huang, X. High-Performance Green-Emitting AIE Nanoparticles for Lateral Flow Immunoassay Applications. Microchim. Acta 2023, 190, 56. [Google Scholar] [CrossRef] [PubMed]
- Mermiga, E.; Pagkali, V.; Kokkinos, C.; Economou, A. An Aptamer-Based Lateral Flow Biosensor for Low-Cost, Rapid and Instrument-Free Detection of Ochratoxin A in Food Samples. Molecules 2023, 28, 8135. [Google Scholar] [CrossRef]
- Contreras Alvarez, L.A.; Lazo Jara, M.D.; Campos, F.V.; de Oliveira, J.P.; Guimarães, M.C.C. Barcode-style lateral flow immunochromatographic strip for the semi-quantitative detection of ochratoxin A in coffee samples. Food Addit. Contam. Part A 2024, 41, 424–437. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, L.; Xie, L.; Wu, Q.; Liu, Y.; Zhao, Q.; Zhang, Y.; Jiao, B.; He, Y. Gold Nanobipyramid-Based Photothermal Immunoassay for Portable Detection of Ochratoxin A in Maize and Grape Juice. ACS Appl. Nano Mater. 2023, 6, 17858–17868. [Google Scholar] [CrossRef]
- Xiang, Y.; Wu, G.; Hu, C.; Liu, H.; Li, Y. Three-in-One Lateral Flow Aptasensor Based on Magnetic Nanoparticles for On-Site Detection of Ochratoxin A in Astragalus membranaceus. Sens. Actuators B Chem. 2024, 408, 135545. [Google Scholar] [CrossRef]
- Hao, L.; Chen, J.; Chen, X.; Ma, T.; Cai, X.; Duan, H.; Leng, Y.; Huang, X.; Xiong, Y. A Novel Magneto-Gold Nanohybrid-Enhanced Lateral Flow Immunoassay for Ultrasensitive and Rapid Detection of Ochratoxin A in Grape Juice. Food Chem. 2021, 336, 127710. [Google Scholar] [CrossRef]
- Wu, S.; Liu, L.; Duan, N.; Wang, W.; Yu, Q.; Wang, Z. A Test Strip for Ochratoxin A Based on the Use of Aptamer-Modified Fluorescence Upconversion Nanoparticles. Microchim. Acta 2018, 185, 497. [Google Scholar] [CrossRef]
- Jiang, H.; Li, X.; Xiong, Y.; Pei, K.; Nie, L.; Xiong, Y. Silver Nanoparticle-Based Fluorescence-Quenching Lateral Flow Immunoassay for Sensitive Detection of Ochratoxin A in Grape Juice and Wine. Toxins 2017, 9, 83. [Google Scholar] [CrossRef]
- Majdinasab, M.; Sheikh-Zeinoddin, M.; Soleimanian-Zad, S.; Li, P.; Zhang, Q.; Li, X.; Tang, X.; Li, J. A Reliable and Sensitive Time-Resolved Fluorescent Immunochromatographic Assay (TRFICA) for Ochratoxin A in Agro-Products. Food Control 2015, 47, 126–134. [Google Scholar] [CrossRef]
- Bu, T.; Zhang, M.; Sun, X.; Tian, Y.; Bai, F.; Jia, P.; Bai, Y.; Zhe, T.; Wang, L. Gold nanoparticles-functionalized microorganisms assisted construction of immunobiosensor for sensitive detection of ochratoxin A in food samples. Sens. Actuators B Chem. 2019, 299, 126969. [Google Scholar] [CrossRef]
- Tian, Y.; Hu, X.; Jiang, J.; Tang, X.; Tian, Z.; Zhang, Z.; Li, P. Smartphone-Based Quantitative Detection of Ochratoxin A in Wheat via a Lateral Flow Assay. Foods 2023, 12, 431. [Google Scholar] [CrossRef] [PubMed]
- Di Nardo, F.; Alladio, E.; Baggiani, C.; Cavalera, S.; Giovannoli, C.; Spano, G.; Anfossi, L. Colour-Encoded Lateral Flow Immunoassay for the Simultaneous Detection of Aflatoxin B1 and Type-B Fumonisins in a Single Test Line. Talanta 2019, 192, 288–294. [Google Scholar] [CrossRef]
- Yu, Q.; Li, H.; Li, C.; Zhang, S.; Shen, J.; Wang, Z. Gold Nanoparticles-Based Lateral Flow Immunoassay with Silver Staining for Simultaneous Detection of Fumonisin B1 and Deoxynivalenol. Food Control 2015, 54, 347–352. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, Y.; Luo, Y.; Yang, X.; Li, M.; Song, Q. Double Detection of Mycotoxins Based on SERS Labels Embedded Ag@Au Core–Shell Nanoparticles. ACS Appl. Mater. Interfaces 2015, 7, 21780–21786. [Google Scholar] [CrossRef]
- Cai, X.; Ma, F.; Jiang, J.; Yang, X.; Zhang, Z.; Jian, Z.; Liang, M.; Li, P.; Yu, L. Fe–NC Single-Atom Nanozyme for Ultrasensitive, On-Site and Multiplex Detection of Mycotoxins Using Lateral Flow Immunoassay. J. Hazard. Mater. 2023, 441, 129853. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Q.; Han, M.; Zhou, J.; Gong, L.; Niu, Y.; Zhang, Y.; He, L.; Zhang, L. Development and Optimization of a Multiplex Lateral Flow Immunoassay for the Simultaneous Determination of Three Mycotoxins in Corn, Rice and Peanut. Food Chem. 2016, 213, 478–484. [Google Scholar] [CrossRef]
- Tang, Y.; Ma, P.; Khan, I.M.; Cao, W.; Zhang, Y.; Wang, Z. Lateral Flow Assay for Simultaneous Detection of Multiple Mycotoxins Using Nanozyme to Amplify Signals. Food Chem. 2024, 460, 140398. [Google Scholar] [CrossRef]
- Jiang, H.; Su, H.; Wu, K.; Dong, Z.; Li, X.; Nie, L.; Leng, Y.; Xiong, Y. Multiplexed Lateral Flow Immunoassay Based on Inner Filter Effect for Mycotoxin Detection in Maize. Sens. Actuators B Chem. 2023, 374, 132793. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, Z.; Zhang, Q.; Zhang, W.; Yu, L.; Wang, D.; Li, H.; Li, P. An on-site simultaneous semi-quantification of aflatoxin B1, zearalenone, and T-2 toxin in maize-and cereal-based feed via multicolor immunochromatographic assay. Toxins 2018, 10, 87. [Google Scholar] [CrossRef]
- Shen, Y.; Chen, Y.; Wang, H.; Zhao, X.; Lu, H.; Zhu, J. Immunochromatographic Assay Integrated Smartphone-Based Device for Simultaneous Detection of Multiple Mycotoxins Using Core-Shell Up-Conversion Nanoparticles. Sens. Actuators B Chem. 2024, 398, 134783. [Google Scholar] [CrossRef]
- Girmatsion, M.; Tang, X.; Zhang, Q.; Jiang, J.; Li, P. Phycocyanin-Based Rapid Fluorometric Immunoassay for the Determination of Aflatoxin B1, Deoxynivalenol, and Zearalenone in Food and Feed Matrices. Food Control 2024, 164, 110585. [Google Scholar] [CrossRef]
- Liang, J.; Li, X.; Huang, B.; Pan, Y.; Zhuang, Z.; Ye, Q.; Peng, C.; Deng, H.; Yi, Y.; Zhang, B.; et al. Rapid, On-Site Quantitative Determination of Mycotoxins in Grains Using a Multiple Time-Resolved Fluorescent Microsphere Immunochromatographic Test Strip. Biosensors 2024, 258, 116357. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Wang, H.; Sun, C.; Zhao, Y.; He, Y.; Nisar, M.S.; Wei, W.; Kang, H.; Xie, X.; Du, C.; et al. Au@ SiO2 SERS Nanotags Based Lateral Flow Immunoassay for Simultaneous Detection of Aflatoxin B1 and Ochratoxin A. Talanta 2023, 258, 124401. [Google Scholar] [CrossRef]
- Yin, L.; Cai, J.; Ma, L.; You, T.; Arslan, M.; Jayan, H.; Zou, X.; Gong, Y. Dual Function of Magnetic Nanocomposites-Based SERS Lateral Flow Strip for Simultaneous Detection of Aflatoxin B1 and Zearalenone. Food Chem. 2024, 446, 138817. [Google Scholar] [CrossRef]
- Hou, S.; Ma, J.; Cheng, Y.; Wang, H.; Sun, J.; Yan, Y. One-Step Rapid Detection of Fumonisin B1, Dexyonivalenol and Zearalenone in Grains. Food Control 2020, 117, 107107. [Google Scholar] [CrossRef]
- Jin, Z.; Sheng, W.; Ren, L.; Bai, D.; Sun, M.; Wang, S.; Ya, T.; Tang, X.; Wang, Z. Homogeneous Fluorescence Immunoassay Based on AuNPs Quenching Dendritic Silica Assembled with Multicolor QDs for the Simultaneous Determination of Four Mycotoxins in Cereals. Chem. Eng. J. 2024, 480, 148247. [Google Scholar] [CrossRef]
- Huang, N.; Sheng, W.; Bai, D.; Sun, M.; Ren, L.; Wang, S.; Zhang, W.; Jin, Z. Multiplex Bio-Barcode Based Fluorometric Immunoassay for Simultaneous Determination of Zearalenone, Fumonisin B1, Ochratoxin A, and Aflatoxin B1 in Cereals. Food Control 2023, 150, 109759. [Google Scholar] [CrossRef]
- Duan, H.; Li, Y.; Shao, Y.; Huang, X.; Xiong, Y. Multicolor Quantum Dot Nanobeads for Simultaneous Multiplex Immunochromatographic Detection of Mycotoxins in Maize. Sens. Actuators B Chem. 2019, 291, 411–417. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, Y.; Wang, X.; Zhou, X.; Qiu, Y.; Qin, W.; ShenTu, X.; Wang, S.; Yu, X.; Ye, Z. Dosage-Sensitive and Simultaneous Detection of Multiple Small-Molecule Pollutants in Environmental Water and Agri-Products Using Portable SERS-Based Lateral Flow Immunosensor. Sci. Total Environ. 2024, 912, 169440. [Google Scholar] [CrossRef]
- Huang, Q.; Yang, H.; Wang, W.; Zhang, Y. Multi-Target Photothermal Immunochromatography for Simultaneous Detection of Three Mycotoxins in Foods. Anal. Chim. Acta 2023, 1279, 341784. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, X.; Wen, K.; Li, C.; Mujtaba Mari, G.; Jiang, H.; Shi, W.; Shen, J.; Wang, Z. Multiplex Lateral Flow Immunoassays Based on Amorphous Carbon Nanoparticles for Detecting Three Fusarium Mycotoxins in Maize. J. Agric. Food Chem. 2017, 65, 8063–8071. [Google Scholar] [CrossRef]
- Li, R.; Meng, C.; Wen, Y.; Fu, W.; He, P. Fluorometric Lateral Flow Immunoassay for Simultaneous Determination of Three Mycotoxins (Aflatoxin B1, Zearalenone and Deoxynivalenol) Using Quantum Dot Microbeads. Microchim. Acta 2019, 186, 748. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yang, Z.; Zhang, J.; Shen, X.; Xu, Z.; Li, X.; Lei, H. High Bioaffinity Controllable Assembly Nanocarrier UiO-66-NH2@ Quantum Dot-Based Immunochromatographic Assay for Simultaneous Detection of Five Mycotoxins in Cereals and Feed. J. Agric. Food Chem. 2023, 71, 16797–16806. [Google Scholar] [CrossRef]
- Chen, Y.; Shen, Y.; Wang, H.; Zhang, J.; Zhu, J. A Novel Dual-Channel Immunochromatographic Strip Using Up-Conversion Nanoparticles for Simultaneous Detection of AFB1 and ZEN in Maize. Anal. Bioanal. Chem. 2023, 415, 4935–4947. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Li, P.; Zhang, Q.; Zhang, Z.; Zhang, W.; Jiang, J. Time-Resolved Fluorescence Immunochromatographic Assay Developed Using Two Idiotypic Nanobodies for Rapid, Quantitative, and Simultaneous Detection of Aflatoxin and Zearalenone in Maize and Its Products. Anal. Chem. 2017, 89, 11520–11528. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, X.; Wang, S.; Fotina, H.; Wang, Z. A Novel Lateral Flow Immunochromatographic Assay for Rapid and Simultaneous Detection of Aflatoxin B1 and Zearalenone in Food and Feed Samples Based on Highly Sensitive and Specific Monoclonal Antibodies. Toxins 2022, 14, 615. [Google Scholar] [CrossRef]
- Wu, M.; Xia, J.; Liu, T.; Xue, G.; Fang, X.; Lai, W.; Peng, J. Simultaneous Detection of OTA and AFB1 in Cereals Based on Bispecific Monoclonal Antibody Using Quantum Dot Nanobead Lateral Flow Immunoassay. Food Agric. Immunol. 2023, 34, 48–66. [Google Scholar] [CrossRef]
- Xue, G.; Wu, M.; Liu, T.; Fang, X.; Yin, J.; Lai, W.; Peng, J. A Multiple Lateral Flow Immunoassay Based on AuNP for the Detection of 5 Chemical Contaminants in Milk. J. Dairy Sci. 2023, 106, 3856–3867. [Google Scholar] [CrossRef]
- Goryacheva, O.A.; Guhrenz, C.; Schneider, K.; Beloglazova, N.V.; Goryacheva, I.Y.; De Saeger, S.; Gaponik, N. Silanized Luminescent Quantum Dots for the Simultaneous Multicolor Lateral Flow Immunoassay of Two Mycotoxins. ACS Appl. Mater. Interfaces 2020, 12, 24575–24584. [Google Scholar] [CrossRef]
- Beloglazova, N.V.; Sobolev, A.M.; Tessier, M.D.; Hens, Z.; Goryacheva, I.Y.; De Saeger, S. Fluorescently Labelled Multiplex Lateral Flow Immunoassay Based on Cadmium-Free Quantum Dots. Methods 2017, 116, 141–148. [Google Scholar] [CrossRef]
- Foubert, A.; Beloglazova, N.V.; De Saeger, S. Comparative Study of Colloidal Gold and Quantum Dots as Labels for Multiplex Screening Tests for Multi-Mycotoxin Detection. Anal. Chim. Acta 2017, 955, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Hua, Q.; Wang, J.; Liang, Z.; Li, J.; Wu, J.; Shen, X.; Lei, H.; Li, X. A smartphone-based dual detection mode device integrated with two lateral flow immunoassays for multiplex mycotoxins in cereals. Biosens. Bioelectron. 2020, 158, 112178. [Google Scholar] [CrossRef]
- Zhou, S.; Zhu, X.; Song, S.; Sun, M.; Kuang, H.; Xu, C.; Guo, L. Rapid and Simultaneous Detection of Five Mycotoxins and Their Analogs with a Gold Nanoparticle-Based Multiplex Immuno-Strip Sensor. Food Microbiol. 2024, 121, 104510. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, E. Gold Nanoparticle-Based Plasmonic Biosensors. Biosensors 2023, 13, 411. [Google Scholar] [CrossRef]
- Sadiq, Z.; Safiabadi Tali, S.H.; Hajimiri, H.; Al-Kassawneh, M.; Jahanshahi-Anbuhi, S. Gold Nanoparticles-Based Colorimetric Assays for Environmental Monitoring and Food Safety Evaluation. Crit. Rev. Anal. Chem. 2023, 54, 2209–2244. [Google Scholar] [CrossRef]
- Chang, C.C.; Chen, C.P.; Wu, T.H.; Yang, C.H.; Lin, C.W.; Chen, C.Y. Gold Nanoparticle-Based Colorimetric Strategies for Chemical and Biological Sensing Applications. Nanomaterials 2019, 9, 861. [Google Scholar] [CrossRef]
- Shao, B.; Ma, X.; Zhao, S.; Lv, Y.; Hun, X.; Wang, H.; Wang, Z. Nanogapped Au(core)@Au–Ag(shell) Structures Coupled with Fe3O4 Magnetic Nanoparticles for the Detection of Ochratoxin A. Anal. Chim. Acta 2018, 1033, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Leng, Y.; Zeng, L.; Chen, X.; Chen, J.; Duan, H.; Huang, X.; Xiong, Y.; Chen, X. Core–Shell-Heterostructured Magnetic–Plasmonic Nanoassemblies with Highly Retained Magnetic–Plasmonic Activities for Ultrasensitive Bioanalysis in Complex Matrix. Adv. Sci. 2019, 7, 1902433. [Google Scholar] [CrossRef]
- Mills, A.M.; Strzalka, J.; Bernat, A.; Rao, Q.; Hallinan, D.T., Jr. Magnetic-Core/Gold-Shell Nanoparticles for the Detection of Hydrophobic Chemical Contaminants. Nanomaterials 2022, 12, 1253. [Google Scholar] [CrossRef]
- Kumar, R.; Shafique, M.S.; Chapa, S.O.M.; Madou, M.J. Recent Advances in MOF-Based Materials for Biosensing Applications. Sensors 2025, 25, 2473. [Google Scholar] [CrossRef]
- Hu, H.; Wang, Y. Recent Advances in Metal–Organic Frameworks as Emerging Platforms for Immunoassays. TrAC Trends Anal. Chem. 2024, 171, 117520. [Google Scholar] [CrossRef]
- Dai, Y.; Huang, K.; Zhang, B.; Zhu, L.; Xu, W. Aflatoxin B1-induced Epigenetic Alterations: An Overview. Food Chem. Toxicol. 2017, 109, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Kew, M.C. Aflatoxins as a Cause of Hepatocellular Carcinoma. J. Gastrointest. Liver Dis. 2013, 22, 305–310. [Google Scholar]
- Khoi, C.S.; Chen, J.H.; Lin, T.Y.; Chiang, C.K.; Hung, K.Y. Ochratoxin A-induced nephrotoxicity: Up-to-date evidence. Int. J. Mol. Sci. 2021, 22, 11237. [Google Scholar] [CrossRef]
- Kamle, M.; Mahato, D.K.; Gupta, A.; Pandhi, S.; Sharma, B.; Dhawan, K.; Vasundhara; Mishra, S.; Kumar, M.; Tripathi, A.D.; et al. Deoxynivalenol: An overview on occurrence, chemistry, biosynthesis, health effects and its detection, management, and control strategies in food and feed. Microbiol. Res. 2022, 13, 292–314. [Google Scholar] [CrossRef]
- Chen, J.; Wen, J.; Tang, Y.; Shi, J.; Mu, G.; Yan, R.; Cai, J.; Long, M. Research Progress on Fumonisin B1 Contamination and Toxicity: A Review. Molecules 2021, 26, 5238. [Google Scholar] [CrossRef]
- Gelineau-van Waes, J.; Voss, K.A.; Stevens, V.L.; Speer, M.C.; Riley, R.T. Maternal fumonisin exposure as a risk factor for neural tube defects. Adv. Food Nutr. Res. 2009, 56, 145–181. [Google Scholar] [CrossRef]
- Pechey, R.; Monsivais, P. Socioeconomic Inequalities in the Healthiness of Food Choices: Exploring the Contributions of Food Expenditures. Prev. Med. 2016, 88, 203–209. [Google Scholar] [CrossRef]
- Mu, P.; Xu, M.; Zhang, L.; Wu, K.; Wu, J.; Jiang, J.; Chen, Q.; Wang, L.; Tang, X.; Deng, Y. Proteomic changes in chicken primary hepatocytes exposed to T-2 toxin are associated with oxidative stress and mitochondrial enhancement. Proteomics 2013, 13, 3175–3188. [Google Scholar] [CrossRef]
- Shahjahan, T.; Javed, B.; Sharma, V.; Tian, F. Overview of Various Components of Lateral-Flow Immunochromatography Assay for the Monitoring of Aflatoxin and Limit of Detection in Food Products: A Systematic Review. Chemosensors 2023, 11, 520. [Google Scholar] [CrossRef]
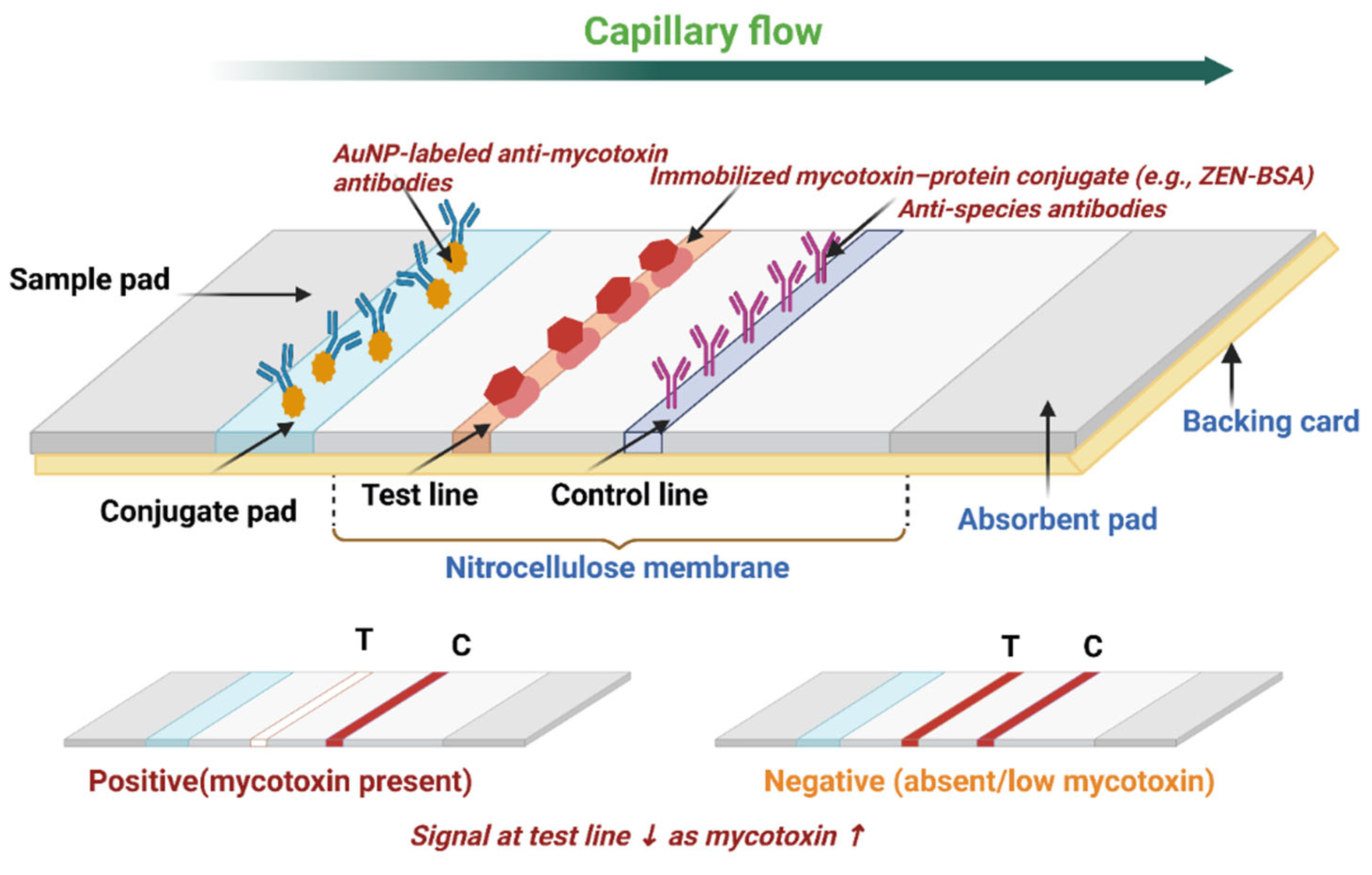
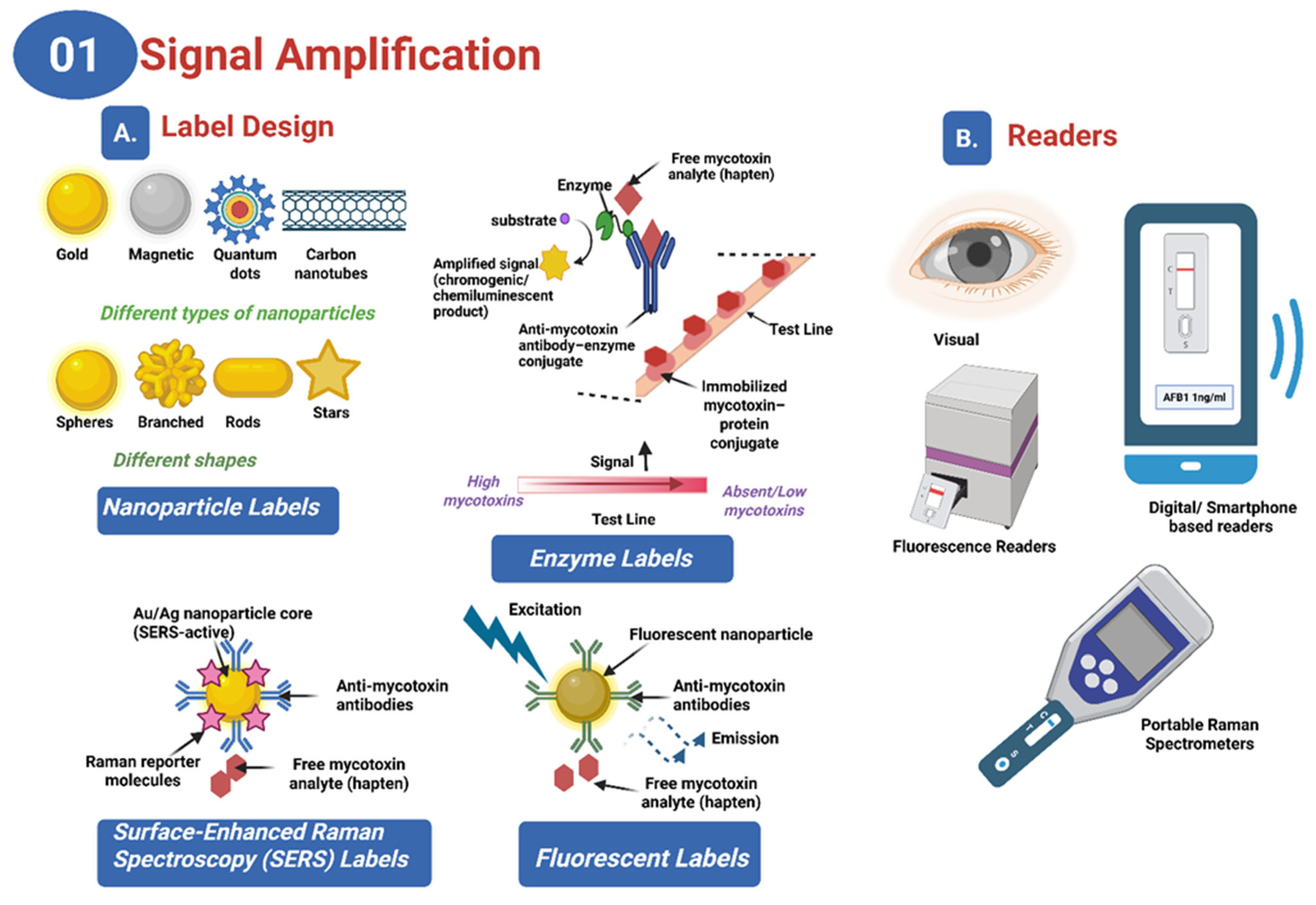
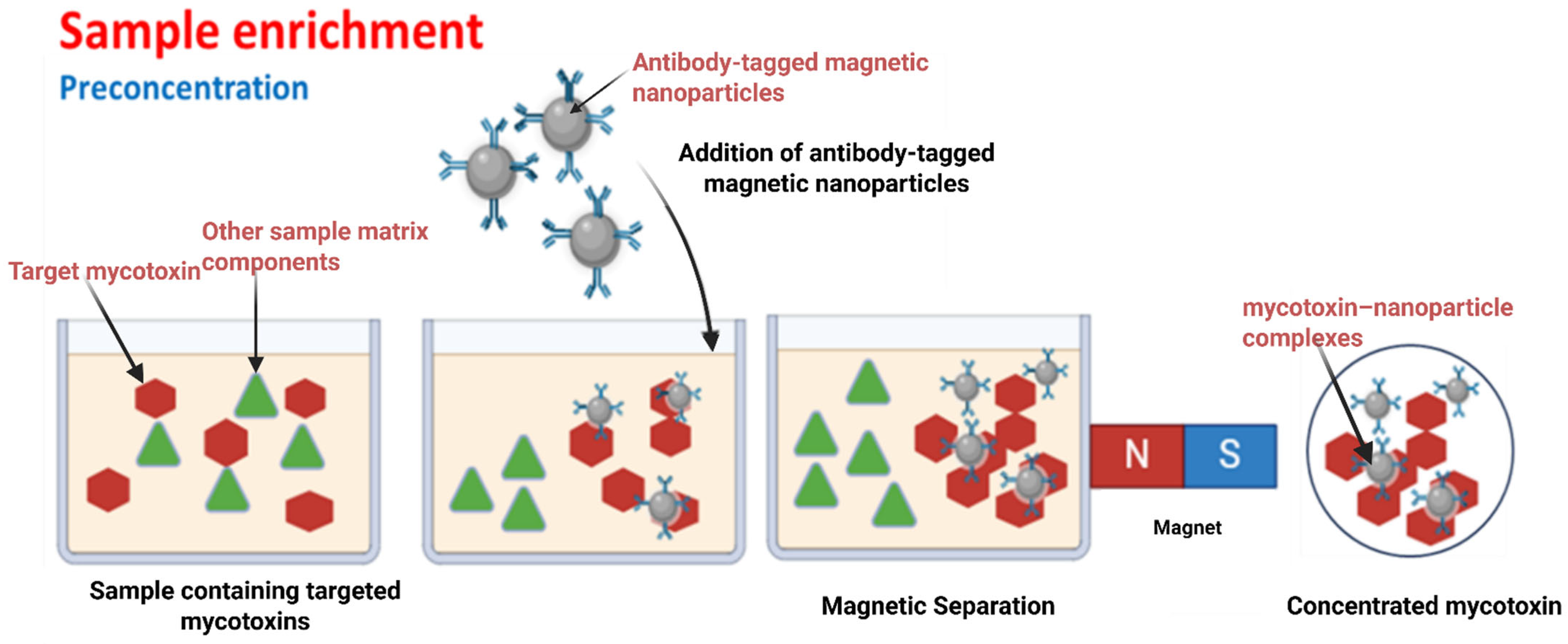
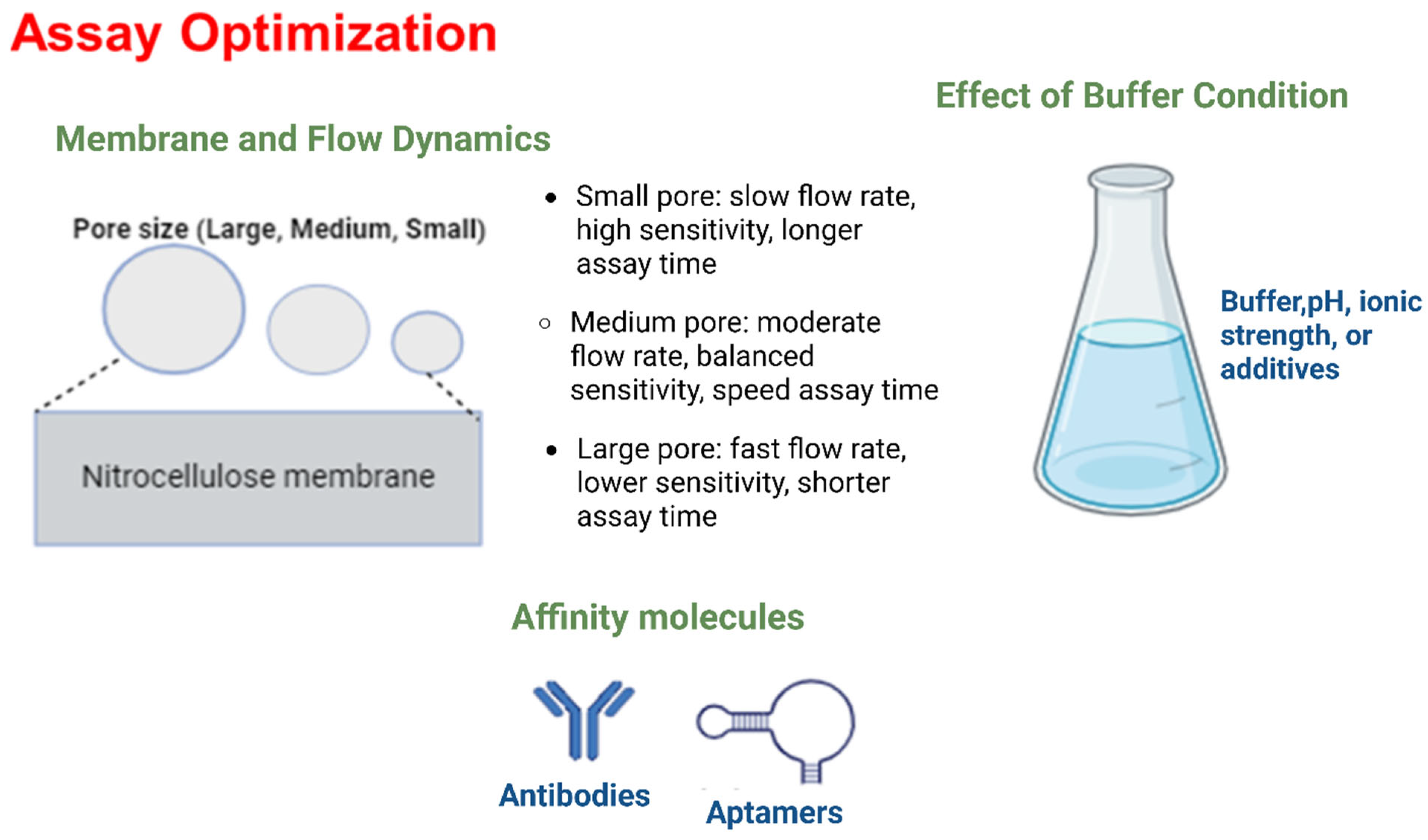






| Detection Method | Sample | Target Analyte | Nanoparticle | Advancement | Sensitivity | Year | Reference |
|---|---|---|---|---|---|---|---|
| Individual detection | Rice extract | AFB1 | Blue gold nanoflowers (AuNFs) | 75 ± 5 nm | 0.32 pg/mL | 2015 | [122] |
| Suspicious Fungi-Contaminated Food Samples | AFB1 | AuNPs with BSA and AFB1 antibody | Rapid and sensitive AuNP immunochromatographic strip | 2 ng/mL | 2019 | [123] | |
| Maize | AFB1 | Zn-CN (pyrolyzed ZIF-8 metal–carbon nanomaterial) | Colorimetric, fluorescent, and photothermal detection | 0.0012 ng/mL (Colorimetric), 0.0094 ng/mL (Fluorescent), 0.252 ng/mL (Photothermal) | 2023 | [124] | |
| Various Food Samples | AFB1 | AuNPs@SH-poly A-cDNA | Turn on mode-based aptamer sensor AuNPs@SH-poly A-cDNA nanoprobes | 0.1 μg/kg | 2023 | [125] | |
| Food samples | AFB1 | CuCo@PDA nanozyme (Copper-Cobalt polydopamine nanozyme) | Dual-readout (naked eye/smartphone), catalytic colorimetric amplification using peroxidase-like nanozyme | 2.2 pg/mL | 2024 | [126] | |
| Distillers’ grains | AFB1 | Red Fluorescent Microspheres | Enables rapid, sensitive, and accurate on-site detection with cost-effective, large-scale applicability. | 3.4 μg/kg | 2021 | [127] | |
| Potable Water Samples | AFB1 | AuNPs | Lateral flow immunostrip effective for 3 months at 4 °C. | 0.5 ppb | 2019 | [128] | |
| Food and Feed | AFB1 | AuNPs | Dot-blot assay using octapeptide-conjugated AuNPs | 0.39 μg/kg | 2023 | [129] | |
| Soybean Sauce | AFB1 | Eu-nanospheres | Time-resolved fluorescence immunochromatography | 0.1 µg·kg−1 | 2016 | [130] | |
| Rice and Peanut | Total Aflatoxin | Quantum Dot Nanobeads (QDNBs) | On-site, ultra-sensitive, and quantitative test strip rapid detection with high sensitivity. | 1.4 pg/mL (rice), 2.9 pg/mL (peanut) | 2017 | [131] | |
| Maize, Lotus seed | AFB1 | Polydopamine-coated HKUST MOFs (HKUST@PDA) | HKUST@PDA as a signal amplification marker. | 0.01 ng/mL | 2023 | [132] | |
| Maize, Lotus seed | AFB1 | UiOL@AIEgens nanocomposites (MOF with AIEgens) | Dual-modal (visual + quantitative), signal-enhanced detection using MOF-AIEgens hybrid | 0.003 ng/mL | 2024 | [133] | |
| Soy-based foods (soy protein, soy milk) | Aflatoxins (B1, M1, G1, G2, B2) | AuNPs | Monoclonal antibody (3B6) for rapid detection of multiple aflatoxins | 0.5 μg/kg | 2017 | [134] | |
| Raw milk | AFM1 | Immunomagnetic nanobeads | Two types of IMNBs to enhance sensitivity and eliminate sample pretreatment. | 0.02 μg/L | 2015 | [135] | |
| Milk | AFM1 | Fluorescent Nanocomposites | Ratiometric hue-based visual & quantitative detection | 0.0012 ng/mL | 2022 | [136] | |
| Maize flour | Fumonisins | CdSe/ZnS, QDs + AgNPs + AuNPs | Fluorescence quenching and recovery to enhance sensitivity | 1.56 ng/mL | 2018 | [137] | |
| Corn samples | FB1 | Colloidal AuNP | Rapid, specific, and low-cost immunoassay | 2.5 ng/mL | 2015 | [138] | |
| Grains | FB1 | Urchin-like AuNPs | ICS with UGNs for enhanced sensitivity and rapid detection | 5 ng/mL | 2015 | [139] | |
| Corn | FB1 | Europium (Eu) Nanoparticles | Indirect signal amplification, higher sensitivity | 0.025 ng/mL | 2022 | [140] | |
| Maize grains | Fumonisins | AuNPs | Optimized IgY-based assay with improved specificity and sensitivity | 4000 µg/kg | 2019 | [141] | |
| Corn | ZEN | Pt@AuNF nanozyme and horseradish peroxidase (HRP) | Dual enzyme catalytic signal amplification strategy | 0.052 ng/mL | 2023 | [142] | |
| Cereals | ZEN | AuNPs loaded black phosphorus (BP-Au) nanocomposite | Photothermal LFIA, high sensitivity, excellent photothermal conversion efficiency, and effective on-site monitoring. | 4.3 pg/mL | 2023 | [143] | |
| Cereals | ZEN | Carboxyl group-coated Fe3O4 nanoparticles (MNPs) | Portable, dual detection mode, multi-channel ICA with a smartphone-based readout device, Magnetic enrichment for improved sensitivity and robustness | 0.06 μg kg−1 | 2020 | [144] | |
| Wheat | ZEN | Colloidal AuNP (30 nm) | Rapid ICS test; optimized antigen and antibody concentrations; completed in 5 min, Millipore 135 NC membrane | 15 ng/mL | 2017 | [145] | |
| Cereals | ZEN | 30% Lu3+-doped UCNPs | Novel UCNPs-ICA with optimized optical properties; high specificity; quick detection | 0.16 μg/kg | 2023 | [146] | |
| Cereals | ZEN | Prussian Blue Nanoparticles (PBNPs) | Portable smartphone-based readout with quantitative analysis | 0.12 μg/kg | 2022 | [147] | |
| Corn | ZEN | AuNPs | Rapid detection in 5 min, competitive assay with aptamer and complementary sequence | 5–200 ng/mL | 2018 | [148] | |
| Corn | ZEN | QDs | Fluorescent quenching lateral flow assay | 0.58 ng/mL | 2019 | [149] | |
| Cereals | T-2 | AuNP + selenium nanoparticles (SeNPs) | SeNPs (Se-ICS) and dual AuNPs (Duo-ICS) for improved sensitivity | Duo-ICS: 1 ng/mL; Se-ICS: 0.25 ng/mL | 2023 | [150] | |
| Rice, chicken feed | T-2 | Colloidal Gold (CG) and Fluorescent Microspheres (FMs) | Comparison of CG and FMs as labels in LFIA, with optimized cut-off values | 0.23 μg/kg (rice), 0.41 μg/kg (chicken feed) | 2015 | [151] | |
| Rice, maize, feed | T-2 | Eu(III) nanoparticles | Time-resolved fluorescence for ultrasensitive detection | Rice 0.09 ng/g Feed 0.17 ng/g | 2015 | [152] | |
| Food samples | DON | Cauliflower-like ReS2@Pt core–shell nanospheres | Colorimetric-catalytic dual-mode, peroxidase-mimicking nanozyme with enhanced antibody affinity | 6.5 pg/mL | 2024 | [153] | |
| Corn, wheat, naturally contaminated cereals and feed | DON | Core–shell up-conversion nanoparticles | Enhanced up-conversion luminescence for highly sensitive and specific DON detection within 5 min | 0.1 ng/mL | 2024 | [154] | |
| Wheat | DON | AuNPs | Single strip with three test lines (TTLS) for semi-quantitative and quantitative determination | 200 µg/kg | 2023 | [155] | |
| Corn | DON | AuNPs | Aptamer-based lateral flow assay | 24.11 ng/mL | 2022 | [156] | |
| Grain | DON | AuNR@Ag@SiO2-AuNP core–shell-satellite nanoassembly | Highly SERS-active, stable, antibody-modified core–shell-satellite structure | 0.053 fg/mL | 2024 | [157] | |
| Real maize samples | OTA | Ultrabright green-emissive AIE nanoparticles (AIENPs) | Enhanced detectability of LFIA with ultrabright AIENPs; applicability for small molecules and macromolecules | 0.043 ng/mL | 2023 | [158] | |
| Wine, beer, apple juice, milk samples | OTA | Aptamer-conjugated AuNPs | Aptasensor strip for rapid detection; competitive format; visual and semi-quantitative detection | Visual LOD: 0.05 ng/mL Semi-quantitative LOD: 0.02 ng/mL | 2023 | [159] | |
| Coffee samples | OTA | AuNPs | Barcode-style lateral flow assay for semi-quantitative detection; distinct color patterns for different OTA concentrations | 2.5 µg/L | 2024 | [160] | |
| Maize and grape juice | OTA | AuNP nanobipyramids | Photothermal immunoassay with Alkaline phosphatase-mediated in situ growth of AuNBPs; sensitive detection using a thermometer | 020 ng/mL | 2023 | [161] | |
| Astragalus membranaceus | OTA | Aptamer-modified MNPs | Three-in-one lateral flow aptasensor using aptamer-MNPs for purification, enrichment, and detection | 0.053 ng/mL | 2024 | [162] | |
| Grape juice | OTA | Magneto-gold nanohybrid (MGNH) | Novel MGNH integrated into LFIA for simultaneous magnetic separation and colorimetric target sensing | 0.094 ng mL−1 | 2021 | [163] | |
| Wheat, beer | OTA | Ytterbium-doped sodium yttrium fluoride (NaYF4:Yb,Er) UCNPs | Aptamer-based upconversion fluorescent strip | 1.86 ng/mL | 2018 | [164] | |
| Grape Juice, Wine | OTA | Silver nanoparticles | Silver nanoparticle-based fluorescence-quenching lateral flow immunoassay | 0.06 µg/L | 2017 | [165] | |
| Wheat, Maize, Soybean, Rice | OTA | Fluorescent europium (III) [Eu (III)] nanoparticles (EuNPs) | Time-resolved fluorescent ICA | 1.0 μg kg−1 | 2015 | [166] | |
| Rice, Corn, Ginger, Green Bean | OTA | Microorganism-loaded AuNPs | Use of Yeast/Lactobacillus as reducers and carriers for AuNP synthesis, enhancing adsorption and lowering antibody usage | 0.1 ng/mL | 2019 | [167] | |
| Wheat | OTA | Europium nanospheres | Smartphone-enabled iPOCT for rapid detection; fluorescent lateral flow assay; cloud-based result sharing | 0.02 ng/mL | 2023 | [168] | |
| Multiplexing | Maize Flour | AFB1, FB1 | Desert rose-like gold nanoparticles (DR-GNPs), Red spherical GNPs | Multicolor ICST strip test employing DR-GNPs and red spherical GNPs | 2 μg/kg (AFB1) 1000 μg/kg (FB1) | 2019 | [169] |
| Maize | FB1, DON | AuNPs | Silver staining for signal amplification | 2.0 ng/mL (FB1), 40 ng/mL (DON) | 2015 | [170] | |
| Maize meal | AFB1, OTA | Ag@Au Core–Shell NPs | SERS labels embedded Ag@Au core–shell NPs for sensitive double detection without nucleic acid amplification | 0.006 ng/mL (AFB1) 0.03 ng/mL (OTA) | 2015 | [171] | |
| Food sample | AFB1, FB1 | Fe-N-C single-atom nanozymes (SAzymes) | Ultra-sensitive detection, dual-function label & catalyst, smartphone readout | 2.8 pg/mL AFB1), 13.9 pg/mL (FB1) | 2023 | [172] | |
| Corn, Rice, Peanut | AFB1, ZEN, OTA | AuNPs | Systematic optimization of antibody-AuNP conjugates, nanoparticle size, and capture antigen position for improved detection | 0.10–0.13 μg/kg (AFB1), 0.42–0.46 μg/kg (ZEN), 0.19–0.24 μg/kg (OTA) | 2016 | [173] | |
| Milk, Maize and Wheat | AFB1, AFM1, OTA | AuNP iridium nanozyme | Three-channel aptamer-based lateral flow assay (Apt-LFA), Catalytic chromogenic substrate & fluorescence-based optimization | 0.39 ng/mL (AFM1), 0.36 ng/mL (AFM1), 0.82 ng/mL (OTA) | 2024 | [174] | |
| Maize | AFB1, OTA, ZEN | Flower-like AuNPs and red-emitting quantum dots | Multiplexed competitive lateral flow immunoassay (cLFIA) based on inner filter effect (IFE) | 0.005 μg/L (AFB1), 0.04 μg/L (OTA), 0.4 μg/L (ZEN) | 2022 | [175] | |
| Maize- and cereal-based animal feeds | AFB1, ZEN, T-2 | AuNPs | Multi-color nanoparticles in an immunochromatographic strip for the simultaneous detection | 0.5 ng/Ml (AFB1), 2 ng/mL (ZEN), 30 ng/mL (T-2) | 2018 | [176] | |
| Corn, Wheat | AFB1, DON, ZEN | Core–shell up-conversion nanoparticle | Smartphone-integrated UCNPs for portable, simultaneous multi-mycotoxin detection | DON: 0.25 ng/mL, AFB1: 0.05 ng/mL, ZEN: 0.1 ng/mL | 2024 | [177] | |
| Wheat, Corn, Animal Feed | AFB1, DON, ZEN | Carboxylated latex nanospheres with phycocyanin and mAbs | Fluorescent multiplex detection using Phycocyanin-labeled LNS, visualized via LED UV and smartphone in <25 min | AFB1 Wheat: 1.04 ng/mL Corn: 1.6 ng/mL Feed: 2.08 ng/mL DON Wheat: 2.2 ng/mL Corn: 6.45 ng/mL Feed: 2.9 ng/mL ZEN Wheat: 1.74 ng/mL Corn: 1.67 ng/mL Feed: 2.11 ng/mL | 2024 | [178] | |
| Grains | AFB1, ZEN, DON | Carboxylated Eu(III)-chelate-doped polystyrene nanobeads | Time-resolved fluorescence to reduce background noise; quantitative multiplex detection with portable reader | AFB1: 0.03 ng/g, ZEN: 0.11 ng/g, DON: 0.81 ng/g | 2024 | [179] | |
| Corn, Rice, Wheat | AFB1, OTA | Au@SiO2 SERS nanotags | Multiplex and ultrasensitive detection using SERS-based LFIA; high sensitivity and biocompatibility | AFB1: 0.24 pg/mL, OTA: 0.37 pg/mL | 2023 | [180] | |
| Corn | AFB1, ZEN | Magnetic Fe3O4@PEI/AuMBA@Ag-MBA nanocomposites | Bi-channel SERS-based LFIA strip for simultaneous detection, magnetic enrichment improves sensitivity | 0.1–10 μg/kg (AFB1) 4–400 μg/kg (ZEN) | 2024 | [181] | |
| Wheat & Corn | FB1, DON, ZEN | AuNPs | Multiplex qualitative detection, High specificity | 60 ng/mL (FB1), 12.5 ng/mL (DON), 6 ng/mL (ZEN) | 2020 | [182] | |
| Cereal | OTA, AFB1, FB1, ZEN | QDs, AuNPs, Dendritic mesoporous silica nanoparticles (DMSNs) | Homogeneous fluorescence immunoassay for simultaneous detection of four mycotoxins | 0.0001 μg/L (OTA) 0.0008 μg/L (AFB1) 0.001 μg/L (FB1) 0.0006 μg/L(ZEN) | 2023 | [183] | |
| Cereal | ZEN, FB1, OTA, AFB1 | AuNPs with fluorophore-labeled ssDNA | Simultaneous quantitative detection of four mycotoxins using a single test process with fluorescence recovery | 0.02 μg/kg (ZEN) 2.42 μg/kg (FB1) 0.03 μg/kg (OTA) 0.065 μg/kg (AFB1) | 2023 | [184] | |
| Corn | ZEN, OTA, FB1 | Tricolor QBs | Simultaneous qualitative detection of multiple mycotoxins | 5 ng/mL (OTA), 20 ng/mL (FB1), 10 ng/mL (ZEN) | 2019 | [185] | |
| Environmental water | Pesticide residues (imidacloprid, pyraclostrobin) and mycotoxin (AFB1) | SERS nanotags | Dosage-sensitive and simultaneous quantitative SERS-based LFIA for multiple pollutants | 8.6 pg/Ml (imidacloprid) 97.4 pg/mL (pyraclostrobin) 8.9 pg/mL (AFB1) | 2023 | [186] | |
| Foods | DON, AFB1, ZEN | Cu2-xSe-Au nanocomposites | Multi-target photothermal immunochromatography for simultaneous detection | 73 ng/L (DON) 45 ng/L (AFB1) 43 ng/L (ZEN) | 2023 | [187] | |
| Maize | DON, T-2, ZEN | Amorphous carbon nanoparticles | Utilization of amorphous carbon nanoparticles (ACNPs) as detection labels | 20 μg/kg (DON), 13 μg/kg (T-2), 1 μg/kg (ZEN) | 2017 | [188] | |
| Feedstuff, naturally contaminated | AFB1, ZEN, DON | CdSe/SiO2 QBs | Simultaneous determination, Quick analysis. | 10 pg mL−1 (AFB1) 80 pg mL−1 (ZEN) 500 pgmL−1 (DON) | 2019 | [189] | |
| Cereals and feed (naturally contaminated) | AFB1, FB1, DON, T-2, ZEN | UiO-66-NH2@quantum dot (NU66@QD) nanocomposites | High bio affinity and controllable assembly nanocarrier for simultaneous detection of five mycotoxins | 0.04 μg/kg (AFB1) 0.28 μg/kg (FB1) 0.25 μg/kg (DON) 0.09 μg/kg (T-2) 0.08 μg/kg (ZEN) | 2023 | [190] | |
| Maize | AFB1, ZEN | Lu3+-doped UCNPs | Synthesis and functionalization of Lu3+-doped UCNPs with larger size, more regular structure, and significantly brighter fluorescence intensity | LOD: 0.01 ng/mL AFB1, LOD: 0.1 ng/mL ZEN | 2023 | [191] | |
| Maize and its products | AFB1, ZEN | Eu/Tb(III) nanospheres | Time-resolved fluorescence immunochromatographic assay (TRFICA) using anti-idiotypic nanobody (AIdnb) and monoclonal antibody (mAb) | Aflatoxin B1 (AFB1): 0.05 ng·mL−1 Zearalenone (ZEN): 0.07 ng·mL−1 | 2017 | [192] | |
| Food and feed | AFB1, ZEN | AuNPs | Dual lateral flow immunochromatographic assay for simultaneous detection | 0.23 μg/L (AFB1) 1.53 μg/L (ZEN) | 2022 | [193] | |
| Soybean, corn, rice | AFB1, OTA | Quantum dot nanobeads | Bispecific monoclonal antibody-based multiplex LFIA | 0.037 μg/kg (AFB1), 1.19 μg/kg (OTA) | 2016 | [194] | |
| Milk | Melamine (MEL), Enrofloxacin (ENR), Sulfamethazine (SMZ), Tetracycline (TC), AFM1 | AuNPs | Multiple lateral flow immunoassay (LFIA) for simultaneous detection of 5 chemical contaminants | 0.173 ng/mL (MEL) 0.078 ng/mL(ENR) 0.059 ng/mL (SMZ) 0.082 ng/mL(TC), 0.0064 ng/mL (AFM1) | 2023 | [195] | |
| Maize and wheat samples, naturally contaminated | ZEN, DON | CdSe/CdS & CdSe/CdS/ZnS core–shell heterostructures | Multicolor lateral flow immunoassay using QD bioconjugates | 40 μg kg−1 (ZEN), 400 μg kg−1 (DON) | 2020 | [196] | |
| Maize, Wheat | ZEN DON | Indium Phosphide (InP) QDs | Water-soluble InP/ZnS QDs-based fluorescent nanostructures (QD@SiO2) for simultaneous detection | 50 µg/kg (ZEN) 500 µg/kg (DON) | 2017 | [197] | |
| Wheat | DON, ZEN, T2/HT2 | CdSe/ZnS QDs, Colloidal gold (CG) | QD-based LFIA consumed less immunoreagents, more sensitive, lower false negative rate; CG-based LFIA developed for comparison | 1000 μg/kg (DON), 80 μg/kg (ZEN), 80 μg/kg (T2/HT2) | 2017 | [198] | |
| Cereals | AFB1, ZEN, DON, T-2, FB1 | AuNPs + Time resolved fluorescence microspheres | Smartphone-based dual detection mode device; multiplex detection; integrated visible light and fluorescence detection | qLODs: 0.59/0.24/0.32/0.9/0.27 μg/kg (AuNPs), 0.42/0.10/0.05/0.75/0.04 μg/kg (TRFMs) | 2020 | [199] | |
| Cereals | DON, ZEN, T-2, TEA, AOH (Total: 15 mycotoxins) | AuNPs | Simultaneous detection of 15 mycotoxins in a single test | DON: 0.91, ZEN: 0.04, T-2: 0.11, TEA: 0.12, AOH: 0.09–0. | 2024 | [200] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thenuwara, G.; Akhtar, P.; Javed, B.; Singh, B.; Byrne, H.J.; Tian, F. Recent Advancements in Lateral Flow Assays for Food Mycotoxin Detection: A Review of Nanoparticle-Based Methods and Innovations. Toxins 2025, 17, 348. https://doi.org/10.3390/toxins17070348
Thenuwara G, Akhtar P, Javed B, Singh B, Byrne HJ, Tian F. Recent Advancements in Lateral Flow Assays for Food Mycotoxin Detection: A Review of Nanoparticle-Based Methods and Innovations. Toxins. 2025; 17(7):348. https://doi.org/10.3390/toxins17070348
Chicago/Turabian StyleThenuwara, Gayathree, Perveen Akhtar, Bilal Javed, Baljit Singh, Hugh J. Byrne, and Furong Tian. 2025. "Recent Advancements in Lateral Flow Assays for Food Mycotoxin Detection: A Review of Nanoparticle-Based Methods and Innovations" Toxins 17, no. 7: 348. https://doi.org/10.3390/toxins17070348
APA StyleThenuwara, G., Akhtar, P., Javed, B., Singh, B., Byrne, H. J., & Tian, F. (2025). Recent Advancements in Lateral Flow Assays for Food Mycotoxin Detection: A Review of Nanoparticle-Based Methods and Innovations. Toxins, 17(7), 348. https://doi.org/10.3390/toxins17070348









