Molecular Diagnosis in Hymenoptera Allergy: Comparison of Euroline DPA-Dx and ImmunoCAP
Abstract
1. Introduction
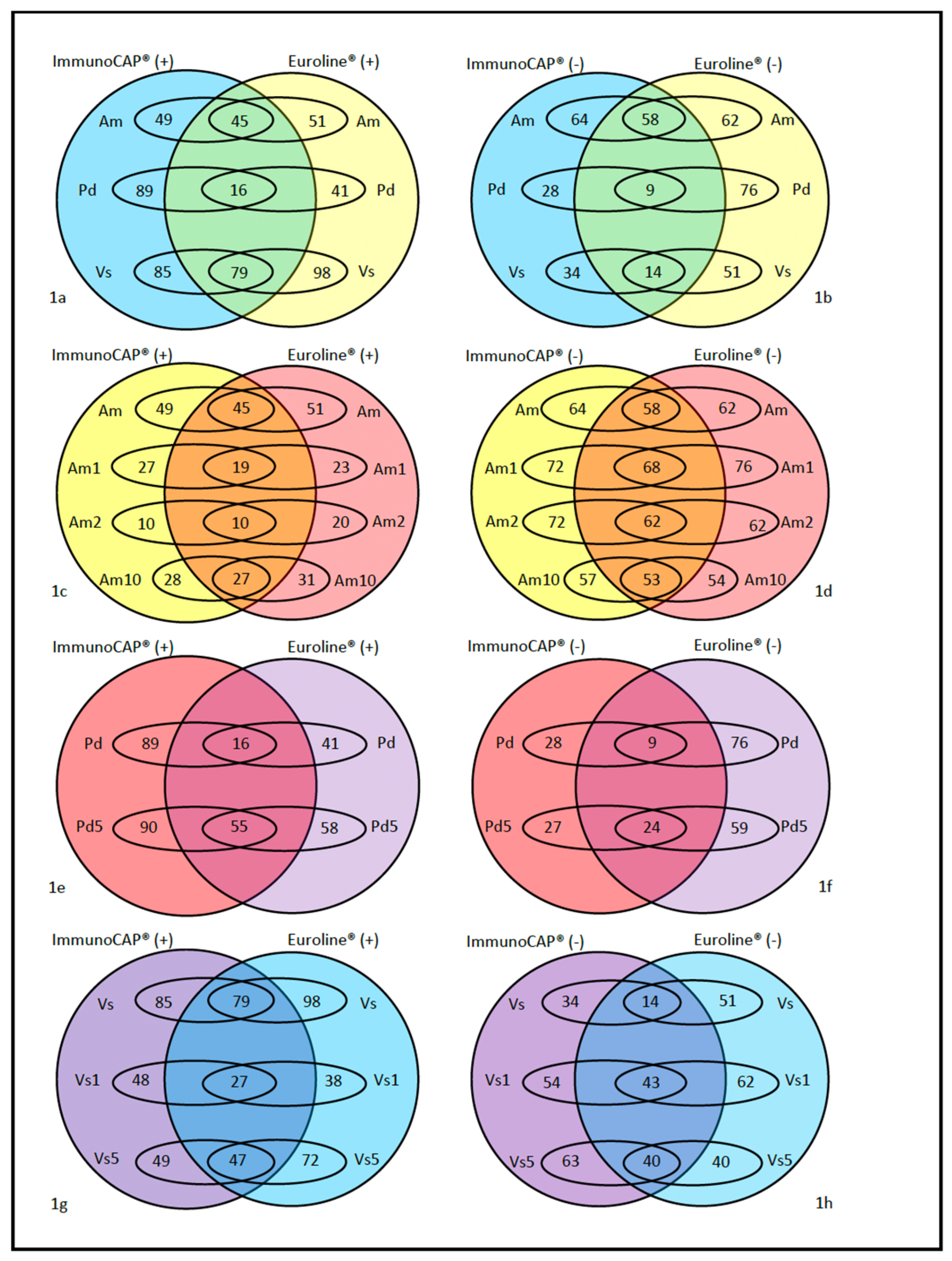
2. Results
2.1. Discrepancies Between Techniques
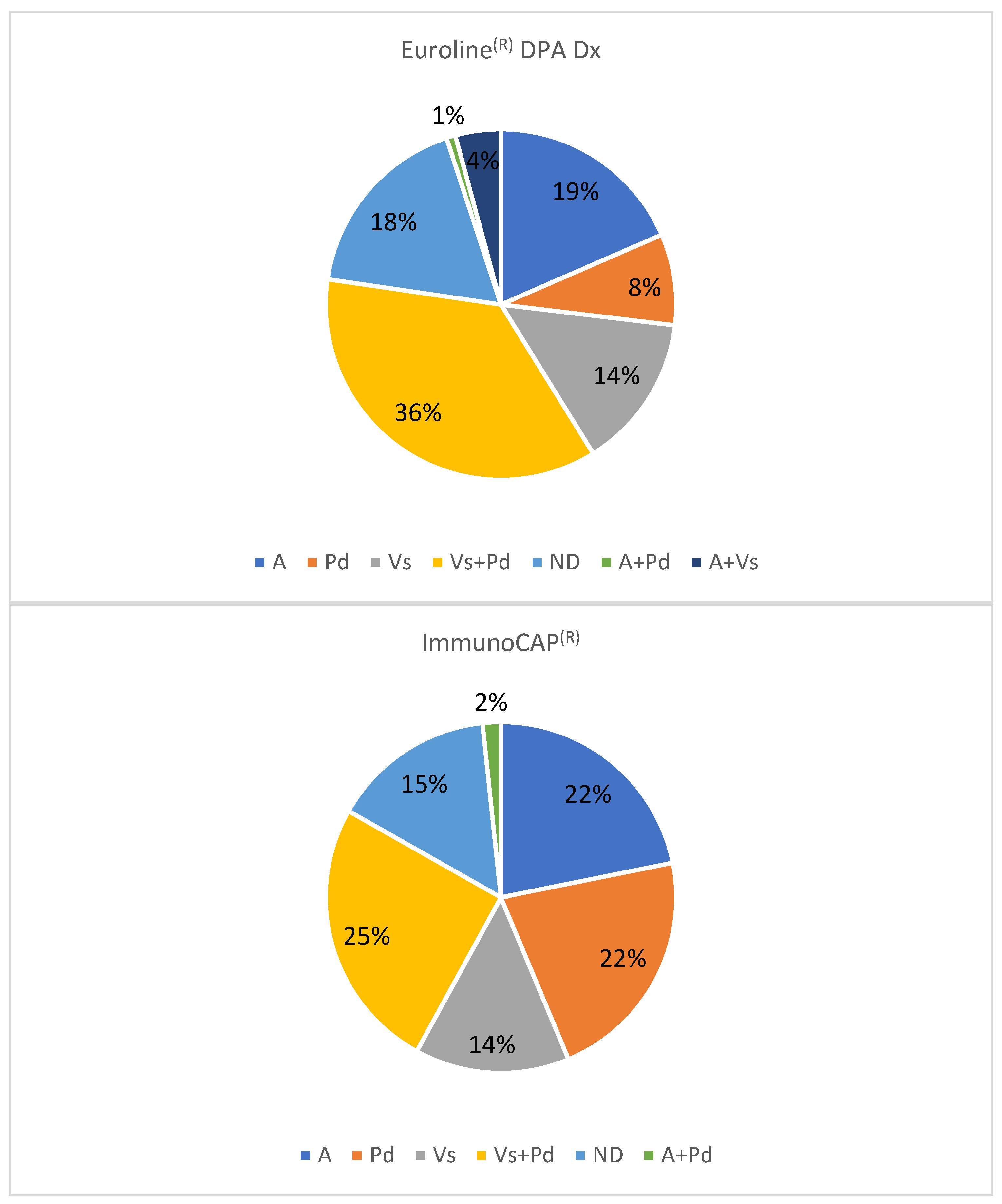
2.2. Other Comparisons
2.3. Other Results
3. Discussion
4. Conclusions
5. Materials and Methods
- The insect identified by the patient, wherever possible, especially if the patient was a beekeeper or described close paper nests.
- The result of skin tests.
- In cases of suspected allergy to Vs or Pd, the presence of sIgE against Am with no other component except Api m 5 was not considered indicative of sensitization to bees but detection of sensitization to vespid dipeptidyl peptidases (Ves v 3 and Pol d 3).
- The cases in which CCD was positive and sIgE was positive for Am and Vs, but with a suspicion of allergy to only one of the insects, were assessed as false positives due to cross-reactivity, and the remaining allergenic components were used to confirm the diagnosis.
- The cases of suspected allergy to Vs or Pd with positive sIgE for hyaluronidase (Api m 2), but with no other positive result for Am, were assessed as false positives to bees due to cross-reactivity.
- The Vs and Pd venoms in ImmunoCAP® are enriched with antigen 5 [7].
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sturm, G.J.; Varga, E.-M.; Roberts, G.; Mosbech, H.; Bilò, M.B.; Akdis, C.A.; Antolín-Amérigo, D.; Cichocka-Jarosz, E.; Gawlik, R.; Jakob, T.; et al. EAACI Guidelines on Allergen Immunotherapy: Hymenoptera Venom Allergy. Allergy 2018, 73, 744–764. [Google Scholar] [CrossRef] [PubMed]
- Alfaya, T.; Soriano, V.; Soto, T.; Vega, A.; Vega, J.M.; Alonso, A.; Antolín, D.; Carballada, F.J.; Dominguez, C.; Gutierrez, D.; et al. Key Issues in Hymenoptera Venom Allergy: An Update. J. Investig. Allergol. Clin. Immunol. 2017, 27, 19–31. [Google Scholar] [CrossRef]
- Berge, M.; Bertilsson, L.; Hultgren, O.; Hugosson, S.; Saber, A. Qualitative and Quantitative Comparison of Allergen Component-Specific to Birch and Grass Analyzed by ImmunoCAP Assay and Euroline Immunoblot Test. Eur. Ann. Allergy Clin. Immunol. 2023, 55, 68–77. [Google Scholar] [CrossRef]
- Fraia, M.D.; Arasi, S.; Castelli, S.; Dramburg, S.; Potapova, E.; Villalta, D.; Tripodi, S.; Sfika, I.; Zicari, A.M.; Villella, V.; et al. A New Molecular Multiplex IgE Assay for the Diagnosis of Pollen Allergy in Mediterranean Countries: A Validation Study. Clin. Exp. Allergy 2019, 49, 341–349. [Google Scholar] [CrossRef]
- Wongpiyabovorn, J.; Suratannon, N.; Boonmee, S.; Chatchatee, P. Comparison of Specific IgE Detection by Immunoblotting and Fluorescence Enzyme Assay with in Vivo Skin Prick Test. Asian Pac. J. Allergy 2017, 36, 159–165. [Google Scholar] [CrossRef]
- Jovanovic, D.; Peric-Popadic, A.; Andrejevic, S.; Stojanovic, M.; Bonaci-Nikolic, B. The Diagnostic Importance of Recombinant Allergen IgE Testing in Patients with Hymenoptera Venom Allergy: Comparison of Two Methods. Iran. J. Allergy Asthma Immunol. 2021, 20, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Vos, B.; Köhler, J.; Müller, S.; Stretz, E.; Ruëff, F.; Jakob, T. Spiking Venom with RVes v 5 Improves Sensitivity of IgE Detection in Patients with Allergy to Vespula Venom. J. Allergy Clin. Immunol. 2012, 131, 1225–1227. [Google Scholar] [CrossRef]
- Monsalve, R.I.; Vega, A.; Marqués, L.; Miranda, A.; Fernández, J.; Soriano, V.; Cruz, S.; Domínguez-Noche, C.; Sánchez-Morillas, L.; Armisen-Gil, M.; et al. Component-resolved Diagnosis of Vespid Venom-allergic Individuals: Phospholipases and Antigen 5s Are Necessary to Identify Vespula or Polistes Sensitization. Allergy 2012, 67, 528–536. [Google Scholar] [CrossRef]
- Ruiz-Leon, B.; Serrano, P.; Vidal, C.; Moreno-Aguilar, C. Management of Double Sensitization to Vespids in Europe. Toxins 2022, 14, 126. [Google Scholar] [CrossRef]
- Cabrera, C.; Palacios-Cañas, A.; Joyanes-Romo, J.; Urra, J.; Mur, P. Basophil Activation Test as Alternative Method to CAP-Inhibition in Patients with Double Sensitization to Vespid Venoms. Mol. Immunol. 2022, 149, 59–65. [Google Scholar] [CrossRef]
- Hamilton, R.G.; Hemmer, W.; Nopp, A.; Kleine-Tebbe, J. Advances in IgE Testing for Diagnosis of Allergic Disease. J. Allergy Clin. Immunol. Pract. 2020, 8, 2495–2504. [Google Scholar] [CrossRef]
- Blank, S.; Bilò, M.B.; Grosch, J.; Schmidt-Weber, C.B.; Ollert, M.; Jakob, T. Marker Allergens in Hymenoptera Venom allergy—Characteristics and Potential Use in Precision Medicine. Allergo J. Int. 2021, 30, 26–38. [Google Scholar] [CrossRef]
- Goikoetxea, M.; Sanz, M.; García, B.; Mayorga, C.; Longo, N.; PM, P.G. Recommendations for the Use of In Vitro Methods to Detect Specific Immunoglobulin E: Are They Comparable? J. Investig. Allergol. Clin. Immunol. 2013, 23, 448–454. [Google Scholar]
- Schrautzer, C.; Bokanovic, D.; Hemmer, W.; Lang, R.; Hawranek, T.; Schwarz, I.; Aberer, W.; Sturm, E.; Sturm, G.J. Sensitivity and Specificity of Hymenoptera Allergen Components Depend on the Diagnostic Assay Employed. J. Allergy Clin. Immun. 2016, 137, 1603–1605. [Google Scholar] [CrossRef]
- Jakob, T.; Spillner, E. Comparing Sensitivity of Hymenoptera Allergen Components on Different Diagnostic Assay Systems: Comparing Apples and Oranges? J. Allergy Clin. Immun. 2017, 139, 1066–1067. [Google Scholar] [CrossRef] [PubMed]
- Pascal, M.; Moreno, C.; Dávila, I.; Tabar, A.I.; Bartra, J.; Labrador, M.; Luengo, O. Integration of in Vitro Allergy Test Results and Ratio Analysis for the Diagnosis and Treatment of Allergic Patients (INTEGRA). Clin. Transl. Allergy 2021, 11, e12052. [Google Scholar] [CrossRef]
- Jakob, T.; Forstenlechner, P.; Matricardi, P.; Kleine-Tebbe, J. Molecular Allergy Diagnostics Using Multiplex Assays: Methodological and Practical Considerations for Use in Research and Clinical Routine. Allergo J. Int. 2015, 24, 320–332. [Google Scholar] [CrossRef]
- Bilò, M.B.; Martini, M.; Bonadonna, P.; Cinti, B.; Re, M.D.; Gabrielli, O.; Olivieri, F.; Salgarolo, V.; Zanoni, G.; Villalta, D. Prevalence of Pol d 1 Sensitization in Polistes Dominula Allergy and Its Diagnostic Role in Vespid Double-Positivity. J. Allergy Clin. Immunol. Pract. 2021, 9, 3781–3787. [Google Scholar] [CrossRef]
- Sturm, G.J.; Arzt-Gradwohl, L. An Algorithm for the Diagnosis and Treatment of Hymenoptera Venom Allergy, 2024 Update. Allergy 2024, 79, 2298–2301. [Google Scholar] [CrossRef] [PubMed]
- Caruso, B.; Bonadonna, P.; Severino, M.G.; Manfredi, M.; Dama, A.; Schiappoli, M.; Rizzotti, P.; Senna, G.; Passalacqua, G. Evaluation of the IgE Cross-reactions among Vespid Venoms. A Possible Approach for the Choice of Immunotherapy. Allergy 2007, 62, 561–564. [Google Scholar] [CrossRef]
- Savi, E.; Peveri, S.; Makri, E.; Pravettoni, V.; Incorvaia, C. Comparing the Ability of Molecular Diagnosis and CAP-Inhibition in Identifying the Really Causative Venom in Patients with Positive Tests to Vespula and Polistes Species. Clin. Mol. Allergy 2016, 14, 3. [Google Scholar] [CrossRef] [PubMed]
| Insect | Frequency of Positive Result n (%) | |
|---|---|---|
| Clinical Suspicion | IDST | |
| Am | 39 (25.8) | 45 (34.6) (n = 130) |
| Vespid | 95 (62.9) | NA |
| Vs | 15 (9.9) | 72 (55) (n = 131) |
| Pd | 21 (13.9) | 85 (64.9) (n = 131) |
| Vespa | 5 (3.3) | NA |
| Vs or Pd | 54 (35.8) | NA |
| Non identified | 17 (11.2) | NA |
| Negative | NA | 8 (5.8) (n = 137) |
| Allergen | N | Obs. Agreement | Cohen’s Kappa Index | Discrepancies (Only Euroline®+ vs. Only ImmunoCAP®+, McNemar Test p-Value) |
|---|---|---|---|---|
| Am | 141 | 0.908 | 0.815 [0.719, 0.911] | 8 vs. 5, p = 0.579 |
| Api m 1 | 120 | 0.867 | 0.637 [0.477, 0.798] | 5 vs. 11, p = 0.211 |
| Api m 2 | 89 | 0.854 | 0.528 [0.315, 0.742] | 12 vs. 1, p = 0.006 |
| Api m 10 | 91 | 0.934 | 0.856 [0.745, 0.967] | 5 vs. 1, p = 0.221 |
| Vs | 150 | 0.747 | 0.34 [0.177, 0.504] | 27 vs. 11, p = 0.015 |
| Ves v 1 | 124 | 0.637 | 0.278 [0.119, 0.437] | 12 vs. 33, p = 0.003 |
| Ves v 5 | 140 | 0.814 | 0.638 [0.521, 0.755] | 26 vs. 0, p < 0.001 |
| Pd | 148 | 0.547 | 0.223 [0.127, 0.319] | 2 vs. 65, p < 0.001 |
| Pol d 5 | 139 | 0.799 | 0.598 [0.466, 0.731] | 18 vs. 10, p = 0.186 |
| CCD | 56 | 0.714 | 0.269 [0.017, 0.521] | 13 vs. 3, p = 0.024 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marquès, L.; Vega, A.; de la Roca, F.; Domínguez, C.; Soriano-Gomis, V.; Alfaya, T.; Ferré-Ybarz, L.; Vega, J.-M.; Tubella, M.; Ruiz-León, B. Molecular Diagnosis in Hymenoptera Allergy: Comparison of Euroline DPA-Dx and ImmunoCAP. Toxins 2025, 17, 310. https://doi.org/10.3390/toxins17060310
Marquès L, Vega A, de la Roca F, Domínguez C, Soriano-Gomis V, Alfaya T, Ferré-Ybarz L, Vega J-M, Tubella M, Ruiz-León B. Molecular Diagnosis in Hymenoptera Allergy: Comparison of Euroline DPA-Dx and ImmunoCAP. Toxins. 2025; 17(6):310. https://doi.org/10.3390/toxins17060310
Chicago/Turabian StyleMarquès, Lluís, Arantza Vega, Federico de la Roca, Carmen Domínguez, Víctor Soriano-Gomis, Teresa Alfaya, Laia Ferré-Ybarz, José-María Vega, Mario Tubella, and Berta Ruiz-León. 2025. "Molecular Diagnosis in Hymenoptera Allergy: Comparison of Euroline DPA-Dx and ImmunoCAP" Toxins 17, no. 6: 310. https://doi.org/10.3390/toxins17060310
APA StyleMarquès, L., Vega, A., de la Roca, F., Domínguez, C., Soriano-Gomis, V., Alfaya, T., Ferré-Ybarz, L., Vega, J.-M., Tubella, M., & Ruiz-León, B. (2025). Molecular Diagnosis in Hymenoptera Allergy: Comparison of Euroline DPA-Dx and ImmunoCAP. Toxins, 17(6), 310. https://doi.org/10.3390/toxins17060310






