Efficacy and Safety of Letibotulinum Toxin A for the Treatment of Melasma in Two Different Dilutions: A Randomized Double-Blind Split-Face Study
Abstract
1. Introduction
- Botulinum Toxin and Skin Pigmentation
- Melasma Clinical Research
- Significance of this Study
2. Results
2.1. Demographic Data
2.2. Outcomes
- Hemi-modified Melasma Area and Severity Index (Hemi-mMASI);
- Brown spot count via VISIA®;
- Adverse events.
- Red area via VISIA®;
- Sebum level (Sebumeter®);
- Mean pore area and volume (Antera 3D®);
- Subjective evaluation by dermatologists;(Investigator Global Aesthetic Improvement Scale (IGAS));
- Subjective evaluation by participants (Patient Global Assessment (PGA)).
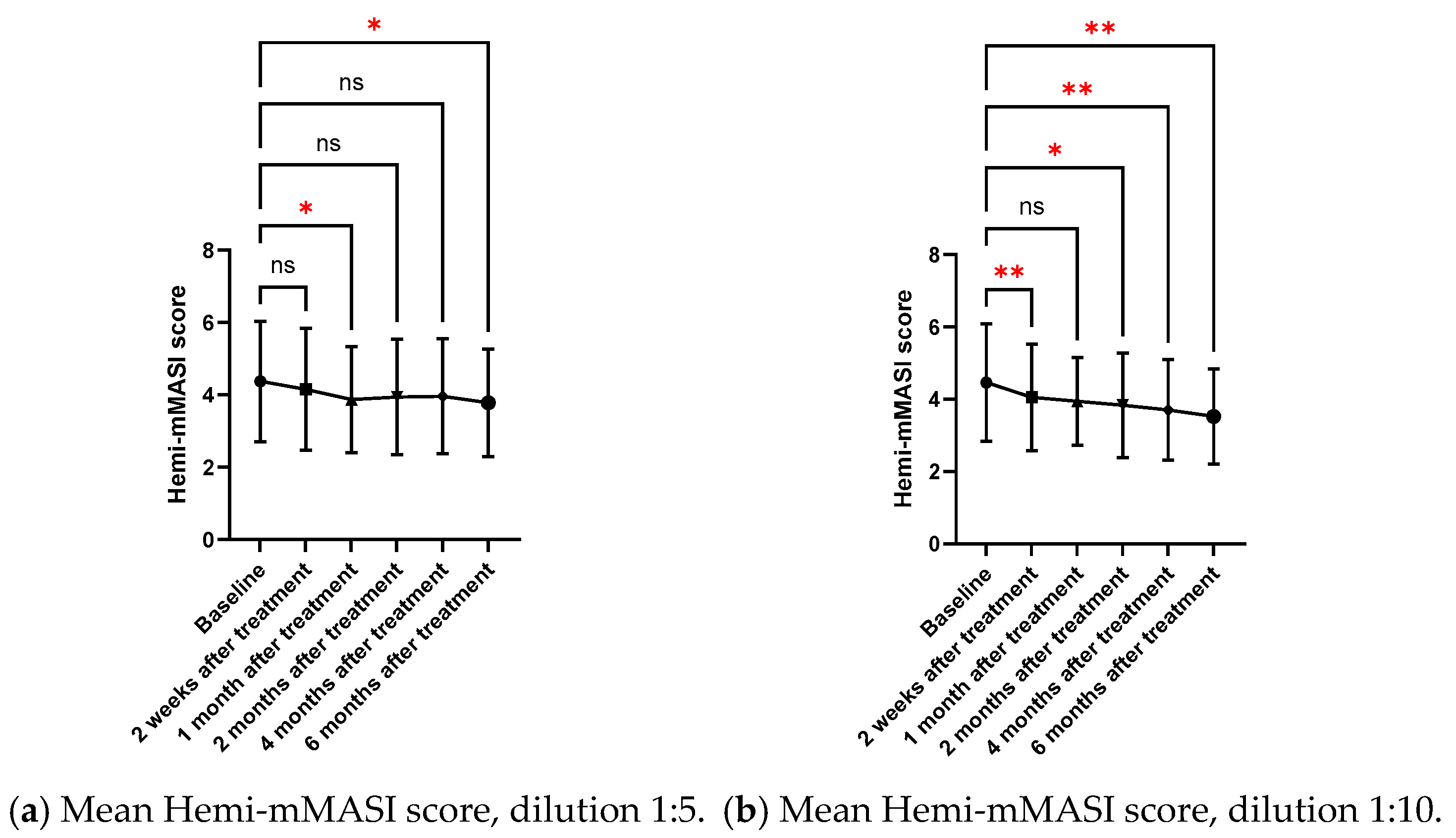
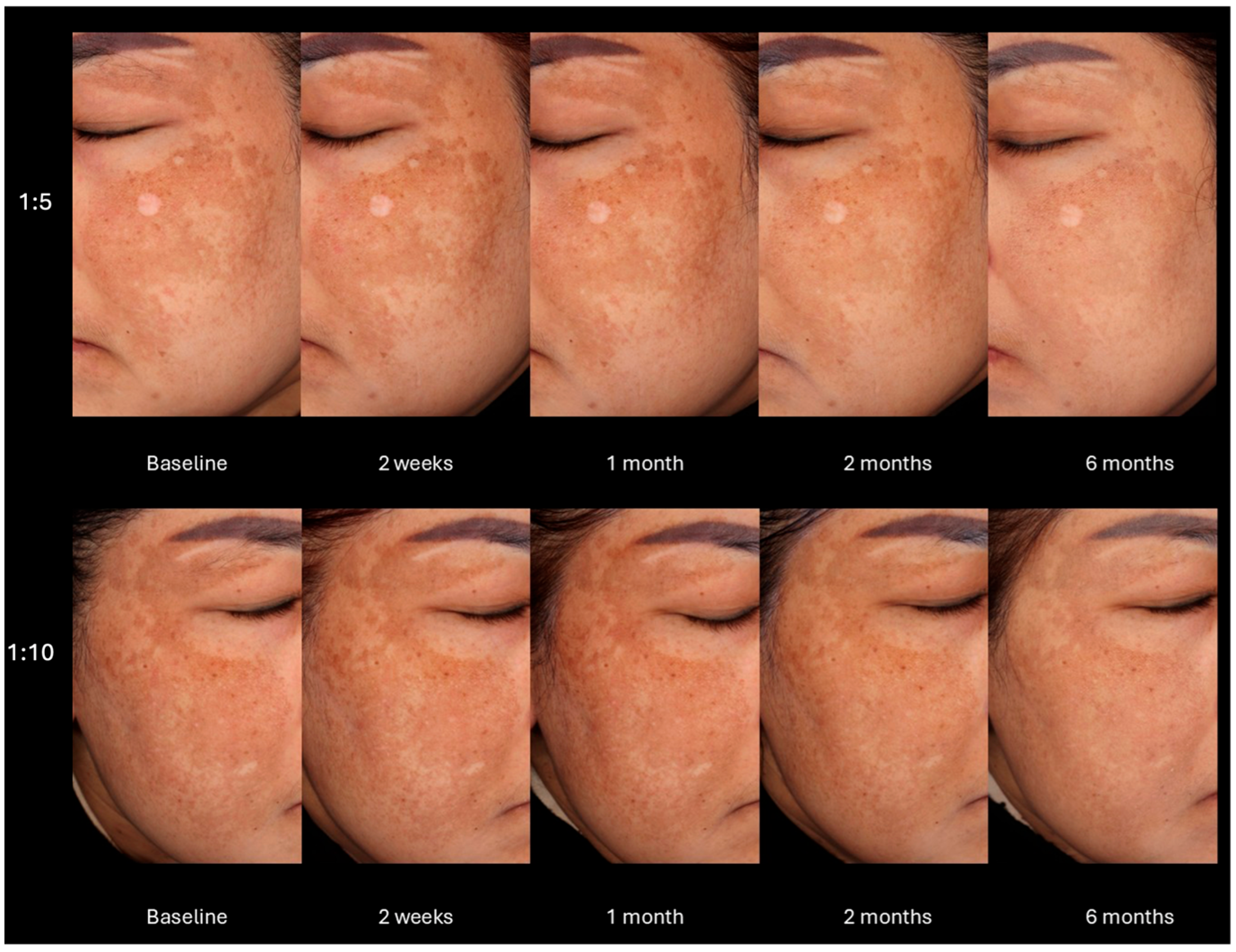
2.2.1. Mean Hemi-MASI Modified Melasma Area and Severity Index (Hemi-mMASI) in the Melasma Area
2.2.2. Brown Spots and Red Areas Assessed with VISIA® in the Melasma Area
2.2.3. Subgroup Analysis
2.2.4. Sebum Level from Measurement with Sebumeter® in the Blemish Area
2.2.5. Mean Pore Area and Mean Pore Volume Based on Antera 3D®
2.2.6. Subjective Evaluation of Treatment Results from Photographs by Two Dermatologists
2.2.7. Subjective Evaluation of Treatment Results by Volunteers
2.3. Adverse Events
3. Discussion
4. Conclusions
5. Materials and Methods
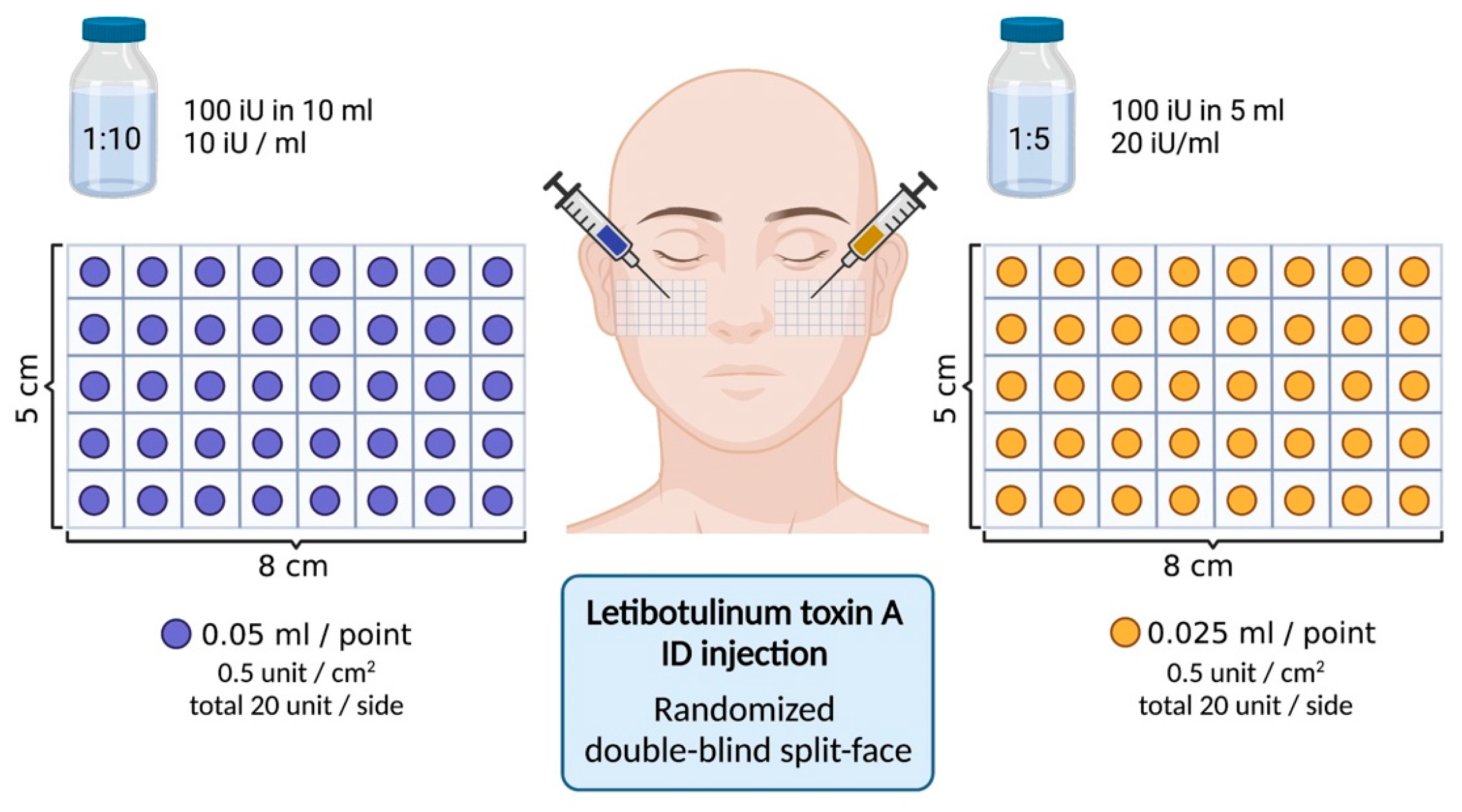
5.1. Study Design and Patient Selection
5.2. Treatment Preparation, Area Selection
5.3. Post-Treatment Care
5.4. Objective Evaluation
5.5. Subjective Evaluation
5.6. Statistical Analysis
6. Study Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chang, M.W. Disorders of hyperpigmentation. Dermatology 2008, 2, 939–964. [Google Scholar]
- Artzi, O.; Horovitz, T.; Bar-Ilan, E.; Shehadeh, W.; Koren, A.; Zusmanovitch, L.; Mehrabi, J.N.; Salameh, F.; Isman Nelkenbaum, G.; Zur, E. The pathogenesis of melasma and implications for treatment. J. Cosmet. Dermatol. 2021, 20, 3432–3445. [Google Scholar] [CrossRef]
- Zhu, J.-W.; Ni, Y.-J.; Tong, X.-Y.; Guo, X.; Wu, X.-P. Activation of VEGF receptors in response to UVB promotes cell proliferation and melanogenesis of normal human melanocytes. Exp. Cell Res. 2020, 387, 111798. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.H.; Kim, Y.C.; Lee, E.-S.; Kang, H.Y. The vascular characteristics of melasma. J. Dermatol. Sci. 2007, 46, 111–116. [Google Scholar] [CrossRef]
- Gilchrest, B.A.; Park, H.-Y.; Eller, M.S.; Yaar, M. Mechanisms of ultraviolet light-induced pigmentation. Photochem. Photobiol. 1996, 63, 1–10. [Google Scholar] [CrossRef] [PubMed]
- González-Molina, V.; Martí-Pineda, A.; González, N. Topical treatments for melasma and their mechanism of action. J. Clin. Aesthetic Dermatol. 2022, 15, 19. [Google Scholar]
- Flynn, T.C. Botulinum toxin: Examining duration of effect in facial aesthetic applications. Am. J. Clin. Dermatol. 2010, 11, 183–199. [Google Scholar] [CrossRef]
- Torres, S.; Hamilton, M.; Sanches, E.; Starovatova, P.; Gubanova, E.; Reshetnikova, T. Neutralizing antibodies to botulinum neurotoxin type A in aesthetic medicine: Five case reports. Clin. Cosmet. Investig. Dermatol. 2013, 7, 11–17. [Google Scholar] [CrossRef]
- Sharma, R.; Zhao, H.; Al-Saleem, F.H.; Ubaid, A.S.; Puligedda, R.D.; Segan, A.T.; Lindorfer, M.A.; Bermudez, R.; Elias, M.; Adekar, S.P. Mechanisms of enhanced neutralization of botulinum neurotoxin by monoclonal antibodies conjugated to antibodies specific for the erythrocyte complement receptor. Mol. Immunol. 2014, 57, 247–254. [Google Scholar] [CrossRef]
- Carruthers, A.; de Almeida, C.J. Botulinum Toxin. Dermatology 2018, 4, 2661–2672. [Google Scholar] [CrossRef]
- Schlessinger, J.; Gilbert, E.; Cohen, J.L.; Kaufman, J. New uses of abobotulinumtoxinA in aesthetics. Aesthetic Surg. J. 2017, 37, S45–S58. [Google Scholar] [CrossRef]
- Peng Chen, Z.; Morris, J.G., Jr.; Rodriguez, R.L.; Wagle Shukla, A.; Tapia-Nunez, J.; Okun, M.S. Emerging opportunities for serotypes of botulinum neurotoxins. Toxins 2012, 4, 1196–1222. [Google Scholar] [CrossRef] [PubMed]
- Friedland, S.; Burde, R.M. Porcelinizing discolorization of the periocular skin following botulinum A toxin injections. J. Neuro-Ophthalmol. Off. J. N. Am. Neuro-Ophthalmol. Soc. 1996, 16, 70–72. [Google Scholar]
- Jung, J.-A.; Kim, B.-J.; Kim, M.-S.; You, H.-J.; Yoon, E.-S.; Dhong, E.-S.; Park, S.-H.; Kim, D.-W. Protective effect of botulinum toxin against ultraviolet-induced skin pigmentation. Plast. Reconstr. Surg. 2019, 144, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Erdil, D.; Manav, V.; Türk, C.B.; Kara Polat, A.; Koku Aksu, A.E. The clinical effect of botulinum toxin on pigmentation. Int. J. Dermatol. 2023, 62, 250–256. [Google Scholar] [CrossRef]
- Vachiramon, V.; Kositkuljorn, C.; Leerunyakul, K.; Rattananukrom, T.; Jurairattanaporn, N. A Study of Botulinum Toxin A for Ultraviolet-Induced Hyperpigmentation: A Randomized Controlled Trial. Dermatol. Surg. 2021, 47, e174–e178. [Google Scholar] [CrossRef] [PubMed]
- Vachiramon, V.; Anuntrangsee, T.; Palakornkitti, P.; Jurairattanaporn, N.; Harnchoowong, S. Incobotulinum Toxin Type A for Treatment of Ultraviolet-B-Induced Hyperpigmentation: A Prospective, Randomized, Controlled Trial. Toxins 2022, 14, 417. [Google Scholar] [CrossRef]
- Jurairattanaporn, N.; Palakornkitti, P.; Anuntrangsee, T.; Vachiramon, V. Study of botulinum toxin type A for the treatment of ultraviolet B--induced hyperpigmentation: A prospective, randomized, controlled trial. J. Cosmet. Dermatol. 2022, 21, 3343–3350. [Google Scholar] [CrossRef]
- Carruthers, J.; Carruthers, A. The effect of full-face broadband light treatments alone and in combination with bilateral crow’s feet botulinum toxin type A chemodenervation. Dermatol. Surg. 2004, 30, 355–366. [Google Scholar] [CrossRef]
- Yamauchi, P.; Lask, G.; Lowe, N. Botulinum toxin type A gives adjunctive benefit to periorbital laser resurfacing. J. Cosmet. Laser Ther. 2004, 6, 145–148. [Google Scholar] [CrossRef]
- Gugerell, A.; Kober, J.; Schmid, M.; Buchberger, E.; Kamolz, L.-P.; Keck, M. Botulinum toxin A: Dose-dependent effect on reepithelialization and angiogenesis. Plast. Reconstr. Surg.-Glob. Open 2016, 4, e837. [Google Scholar] [CrossRef]
- Dayan, S.H.; Pritzker, R.N.; Arkins, J.P. A new treatment regimen for rosacea: OnabotulinumtoxinA. J. Drugs Dermatol. 2012, 11, e76–e79. [Google Scholar] [PubMed]
- Bloom, B.S.; Payongayong, L.; Mourin, A.; Goldberg, D.J. Impact of intradermal abobotulinumtoxinA on facial erythema of rosacea. Dermatol. Surg. 2015, 41, S9–S16. [Google Scholar] [CrossRef] [PubMed]
- Park, K.Y.; Hyun, M.Y.; Jeong, S.Y.; Kim, B.J.; Kim, M.N.; Hong, C.K. Botulinum toxin for the treatment of refractory erythema and flushing of rosacea. Dermatology 2015, 230, 299–301. [Google Scholar] [CrossRef]
- Dayan, S.H.; Ashourian, N.; Cho, K. A Pilot, Double-Blind, Placebo-Controlled Study to Assess the Efficacy and Safety of IncobotulinumtoxinA Injections in the Treatment of Rosacea. J. Drugs Dermatol. JDD 2017, 16, 549–554. [Google Scholar] [PubMed]
- Park, K.Y.; Kwon, H.J.; Kim, J.M.; Jeong, G.J.; Kim, B.J.; Seo, S.J.; Kim, M.N. A pilot study to evaluate the efficacy and safety of treatment with botulinum toxin in patients with recalcitrant and persistent erythematotelangiectatic rosacea. Ann. Dermatol. 2018, 30, 688–693. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, J.H.; Cheon, H.I.; Hur, M.S.; Han, S.H.; Lee, Y.W.; Choe, Y.B.; Ahn, K.J. Assessment of skin physiology change and safety after intradermal injections with botulinum toxin: A randomized, double-blind, placebo-controlled, split-face pilot study in rosacea patients with facial erythema. Dermatol. Surg. 2019, 45, 1155–1162. [Google Scholar] [CrossRef]
- Al-Niaimi, F.; Glagoleva, E.; Araviiskaia, E. Pulsed dye laser followed by intradermal botulinum toxin type-A in the treatment of rosacea-associated erythema and flushing. Dermatol. Ther. 2020, 33, e13976. [Google Scholar] [CrossRef]
- Zhang, H.; Tang, K.; Wang, Y.; Fang, R.; Sun, Q. Use of botulinum toxin in treating rosacea: A systematic review. Clin. Cosmet. Investig. Dermatol. 2021, 14, 407–417. [Google Scholar] [CrossRef]
- Calvisi, L.; Diaspro, A.; Sito, G. Microbotox: A prospective evaluation of dermatological improvement in patients with mild--to--moderate acne and erythematotelangiectatic rosacea. J. Cosmet. Dermatol. 2022, 21, 3747–3753. [Google Scholar] [CrossRef]
- Tong, Y.; Luo, W.; Gao, Y.; Liu, L.; Tang, Q.; Wa, Q. A randomized, controlled, split—Face study of botulinum toxin and broadband light for the treatment of erythematotelangiectatic rosacea. Dermatol. Ther. 2022, 35, e15395. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Liu, C.; Liu, W.; Luo, J.; Cheng, S.; Mu, X. Botulinum toxin A alleviates persistent erythema and flushing in patients with erythema telangiectasia rosacea. Dermatol. Ther. 2022, 12, 2285–2294. [Google Scholar] [CrossRef] [PubMed]
- Suksantilap, S.; Chalermchai, T.; Paichitrojjana, A. The Efficacy of Intradermal Injection of Botulinum Toxin A In The Treatment of Dermal and Mixed Type, Melasma. In Proceedings of the 4th National Academic Conference and Research Presentation Graduate School Conference 2022 iHappiness: Sustainable Happiness and Good Quality of Life in the Digital Age (Suan Sunandha Rajabhat University, 2022), Bangkok, Thailand, 27 May 2022. [Google Scholar]
- Thanasarnaksorn, W.; Supasiri, T.; Panich, U.; Thanachaiphiwat, S.; Salakshna, N. Intradermal Botulinum Toxin A for Melasma: A Randomized Split—Face Study Trial and In Vitro Study of Its Antimelanogenic Effect. Dermatol. Ther. 2025, 2025, 5550483. [Google Scholar] [CrossRef]
- Kania, B.; Lolis, M.; Goldberg, D. Melasma Management: A Comprehensive Review of Treatment Strategies Including BTX—A. J. Cosmet. Dermatol. 2025, 24, e16669. [Google Scholar] [CrossRef]
- Carmichael, N.M.E.; Dostrovsky, J.O.; Charlton, M.P. Peptide-mediated transdermal delivery of botulinum neurotoxin type A reduces neurogenic inflammation in the skin. Pain 2010, 149, 316–324. [Google Scholar] [CrossRef]
- Pomerantz, H.; Christman, M.P.; Bloom, B.S.; Lederhandler, M.; Feng, H.; Holmes, J.; Geronemus, R.G. Dynamic optical coherence tomography of cutaneous blood vessels in melasma and vessel response to oral tranexamic acid. Lasers Surg. Med. 2021, 53, 861–864. [Google Scholar] [CrossRef]
- Kim, Y.J.; Kim, J.-H.; Lee, K.-J.; Choi, M.-M.; Kim, Y.H.; Rhie, G.-e.; Yoo, C.-K.; Cha, K.; Shin, N.-R. Botulinum neurotoxin type A induces TLR2-mediated inflammatory responses in macrophages. PLoS ONE 2015, 10, e0120840. [Google Scholar] [CrossRef]
- Kellogg, D.L., Jr. In vivo mechanisms of cutaneous vasodilation and vasoconstriction in humans during thermoregulatory challenges. J. Appl. Physiol. 2006, 100, 1709–1718. [Google Scholar] [CrossRef]
- Kellogg, D.L., Jr.; Pérgola, P.E.; Piest, K.L.; Kosiba, W.A.; Crandall, C.G.; Grossmann, M.; Johnson, J.M. Cutaneous active vasodilation in humans is mediated by cholinergic nerve cotransmission. Circ. Res. 1995, 77, 1222–1228. [Google Scholar] [CrossRef]
- Zhou, J.; Feng, J.-Y.; Wang, Q.; Shang, J. Calcitonin gene-related peptide cooperates with substance P to inhibit melanogenesis and induces apoptosis of B16F10 cells. Cytokine 2015, 74, 137–144. [Google Scholar] [CrossRef]
- Filipović, B.; Matak, I.; Bach-Rojecky, L.; Lacković, Z. Central action of peripherally applied botulinum toxin type A on pain and dural protein extravasation in rat model of trigeminal neuropathy. PLoS ONE 2012, 7, e29803. [Google Scholar] [CrossRef] [PubMed]
- Picelli, A.; Tamburin, S.; Di Censo, R.; Smania, N.; Filippetti, M. Does the Diffusion Profile Differ Between Botulinum Toxin Type a Formulations? Implications for the Management of Post-Stroke Spasticity. Toxins 2024, 16, 480. [Google Scholar] [CrossRef] [PubMed]
- Brodsky, M.A.; Swope, D.M.; Grimes, D. Diffusion of botulinum toxins. Tremor Other Hyperkinetic Mov. 2012, 2, tre-02-85-417-1. [Google Scholar] [CrossRef]
- Klein, A.W.; Carruthers, A.; Fagien, S.; Lowe, N.J. Comparisons among botulinum toxins: An evidence-based review. Plast. Reconstr. Surg. 2008, 121, 413e–422e. [Google Scholar] [CrossRef]
- Hopewell, S.; Chan, A.-W.; Collins, G.S.; Hróbjartsson, A.; Moher, D.; Schulz, K.F.; Tunn, R.; Aggarwal, R.; Berkwits, M.; Berlin, J.A. CONSORT 2025 statement: Updated guideline for reporting randomised trials. Lancet 2025, 405, 1633–1640. [Google Scholar] [CrossRef] [PubMed]
- Al-Fouzan, A.F.; Mokeem, L.S.; Al-Saqat, R.T.; Alfalah, M.A.; Alharbi, M.A.; Al-Samary, A.E. Botulinum Toxin for the Treatment of Gummv Smile. J. Contemp. Dent. Pract. 2017, 18, 474–478. [Google Scholar] [CrossRef]
- Gong, X.; Tang, H.-N.; Zhang, A.-R.; Wang, Z.; Tang, Z.-H.; Han, X.-F.; Su, J.-Z. Application of botulinum toxin at the Yonsei point for the treatment of gummy smile: A randomized controlled trial. Plast. Reconstr. Surg. 2024, 153, 711e–721e. [Google Scholar] [CrossRef]
- Hur, M.S.; Youn, K.H.; Kim, H.J. New insight regarding the zygomaticus minor as related to cosmetic facial injections. Clin. Anat. 2018, 31, 974–980. [Google Scholar] [CrossRef]
- Mazzuco, R.; Hexsel, D. Gummy smile and botulinum toxin: A new approach based on the gingival exposure area. J. Am. Acad. Dermatol. 2010, 63, 1042–1051. [Google Scholar] [CrossRef]
- Mekawy, K.M.; Sadek, A.; Seddeik Abdel-Hameed, A.K. Micro-needling versus fractional carbon dioxide laser for delivery of tranexamic acid in the treatment of melasma: A split-face study. J. Cosmet. Dermatol. 2021, 20, 460–465. [Google Scholar] [CrossRef]





| Characteristics | Value (n = 30) | |
|---|---|---|
| Age in years | ||
| Mean ± SD * (min–max) | 47 ± 6.60 (32–62) | |
| Sex, n (%) | ||
| Male | 1 (3%) | |
| Female | 29 (96.67%) | |
| Fitzpatrick Skin type, n (%) | ||
| Type III | 4 (13.33%) | |
| Type IV | 21 (70%) | |
| Type V | 5 (16.67%) | |
| Melasma type, n (%) | Non-telangiectatic | 16 (53.33%) |
| Telangiectatic | 14 (46.67%) | |
| Baseline Hemi-mMASI score | ||
| 1:5 dilution | 4.44 ± 1.82 | |
| 1:10 dilution | 4.66 ± 1.85 | |
| Baseline Hemi-mMASI score | ||
| Non-telangiectatic | 1:5 dilution | 3.78 ± 1.45 |
| 1:10 dilution | 3.95 ± 0.90 | |
| Telangiectatic | 1:5 dilution | 5.20 ± 1.96 |
| 1:10 dilution | 5.48 ± 2.30 | |
| Mean pain score | ||
| Mean ± SD * (min–max) | 1:5 dilution | 5.13 ± 2.29 (min = 0, max = 9) |
| 1:10 dilution | 5.23 ± 2.21(min = 0, max = 9) |
| Follow-Up | Mean Hemi-mMASI (1:5) | Mean Hemi-mMASI (1:10) | p-Value Comparing Two Concentrations |
|---|---|---|---|
| Baseline | 4.38 ± 1.66 | 4.47 ± 1.63 | |
| 2-week follow-up | 4.16 ± 1.69 (p = 0.158) | 4.06 ± 1.48 (p = 0.006) ** | >0.99 |
| 1-month follow-up | 3.87 ± 1.46 (p = 0.049) * | 3.94 ± 1.22 (p = 0.082) | >0.99 |
| 2-month follow-up | 3.95 ± 1.60 (p = 0.080) | 3.84 ± 1.45 (p = 0.013) * | >0.99 |
| 4-month follow-up | 3.97 ± 1.59 (p = 0.100) | 3.70 ± 1.39 (p = 0.002) ** | 0.847 |
| 6-month follow-up | 3.78 ± 1.49 (p = 0.043) * | 3.53 ± 1.32 (p = 0.002) ** | >0.99 |
| Follow-Up | Brown Spots (1:5) | Brown Spots (1:10) | Red Areas (1:5) | Red Areas (1:10) |
|---|---|---|---|---|
| Baseline | 17.93 ± 5.79 | 17.95 ± 5.61 | 13.68 ± 11.77 | 14.69 ± 11.43 |
| 2-week follow-up | 16.88 ± 5.23 | 17.02 ± 5.19 | 14.15 ± 11.31 | 15.10 ± 11.25 |
| (0.131) | (0.203) | (>0.99) | (>0.99) | |
| 1-month follow-up | 16.30 ± 4.99 | 16.41 ± 5.27 | 12.61 ± 11.32 | 14.09 ± 11.16 |
| (0.017) * | (0.007) ** | (>0.99) | (>0.99) | |
| 2-month follow-up | 16.24 ± 4.28 | 16.38 ± 4.87 | 12.40 ± 10.66 | 13.05 ± 10.35 |
| (0.001) ** | (0.001) ** | (>0.99) | (0.834) | |
| 4-month follow-up | 16.11 ± 4.61 | 15.94 ± 4.69 | 11.68 ± 10.65 | 12.78 ± 10.14 |
| (0.010) * | (0.001) *** | (0.208) | (0.348) | |
| 6-month follow-up | 15.64 ± 4.48 | 15.5 ± 4.71 | 11.76 ± 10.75 | 12.32 ± 10.45 |
| (0.002) ** | (<0.001) **** | (0.227) | (0.032) * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pongklaokam, J.; Manuskiatti, W.; Wanitphakdeedecha, R.; Maneeprasopchoke, P.; Thongjaroensirikul, P.; Nokdhes, Y.; Abad-Constantino, R.M.R.; Bhorntarakcharoen, W.; Sittiwanaruk, S.; Techapichetvanich, T. Efficacy and Safety of Letibotulinum Toxin A for the Treatment of Melasma in Two Different Dilutions: A Randomized Double-Blind Split-Face Study. Toxins 2025, 17, 349. https://doi.org/10.3390/toxins17070349
Pongklaokam J, Manuskiatti W, Wanitphakdeedecha R, Maneeprasopchoke P, Thongjaroensirikul P, Nokdhes Y, Abad-Constantino RMR, Bhorntarakcharoen W, Sittiwanaruk S, Techapichetvanich T. Efficacy and Safety of Letibotulinum Toxin A for the Treatment of Melasma in Two Different Dilutions: A Randomized Double-Blind Split-Face Study. Toxins. 2025; 17(7):349. https://doi.org/10.3390/toxins17070349
Chicago/Turabian StylePongklaokam, Juthapa, Woraphong Manuskiatti, Rungsima Wanitphakdeedecha, Pitchaya Maneeprasopchoke, Panwadee Thongjaroensirikul, Yanin Nokdhes, Rona Maria R. Abad-Constantino, Woramate Bhorntarakcharoen, Sariya Sittiwanaruk, and Thanya Techapichetvanich. 2025. "Efficacy and Safety of Letibotulinum Toxin A for the Treatment of Melasma in Two Different Dilutions: A Randomized Double-Blind Split-Face Study" Toxins 17, no. 7: 349. https://doi.org/10.3390/toxins17070349
APA StylePongklaokam, J., Manuskiatti, W., Wanitphakdeedecha, R., Maneeprasopchoke, P., Thongjaroensirikul, P., Nokdhes, Y., Abad-Constantino, R. M. R., Bhorntarakcharoen, W., Sittiwanaruk, S., & Techapichetvanich, T. (2025). Efficacy and Safety of Letibotulinum Toxin A for the Treatment of Melasma in Two Different Dilutions: A Randomized Double-Blind Split-Face Study. Toxins, 17(7), 349. https://doi.org/10.3390/toxins17070349






