Differentiating Zeranol Implant Abuse and Fusarium spp. Toxin-Contaminated Corn Intake by Detection and Quantification of Resorcylic Acid Lactones in Bovine Urine
Abstract
1. Introduction
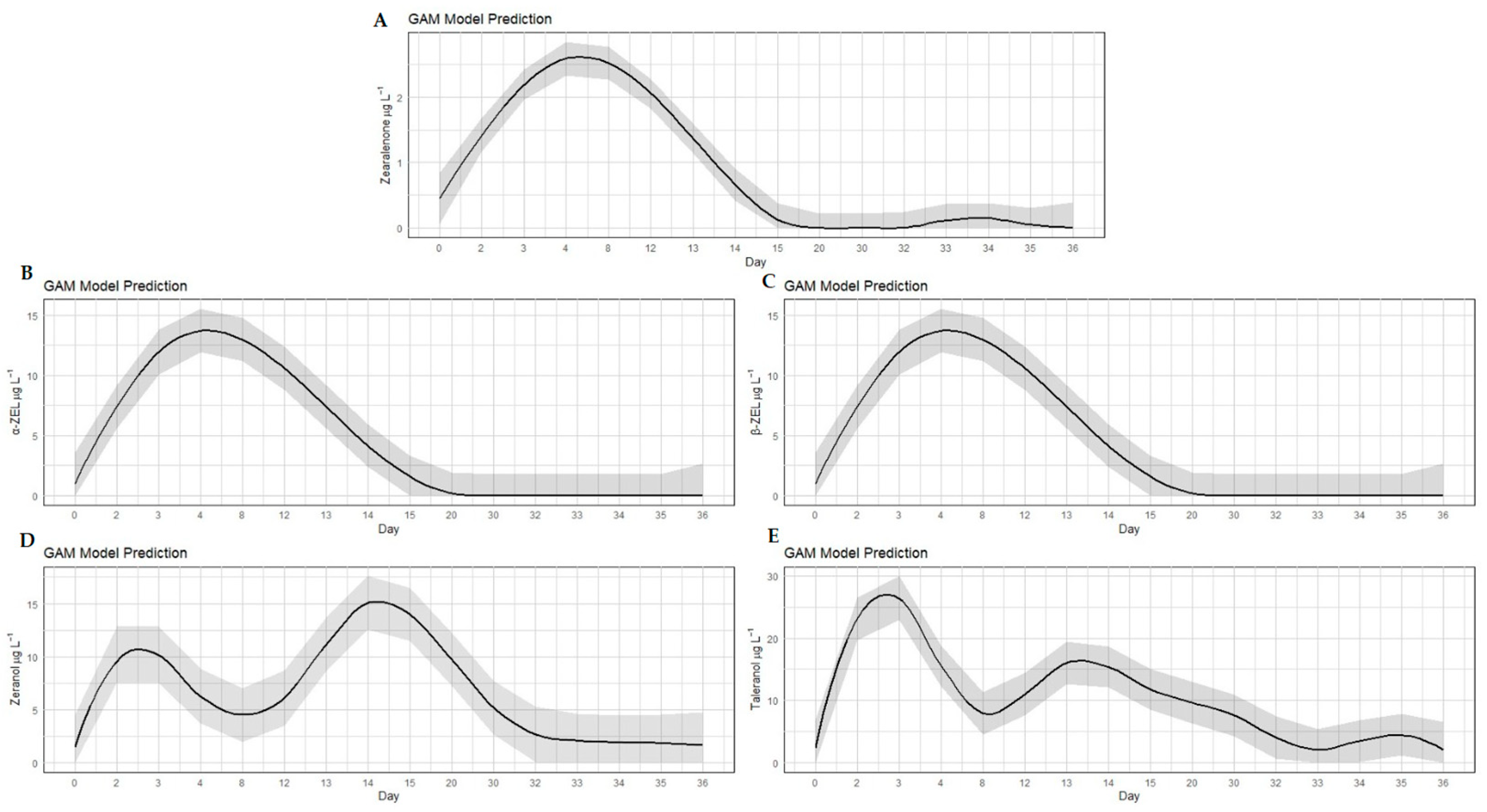
2. Results and Discussion
2.1. Detection and Quantification of RALs
2.2. Discrimination Between Abuse or Natural Contamination
3. Conclusions
4. Materials and Methods
4.1. Experimental Animals
4.2. Treatments
4.3. Detection and Quantification of RALs
4.3.1. Laboratory Analyses
4.3.2. Chemicals and Reagents
4.3.3. Instrumentation
4.3.4. Samples and Extraction Procedure
4.4. Discrimination Abuse or Contamination Through the Statistical Model
4.5. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
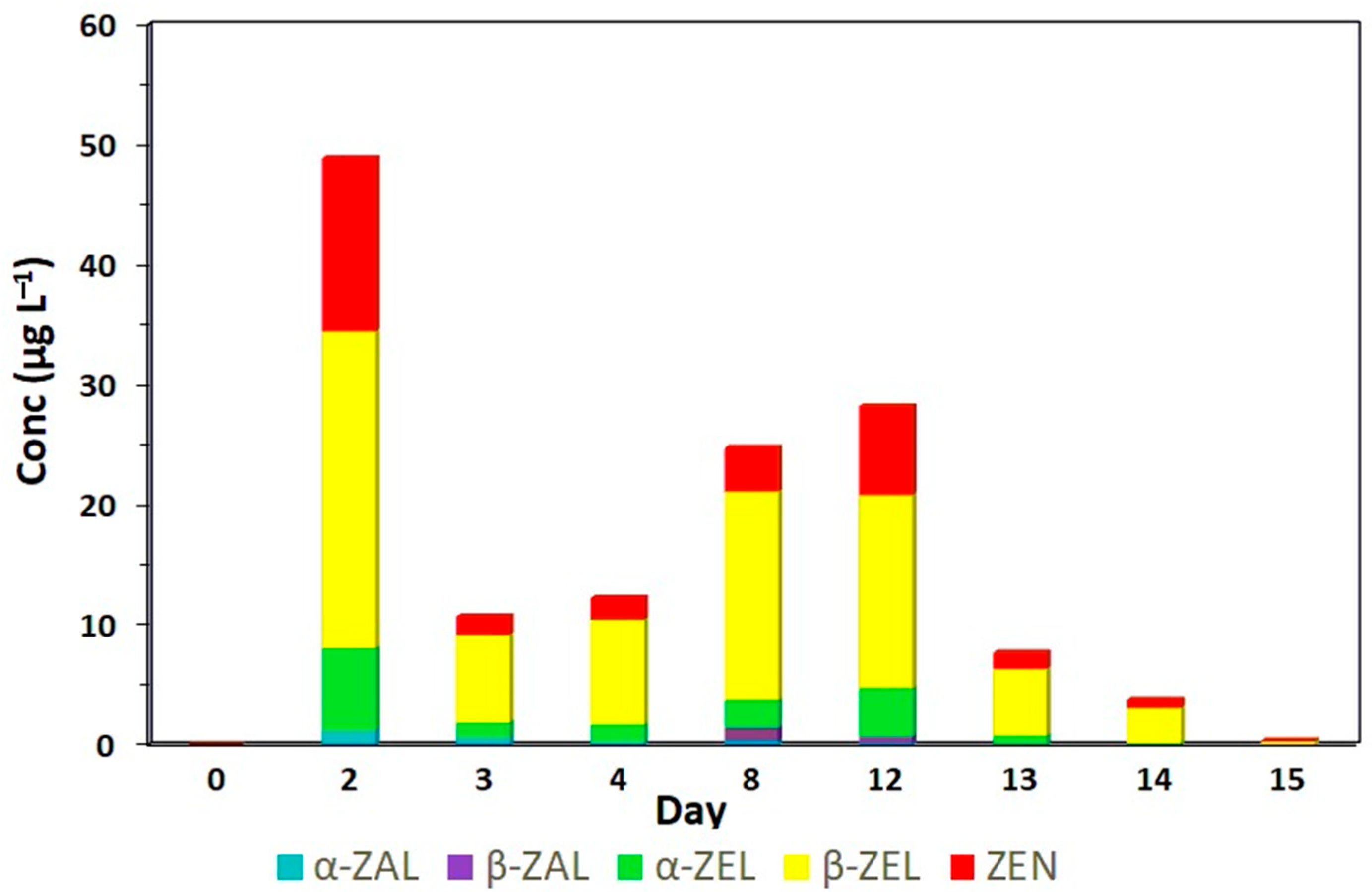
| Resorcylic Acid Lactones | |||||
|---|---|---|---|---|---|
| Day | Zearalenone | α-Zearalenol | β-Zearalenol | Zeranol | Taleranol |
| 0 | ND | ND | ND | ND | ND |
| 2 | 14.45 a | 6.24 a | 24.37 a | 1.08 a | ND |
| 3 | 1.54 b | 1.92 b | 9.10 bcd | 0.65 ab | ND |
| 4 | 1.81 b | 1.28 b | 8.63 bcd | 0.26 bc | ND |
| 8 | 3.63 ab | 2.49 ab | 17.14 ab | 0.32 bc | 1.01 a |
| 12 | 7.40 ab | 3.74 ab | 15.80 abc | 0.19 bc | 0.52 b |
| 13 | 1.34 b | 1.02 b | 6.04 bcd | ND | ND |
| 14 | 0.68 b | 0.13 b | 2.71 cd | ND | ND |
| 15 | 0.07 b | ND | 0.34 d | ND | ND |
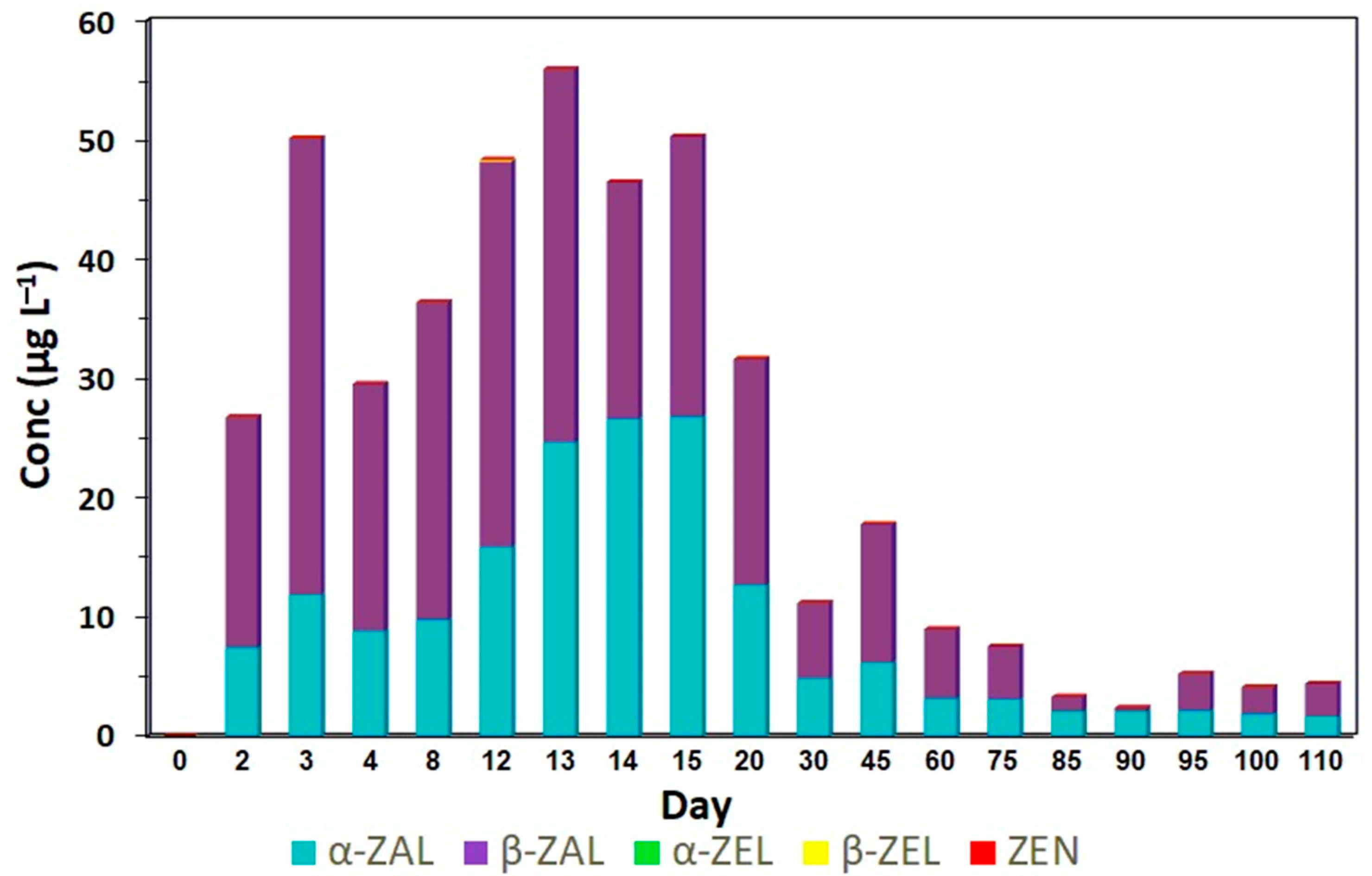
| Resorcylic Acid Lactones | |||||
|---|---|---|---|---|---|
| Day | Zearalenone | α-Zearalenol | β-Zearalenol | Zeranol | Taleranol |
| 0 | ND | ND | ND | ND | ND |
| 2 | ND | ND | ND | 7.13 defgh | 21.35 abc |
| 3 | ND | ND | ND | 10.35 cdef | 31.40 a |
| 4 | ND | ND | ND | 9.80 cdefg | 27.45 a |
| 8 | ND | ND | ND | 10.86 cde | 26.26 a |
| 12 | ND | ND | ND | 16.66 bc | 31.40 a |
| 13 | ND | ND | ND | 23.60 ab | 29.00 a |
| 14 | ND | ND | ND | 27.24 a | 23.56 ab |
| 15 | ND | ND | ND | 23.90 ab | 21.83 abc |
| 20 | ND | ND | ND | 14.43 cd | 16.78 abcd |
| 30 | ND | ND | ND | 6.80 efgh | 10.50 bcde |
| 45 | ND | ND | ND | 4.61 efgh | 8.56 bcde |
| 60 | ND | ND | ND | 3.77 efgh | 6.97 cde |
| 75 | ND | ND | ND | 2.74 fgh | 3.73 de |
| 85 | ND | ND | ND | 2.43 gh | 1.45 de |
| 90 | ND | ND | ND | 2.15 gh | 1.04 de |
| 95 | ND | ND | ND | 2.08 gh | 2.02 de |
| 100 | ND | ND | ND | 2.11 gh | 2.99 de |
| 110 | ND | ND | ND | 1.42 h | 2.39 de |
| Resorcylic Acid Lactones | |||||
|---|---|---|---|---|---|
| Day | Zearalenone | α-Zearalenol | β-Zearalenol | Zeranol | Taleranol |
| 0 | ND | ND | ND | ND | ND |
| 2 | 1.60 cde | 1.38 bc | 7.32 def | 10.33 abc | 26.37 a |
| 3 | 2.40 ab | 1.92 ab | 11.88 abc | 10.20 abc | 22.63 ab |
| 4 | 2.60 ab | 1.92 ab | 13.70 ab | 6.26 bcd | 14.60 bc |
| 8 | 2.40 ab | 1.60 ab | 12.93 ab | 4.52 cd | 9.60 cd |
| 12 | 1.93 bcd | 1.20 abcd | 10.56 bcd | 6.09 bcd | 10.26 cd |
| 13 | 1.30 e | 0.80 cde | 7.40 cde | 11.16 ab | 14.23 bc |
| 14 | 0.64 f | 0.42 e | 4.17 fg | 15.07 a | 16.32 abc |
| 15 | 0.21 f | ND | 1.60 h | 13.99 a | 10.40 cd |
| 20 | ND | ND | ND | 9.75 abc | 10.49 cd |
| 30 | ND | ND | ND | 5.19 bcd | 6.50 cd |
References
- Makinde, O.A.; Soyelu, O.T.; Aderibigbe, A.O. Effect of Zeranol and estradiol-17â on carcass and sensory characteristics of zero-grazed White Fulani bulls. Niger. J. Anim. Prod. 2020, 47, 110–115. [Google Scholar] [CrossRef]
- Ropejko, K.; Twaruzek, M. Zearalenone and Its Metabolites—General Overview, Occurrence, and Toxicity. Toxins 2021, 13, 35. [Google Scholar] [CrossRef]
- Arrizabalaga-Larrañaga, A.A.; Linders, R.; Blokland, M.H.; Sterk, S. Occurrence of resorcyclic acid lactones in porcine urine: Discrimination between illegal use and contamination. Food Addit. Contam. Part A 2023, 40, 838–851. [Google Scholar] [CrossRef]
- Qaid, M.M.; Abdoun, K.A. Safety and concerns of hormonal application in farm animal production: A review. J. Appl. Anim. Res. 2022, 50, 426–439. [Google Scholar] [CrossRef]
- Ronquillo, M.G.; Hernandez, J.C.A. Antibiotic and synthetic growth promoters in animal diets: Review of impact and analytical methods. Food Control 2017, 72, 255–267. [Google Scholar] [CrossRef]
- Brasil, Instrução Normativa n.º 55, de 01 de Dezembro de 2011. Proibe a Importação, a Produção, a Comercialização e o uso de Substâncias Naturais ou Artificiais, com Atividade Anabolizantes Hormonais, para fins de Crescimento e Ganho de peso em Bovinos de Abate. Off. Gaz. Fed. Govern. 2011. Available online: https://www.gov.br/agricultura/pt-br/assuntos/inspecao/produtos-animal/plano-de-nacional-de-controle-de-residuos-e-contaminantes (accessed on 17 April 2025).
- European Commission. Commission Delegated Regulation (EU) 2022/1644 of 7 July 2022, supplementing Regulation (EU) 2017/625 of the European Parliament and of the Council with specific requirements for the performance of official controls on he use of pharmacologically active substances authorised as veterinary medicinal products or as feed additives and of prohibited or unauthorised pharmacologically active substances and residues thereof. Off. J. Europ. Union 2022, L248, 3–17. Available online: http://data.europa.eu/eli/reg_del/2022/1644/oj (accessed on 7 May 2025).
- Widodo, O.S.; Uno, S.; Kokushi, E.; Yamato, O.; Mardianto, M.F.F.; Shinya, U.; Kano, Y.; Kawashima, C.; Fushimi, Y.; Ono, T.; et al. Exposure of Cattle Breeding Herds to Naturally Co-Contaminated Zearalenone and Deoxynivalenol: The Relevance of a Urinary Mycotoxin Monitoring System for Herd Health and Food Safety. Toxins 2024, 16, 402. [Google Scholar] [CrossRef] [PubMed]
- Snethen, C.; Fedoruk, M.; Ahrens, E.; Sobolevskii, T.; Avliyakulov, N.; Johnson, B. Surveillance of Anabolic Agent Residues in US Meat Supply by Liquid Chromatography with High-Resolution Tandem Mass Spectrometry. Drug Test. Anal. 2025. [Google Scholar] [CrossRef]
- Salvat, A.E.; Balbuena, O.; Ricca, A.; Comerio, R.M.; Rosello Brajovich, J.E.; Rojas, D.; Berretta, M.F.; Delssin, E.; Bedascarrasbure, E.; Salerno, J.C. Presencia de zearalenona en pasturas del este de Chaco. Rev. Investig. Agropecu. 2013, 39, 31–36. [Google Scholar]
- Biscoto, G.L.; Salvato, L.A.; Alvarenga, E.R.; Dias, R.R.S.; Pinheiro, G.R.G.; Rodrigues, M.P.; Pinto, P.N.; Freitas, R.P.; Keller, K.M. Mycotoxins in Cattle Feed and Feed Ingredients in Brazil: A Five-Year Survey. Toxins 2022, 14, 552. [Google Scholar] [CrossRef]
- Han, X.; Huangfu, B.; Xu, T.; Xu, W.; Asakiya, C.; Huang, K.; He, X. Research Progress of Safety of Zearalenone: A Review. Toxins 2022, 14, 386. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM); Knutsen, H.-K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Edler, L.; et al. Scientific opinion on risk for animal health related to the presence of Zearalenone and its modified forms in feed. EFSA J. 2017, 15, 4851. [Google Scholar] [CrossRef]
- Raí, A.; Das, M.; Tripathi, A. Occurrence and toxicity of a fusarium mycotoxin, Zearalenone. Crit. Rev. Food. Sci. Nutr. 2019, 60, 2710–2729. [Google Scholar] [CrossRef] [PubMed]
- Zinedine, A.; Soriano, J.M.; Moltó, J.C.; Mañes, J. Review on the toxicity, occurrence, metabolism, detoxification, regulations and intake of Zearalenone: An oestrogenic mycotoxin. Food Chem. Toxicol. 2007, 45, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Takemura, H.; Shim, J.Y.; Sayama, K.; Tsubura, A.; Zhu, B.T.; Shimoi, K. Characterization of the estrogenic activities of zearalenone and zeranol in vivo and in vitro. J. Steroid Biochem. Mol. Biol. 2007, 103, 170–177. [Google Scholar] [CrossRef]
- Dänicke, S.; Keese, C.; Meyer, U.; Starke, A.; Kinoshita, A.; Rehage, J. Zearalenone (ZEN) metabolism and residue concentrations in physiological specimens of dairy cows exposed long-term to ZEN-contaminated diets differing in concentrate feed proportions. Arch. Anim. Nutrit. 2014, 68, 492–506. [Google Scholar] [CrossRef]
- Launay, F.M.; Ribeiro, L.; Alves, P.; Vozikis, V.; Tsitsamis, S.; Alfredsson, G.; Sterk, S.S.; Blokland, M.; Iitia, A.; Lövgren, T.; et al. Prevalence of zeranol, taleranol and Fusarium spp. toxins in urine: Implications for the control of zeranol abuse in the European Union. Food Addit. Contam. 2004, 21, 833–839. [Google Scholar] [CrossRef]
- Falkauskas, R.; Bakutis, B.; Jovaišienė, J.; Vaičiulienė, G.; Gerulis, G.; Kerziene, S.; Jovaišienė, I.; Jacevičius, E.; Baliukoniene, V. Zearalenone and its metabolites inblood serum, urine, and milk of dairy cows. Animals 2022, 12, 1651. [Google Scholar] [CrossRef]
- Kleinova, M.; Zollner, P.; Kahlbacher, H.; Hochsteiner, W.; Lindner, W. Metabolic profiles of the mycotoxin zearalenone and of the growth promoter Zeranol in urine, liver, and muscle of heifers. J. Agricult. Food Chem. 2002, 50, 4769–4776. [Google Scholar] [CrossRef] [PubMed]
- Takagi, M.; Uno, S.; Kokushi, E.; Shiga, S.; Mukai, S.; Kuriyagawa, T.; Takagaki, K.; Hasunuma, H.; Matsumoto, D.; Okamoto, K.; et al. Measurement of urinary zearalenone concentrations for monitoring natural feed contamination in cattle herds: On-farm trials1. J. Anim Sci. 2011, 89, 287–296. [Google Scholar] [CrossRef]
- Gençer, N.; Ergün, A.; Demir, D. In vitro effects of some anabolic compounds on erythrocyte carbonic anhydrase I and II. J. Enzyme Inhib. Med. Chem. 2011, 27, 208–210. [Google Scholar] [CrossRef][Green Version]
- Blokland, M.H.; Sterk, S.S.; Stephany, R.W.; Launay, F.M.; Kennedy, D.G.; van Ginkel, L.A. Determination of resorcylic acid lactones in biological samples by GC–MS. Discrimination between illegal use and contamination with fusarium toxins. Anal. Bioanal. Chem. 2006, 384, 1221–1227. [Google Scholar] [CrossRef] [PubMed]
- Teqja, S.; Mavromati, J. Resorcylic Acid Lactones Interpretation, Discrimination Abuse or Contamination through the Statistical Model. Sch. J. Agric. Vet. Sci. 2024, 11, 77–81. [Google Scholar] [CrossRef]
- Lega, F.; Angeletti, R.; Stella, R.; Rigoni, L.; Biancotto, G.; Giusepponi, D.; Moretti, S.; Saluti, G.; Galarini, R. Abuse of anabolic agents in beef cattle: Could bile be a possible alternative matrix? Food Chem. 2017, 229, 188–197. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission Implementing Regulation (EU) 2023/2783 of 14 December 2023, laying down the methods of sampling and analysis for the control of the levels of plant toxins in food and repealing Regulation (EU) 2015/705. Off. J. Europ. Union 2023, 1–13. Available online: http://data.europa.eu/eli/reg_impl/2023/2783/oj (accessed on 7 May 2025).
- Anastassiades, M.; Lehotay, S.J.; Stajnbaher, D.; Schenck, F.J. Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. J. AOAC Int. 2003, 86, 412–431. [Google Scholar] [CrossRef]
- McConway, K.J.; Jones, M.C.; Taylor, P.C. Statistical Modelling Using Genstat; Arnold in Association with the Open University: London, UK, 1999; pp. 65–102. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 7 May 2025).
- Wood, S.N.; Augustin, N.H. GAMs with integrated model selection using penalized regression splines and applications to environmental modelling. Ecol. Model. 2002, 157, 157–177. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomes, R.S.; Santos, V.G.d.; Silva, C.J.d.; Simões, A.M.N.; Santos, E.A.d.; Lana, M.A.G.; Keller, K.M.; Blokland, M.; Arrizabalaga-Larrañaga, A.; Nicolino, R.R.; et al. Differentiating Zeranol Implant Abuse and Fusarium spp. Toxin-Contaminated Corn Intake by Detection and Quantification of Resorcylic Acid Lactones in Bovine Urine. Toxins 2025, 17, 347. https://doi.org/10.3390/toxins17070347
Gomes RS, Santos VGd, Silva CJd, Simões AMN, Santos EAd, Lana MAG, Keller KM, Blokland M, Arrizabalaga-Larrañaga A, Nicolino RR, et al. Differentiating Zeranol Implant Abuse and Fusarium spp. Toxin-Contaminated Corn Intake by Detection and Quantification of Resorcylic Acid Lactones in Bovine Urine. Toxins. 2025; 17(7):347. https://doi.org/10.3390/toxins17070347
Chicago/Turabian StyleGomes, Rafael Silva, Vanessa Gonçalves dos Santos, Carlos Juliano da Silva, Amanda Martinez Nagato Simões, Eliene Alves dos Santos, Mary Ane Gonçalves Lana, Kelly Moura Keller, Marco Blokland, Ane Arrizabalaga-Larrañaga, Rafael Romero Nicolino, and et al. 2025. "Differentiating Zeranol Implant Abuse and Fusarium spp. Toxin-Contaminated Corn Intake by Detection and Quantification of Resorcylic Acid Lactones in Bovine Urine" Toxins 17, no. 7: 347. https://doi.org/10.3390/toxins17070347
APA StyleGomes, R. S., Santos, V. G. d., Silva, C. J. d., Simões, A. M. N., Santos, E. A. d., Lana, M. A. G., Keller, K. M., Blokland, M., Arrizabalaga-Larrañaga, A., Nicolino, R. R., Souza, M. R. d., Figueiredo, T. C. d., Sterk, S., & Cançado, S. d. V. (2025). Differentiating Zeranol Implant Abuse and Fusarium spp. Toxin-Contaminated Corn Intake by Detection and Quantification of Resorcylic Acid Lactones in Bovine Urine. Toxins, 17(7), 347. https://doi.org/10.3390/toxins17070347






