Assessing Alternaria Species and Related Mycotoxin Contamination in Wheat in Algeria: A Food Safety Risk
Abstract
1. Introduction
2. Results
2.1. Alternaria Contamination in Durum Wheat Samples
2.2. Molecular and Phylogenetic Analyses of Alternaria Strains
2.3. Mycotoxin Production of Alternaria Strains
2.4. Content of Alternaria Mycotoxins in Grain Samples
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Wheat Sampling and Isolation of Alternaria Strains
5.2. Molecular Characterization of Alternaria Strains
5.3. Mycotoxin Detection in Alternaria Culture Materials and Wheat Samples
5.3.1. Solid Phase Extraction (SPE) Clean-Up for TeA
5.3.2. Solid Phase Extraction (SPE) Clean-Up for AME, AOH, ATX-I, TEN and ALT
5.3.3. Recovery Experiments
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saini, P.; Kaur, H.; Tyagi, V.; Saini, P.; Ahmed, N.; Dhaliwal, H.S.; Sheikh, I. Nutritional value and end-use quality of durum wheat. Cereal Res. Commun. 2023, 51, 283–294. [Google Scholar] [CrossRef]
- FAOSTAT. Available online: www.fao.org (accessed on 22 April 2025).
- Moretti, A.; Logrieco, A.F.; Susca, A. Mycotoxins: An Underhand Food Problem. In Mycotoxigenic Fungi: Methods in Molecular Biology; Moretti, A., Susca, A., Eds.; Humana Press: New York, NY, USA, 2017; Volume 1542. [Google Scholar] [CrossRef]
- Masiello, M.; Somma, S.; Susca, A.; Ghionna, V.; Logrieco, A.F.; Franzoni, M.; Ravaglia, S.; Meca, G.; Moretti, A. Molecular identification and mycotoxin production by Alternaria species occurring on durum wheat, showing black point symptoms. Toxins 2020, 12, 275. [Google Scholar] [CrossRef]
- Monaco, C.; Sisterna, M.; Perello, A.; Bello, G.D.; Mo, C. Preliminary studies on biological control of the blackpoint complex of wheat in Argentina. World J. Microbiol. Biotechnol. 2004, 20, 285–290. [Google Scholar] [CrossRef]
- Lee, H.B.; Patriarca, A.; Magan, N. Alternaria in Food: Ecophysiology, Mycotoxin Production and Toxicology. Mycobiology 2018, 43, 93–106. [Google Scholar] [CrossRef] [PubMed]
- Somma, S.; Amatulli, M.T.; Masiello, M.; Moretti, A.; Logrieco, A.F. Alternaria species associated to wheat black point identified through a multilocus sequence approach. Int. J. Food Microbiol. 2019, 293, 34–43. [Google Scholar] [CrossRef]
- Habib, W.; Masiello, M.; Ghorayeb, R.; Gerges, E.; Susca, A.; Meca, G.; Quiles, J.; Logrieco, A.; Moretti, A. Mycotoxin profile and phylogeny of pathogenic Alternaria species isolated from symptomatic tomato plants in Lebanon. Toxins 2021, 13, 513. [Google Scholar] [CrossRef] [PubMed]
- Ramires, F.A.; Masiello, M.; Somma, S.; Villani, A.; Susca, A.; Logrieco, A.F.; Luz, C.; Meca, G.; Moretti, A. Phylogeny and Mycotoxin Characterization of Alternaria Species Isolated from Wheat Grown in Tuscany, Italy. Toxins 2018, 10, 472. [Google Scholar] [CrossRef]
- Amatulli, M.T.; Fanelli, F.; Moretti, A.; Mulè, G.; Logrieco, A.F. Alternaria species and mycotoxins associated to black point of cereals. Mycotoxins 2013, 63, 39–46. [Google Scholar] [CrossRef]
- Simmons, E.G. Alternaria: An Identification Manual; CBS Fungal Biodiversity Center: Utrercht, The Netherlands, 2007. [Google Scholar]
- Andrew, M.; Peever, T.L.; Pryor, B.M. An expanded multilocus phylogeny does not resolve species among the small-spored Alternaria species complex. Mycologia 2009, 101, 95–109. [Google Scholar] [CrossRef]
- Pinto, V.E.F.; Patriarca, A. Alternaria species and their associated mycotoxins. In Mycotoxigenic Fungi: Methods in Molecular Biology; Moretti, A., Susca, A., Eds.; Humana Press: New York, NY, USA, 2017; Volume 1542. [Google Scholar] [CrossRef]
- Li, J.F.; Phookamsak, R.; Jiang, H.B.; Bhat, D.J.; Camporesi, E.; Lumyong, S.; Kumla, J.; Hongsanan, S.; Mortimer, P.E.; Xu, J.; et al. Additions to the Inventory of the Genus Alternaria Section Alternaria (Pleosporaceae, Pleosporales) in Italy. J. Fungi 2022, 8, 898. [Google Scholar] [CrossRef]
- Woudenberg, J.H.C.; Seidl, M.F.; Groenewald, J.Z.; de Vries, M.; Stielow, J.B.; Thomma, B.P.H.J.; Crous, P.W. Alternaria section Alternaria: Species, formae speciales, or pathotypes? Stud. Mycol. 2015, 82, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Aichinger, G.; Del Favero, G.; Warth, B.; Marko, D. Alternaria toxins-Still emerging? Compr. Rev. Food Sci. Food Saf. 2021, 20, 4390–4406. [Google Scholar] [CrossRef]
- Rabaaoui, A.; Masiello, M.; Somma, S.; Crudo, F.; Dall’Asta, C.; Righetti, L.; Susca, A.; Logrieco, A.F.; Namsi, A.; Gdoura, R.; et al. Phylogeny and mycotoxin profiles of pathogenic Alternaria and Curvularia species isolated from date palm in southern Tunisia. Front. Microbiol. 2022, 13, 1034658. [Google Scholar] [CrossRef] [PubMed]
- Somma, S.; Pose, G.; Pardo, A.; Mulè, G.; Fernandez Pinto, V.; Moretti, A.; Logrieco, A.F. AFLP variability, toxin production, and pathogenicity of Alternaria species from Argentinean tomato fruits and puree. Int. J. Food Microbiol. 2011, 145, 414–419. [Google Scholar] [CrossRef]
- Meena, M.; Samal, S. Alternaria host-specific (HSTs) toxins: An overview of chemical characterization, target sites, regulation and their toxic effects. Toxicol. Rep. 2019, 6, 745–758. [Google Scholar] [CrossRef]
- Logrieco, A.; Moretti, A.; Solfrizzo, M. Alternaria toxins and plant diseases: An overview of origin, occurrence and risks. World Mycotoxin J. 2009, 2, 129–140. [Google Scholar] [CrossRef]
- Solhaug, A.; Eriksen, G.S.; Holme, J.A. Mechanisms of Action and Toxicity of the Mycotoxin Alternariol: A Review. Basic Clin. Pharmacol. Toxicol. 2016, 119, 533–539. [Google Scholar] [CrossRef]
- Fehr, M.; Pahlke, G.; Fritz, J.; Christensen, M.O.; Boege, F.; Altemöller, M.; Podlech, J.; Marko, D. Alternariol acts as a topoisomerase poison, preferentially affecting the II α isoform. Mol. Nutr. Food Res. 2009, 53, 441–451. [Google Scholar] [CrossRef]
- EC—European Commission. Commission Recommendation (EC) No 2022/553 of 5 April 2022 on monitoring the presence of Alternaria toxins in food. Off. J. Eur. Communities 2022, 107, 90. Available online: http://data.europa.eu/eli/reco/2022/553/oj (accessed on 16 June 2025).
- Mokhtar, H.; Dehimat, A. Study the impact of Trichoderma harzianum filtrate on vitality of some hard wheat seeds, and on their interior associated fungi. Agric. Biol. J. N. Am. 2013, 4, 2151–7517. [Google Scholar] [CrossRef]
- Abdallah-Nekache, N.; Laraba, I.; Ducos, C.; Barreau, C.; Bouznad, Z.; Boureghda, H. Occurrence of Fusarium head blight and Fusarium crown rot in Algerian wheat: Identification of associated species and assessment of aggressiveness. Eur. J. Plant Pathol. 2019, 154, 499–512. [Google Scholar] [CrossRef]
- Benmahti, H.; Yezli, W.; Mohammed, Z.; Benyettou, I. Biodiversity, molecular identification, and pathogenicity of Fusarium species isolated from wheat in western Algeria. Braz. J. Anim. Environ. Res. 2024, 7, e75234. [Google Scholar] [CrossRef]
- González, H.H.L.; Martínez, E.J.; Pacin, A.; Resnik, S.L.; Sydenham, E.W. Natural co-occurrence of fumonisins, deoxynivalenol, zearalenone and aflatoxins in field trial corn in Argentina. Food Add. Contam. 1999, 16, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Azcarate, M.P.; Patriarca, A.; Terminiello, L.; Fernández Pinto, V. Alternaria toxins in wheat during the 2004 to 2005 Argentinean harvest. J. Food Prot. 2008, 71, 1262–1265. [Google Scholar] [CrossRef] [PubMed]
- Tralamazza, S.M.; Bemvenuti, R.H.; Zorzete, P.; de Souza Garcia, F.; Corrêa, B. Fungal diversity and natural occurrence of deoxynivalenol and zearalenone in freshly harvested wheat grains from Brazil. Food Chem. 2016, 196, 445–450. [Google Scholar] [CrossRef]
- Müller, M.E.H.; Koszinski, S.; Bangs, D.E.; Wehrhan, M.; Ulrich, A.; Verch, G.; Brenning, A. Crop biomass and humidity related factors reflect the spatial distribution of phytopathogenic Fusarium fungi and their mycotoxins in heterogeneous fields and landscapes. Precis. Agric. 2016, 17, 698–720. [Google Scholar] [CrossRef]
- Masiello, M.; El Ghorayeb, R.; Somma, S.; Saab, C.; Meca, G.; Logrieco, A.F.; Habib, W.; Moretti, A. Alternaria species and related mycotoxin detection in Lebanese durum wheat kernels. Phytopathol. Mediterr. 2022, 61, 383–393. [Google Scholar] [CrossRef]
- Siciliano, I.; Franco Ortega, S.; Gilardi, G.; Bosio, P.; Garibaldi, A.; Gullino, M.L. Molecular phylogeny and characterization of secondary metabolite profile of plant pathogenic Alternaria species isolated from basil. Food Microbiol. 2018, 73, 264–274. [Google Scholar] [CrossRef]
- Dettman, J.R.; Eggertson, Q.A.; Kim, N.E. Species diversity and molecular characterization of Alternaria section Alternaria isolates collected mainly from cereal crops in Canada. Front. Microbiol. 2023, 14, 1194911. [Google Scholar] [CrossRef]
- Lawrence, D.P.; Gannibal, P.B.; Dugan, F.M.; Pryor, B.M. Characterization of Alternaria isolates from the infectoria species-group and a new taxon from Arrhenatherum, Pseudoalternaria arrhenatheria sp. nov. Mycol. Prog. 2014, 13, 257–276. [Google Scholar] [CrossRef]
- Bessadat, N.; Hamon, B.; Bataillé-Simoneau, N.; Mabrouk, K.; Simoneau, P. Characterization of New Small-Spored Alternaria Species Isolated from Solanaceae in Algeria. Life 2021, 11, 1291. [Google Scholar] [CrossRef]
- Scott, P.M. Analysis of agricultural commodities and foods for Alternaria mycotoxins. J. AOAC Int. 2001, 84, 1809–1817. [Google Scholar] [CrossRef]
- Di Martino, C.; Torino, V.; Minotti, P.; Pietrantonio, L.; Del Grosso, C.; Palmieri, D.; Palumbo, G.; Crawford, T.W., Jr.; Carfagna, S. Mycorrhized Wheat Plants and Nitrogen Assimilation in Coexistence and Antagonism with Spontaneous Colonization of Pathogenic and Saprophytic Fungi in a Soil of Low Fertility. Plants 2022, 11, 924. [Google Scholar] [CrossRef] [PubMed]
- Hatamzadeh, S.; Rahnama, K.; White, J.F.; Oghaz, N.A.; Nasrollahnejad, S.; Hemmati, K. Investigation of some endophytic fungi from five medicinal plants with growth promoting ability on maize (Zea mays L.). J. Appl. Microbiol. 2023, 134, lxac015. [Google Scholar] [CrossRef]
- Wu, H.; Yang, J.; Shen, P.; Li, Q.; Wu, W.; Jiang, X.; Qin, L.; Huang, J.; Cao, X.; Qi, F. High-Level Production of Indole-3-acetic Acid in the Metabolically Engineered Escherichia coli. J. Agric. Food Chem. 2021, 69, 1916–1924. [Google Scholar] [CrossRef] [PubMed]
- Shabana, Y.M.; Rashad, Y.M.; Ghoneem, K.M.; Arafat, N.S.; Aseel, D.G.; Qi, A.; Richard, B.; Fitt, B.D.L. Biodiversity of Pathogenic and Toxigenic Seed-Borne Mycoflora of Wheat in Egypt and Their Correlations with Weather Variables. Biology 2021, 10, 1025. [Google Scholar] [CrossRef] [PubMed]
- Li, F.Q.; Yoshizawa, T. Alternaria mycotoxins in weathered wheat from China. J. Agric. Food Chem. 2000, 48, 2920–2924. [Google Scholar] [CrossRef]
- Tralamazza, S.M.; Piacentini, K.C.; Iwase, C.H.T.; Rocha, L.O. Toxigenic Alternaria species: Impact in cereals worldwide. Curr. Opin. Food Sci. 2018, 23, 57–63. [Google Scholar] [CrossRef]
- Müller, M.E.H.; Korn, U. Alternaria mycotoxins in wheat—A 10 years survey in the Northeast of Germany. Food Control 2013, 34, 191–197. [Google Scholar] [CrossRef]
- Xu, W.; Han, X.; Li, F.; Zhang, L. Natural Occurrence of Alternaria Toxins in the 2015 Wheat from Anhui Province, China. Toxins 2016, 8, 308. [Google Scholar] [CrossRef]
- Escrivá, L.; Oueslati, S.; Font, G.; Manyes, L. Alternaria Mycotoxins in Food and Feed: An Overview. J Food Qual. 2017, 1569748. [Google Scholar] [CrossRef]
- Steyn, P.S.; Rabie, C.J. Characterization of magnesium and calcium tenuazonate from Phoma sorghina. Phytochemistry 1976, 15, 1977–1979. [Google Scholar] [CrossRef]
- David, S.; Feist, M.; Proske, M.; Koch, M.; Nehls, I. Degradation of the Alternaria mycotoxins alternariol, alternariol monomethyl ether, and altenuene upon bread baking. Agric. Food Chem. 2010, 58, 9622–9630. [Google Scholar] [CrossRef]
- Mašková, Z.; Tančinová, D.; Ballová, M. Alternaria spp. in food commodities of Slovak origin: Occurrence and myco-toxin production abilities. Potravin. Slovak J. Food Sci. 2019, 13, 524–531. [Google Scholar] [CrossRef]
- Woo, S.Y.; Lee, S.Y.; Jeong, T.K.; Park, S.M.; Auh, J.H.; Shin, H.S.; Chun, H.S. Natural occurrence of Alternaria toxins in agricultural products and processed foods marketed in South Korea by LC–MS/MS. Toxins 2022, 14, 824. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, C.; van der Fels-Klerx, H.J. Occurrence, toxicity, dietary exposure, and management of Alternaria mycotoxins in food and feed: A systematic literature review. Compr. Rev. Food Sci. Food. Saf. 2025, 24, e70085. [Google Scholar] [CrossRef]
- Marin, D.E.; Grosu, I.A.; Pistol, G.C.; Bulgaru, C.V.; Pertea, A.M.; Taranu, I. The Combined Effect of Two Alternaria Mycotoxins (Alternariol and Alternariol Monomethyl Ether) on Porcine Epithelial Intestinal Cells. Agriculture 2024, 14, 1478. [Google Scholar] [CrossRef]
- Xu, W.; Han, X.; Zhang, J.; Xu, J.; Bai, L. Occurrence and Co-occurrence of Alternaria toxins in tomato-based products collected in China. Food Control 2024, 155, 110030. [Google Scholar] [CrossRef]
- Beck, H.; Zimmermann, N.; McVicar, T.R.; Vergopolan, N.; Berg, A.; Wood, E.F. Present and future Köppen-Geiger climate classification maps at 1-km resolution. Sci. Data 2018, 5, 180214. [Google Scholar] [CrossRef]
- Hong, S.G.; Cramer, R.A.; Lawrence, C.B.; Pryor, B.M. Alt a 1 allergen homologs from Alternaria and related taxa: Analysis of phylogenetic content and secondary structure. Fungal Genet. Biol. 2005, 42, 119–129. [Google Scholar] [CrossRef]
- Berbee, M.L.; Pirseyedi, M.; Hubbard, S. Cochliobolus phylogenetics and the origin of known, highly virulent pathogens, inferred from ITS and glyceraldehyde-3-phosphate dehydrogenase gene sequences. Mycologia 1999, 91, 964–977. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Solfrizzo, M.; De Girolamo, A.; Vitti, C.; Visconti, A.; van den Bulk, R. Liquid chromatographic determination of Alternaria toxins in carrots. J. AOAC Int. 2004, 87, 101–106. [Google Scholar] [PubMed]
- Li, F.Q.; Toyazaki, N.; Yoshizawa, T. Production of Alternaria mycotoxins by Alternaria alternata isolated from weather-damaged wheat. J. Food Prot. 2001, 64, 567–571. [Google Scholar] [CrossRef]

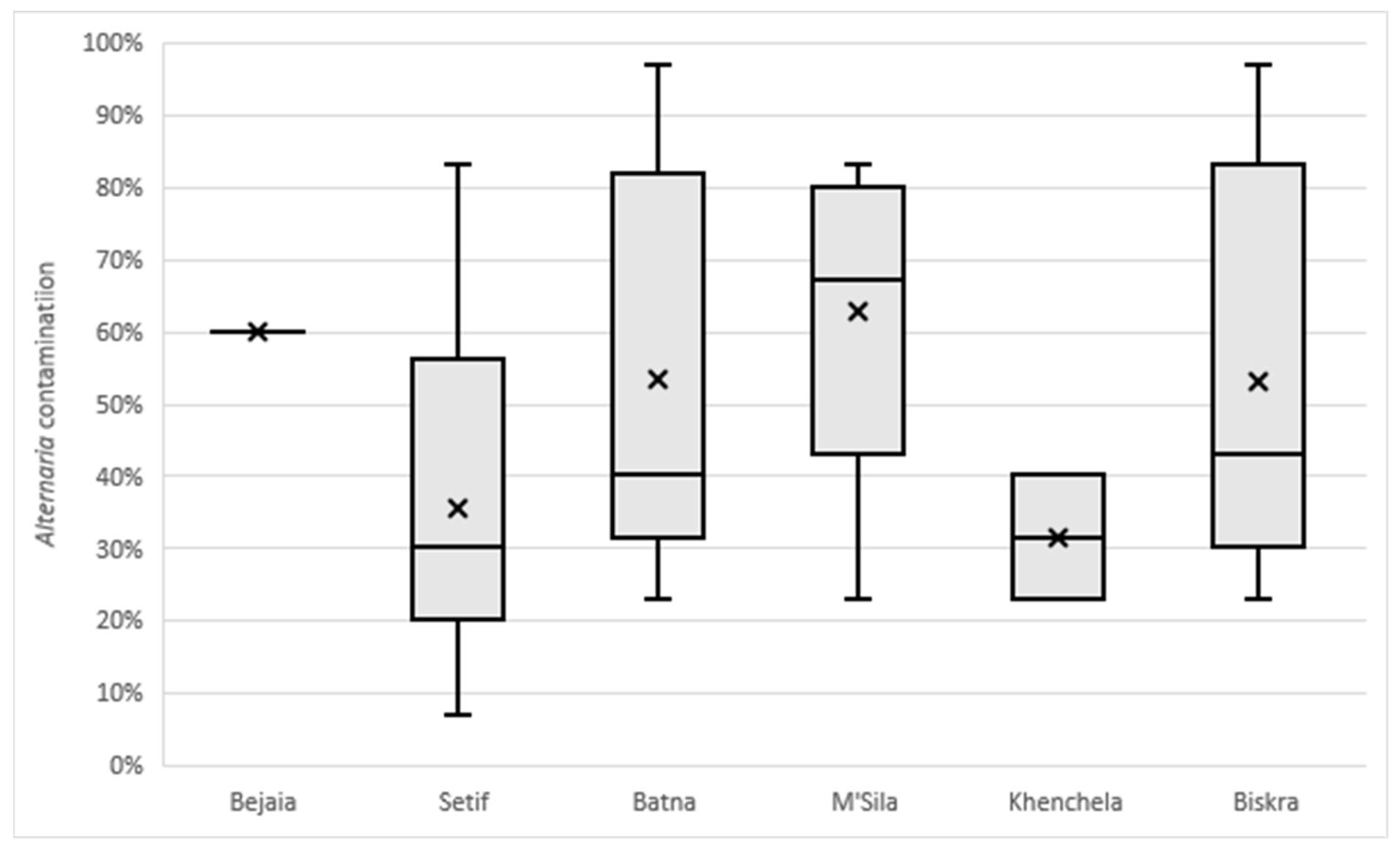
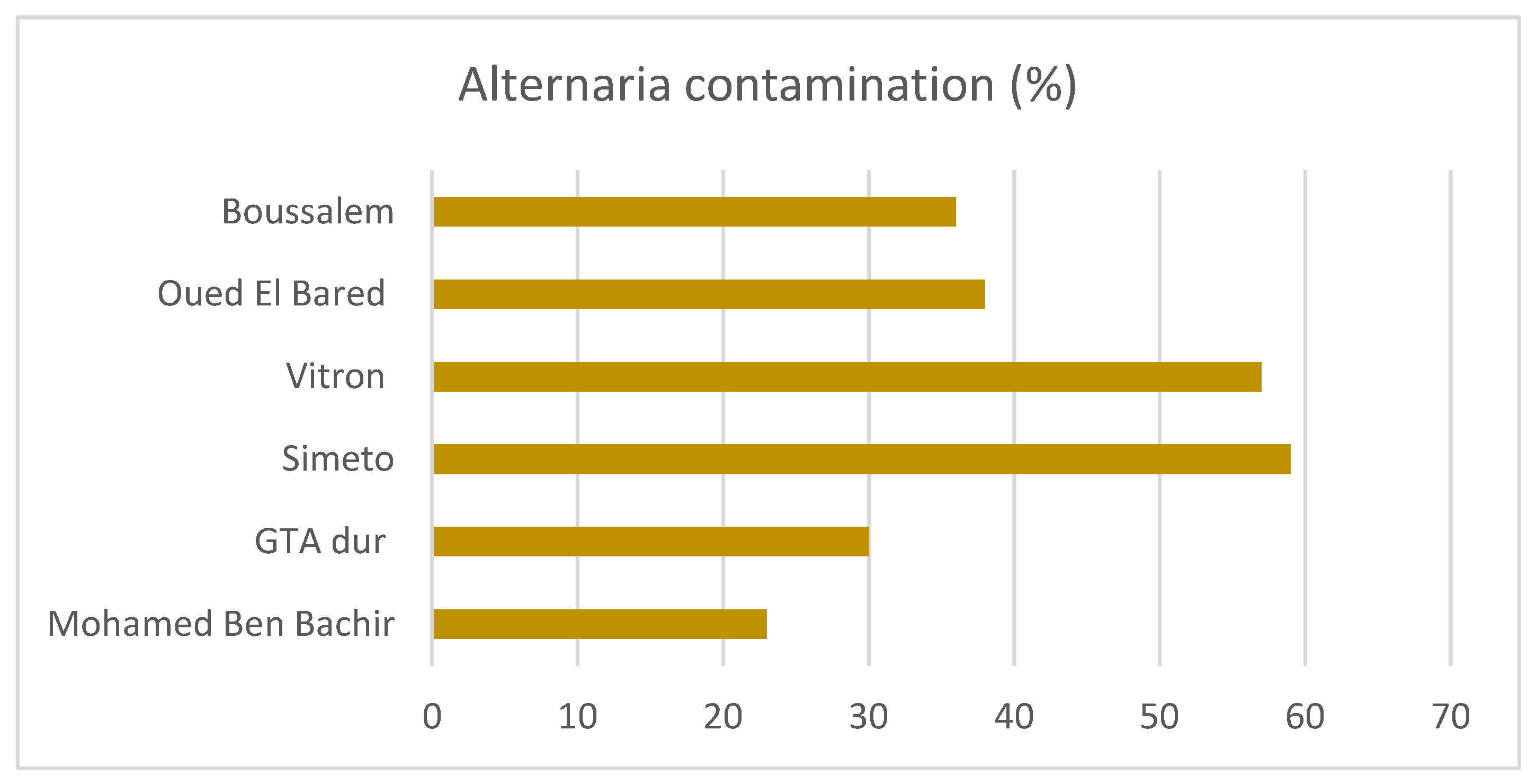

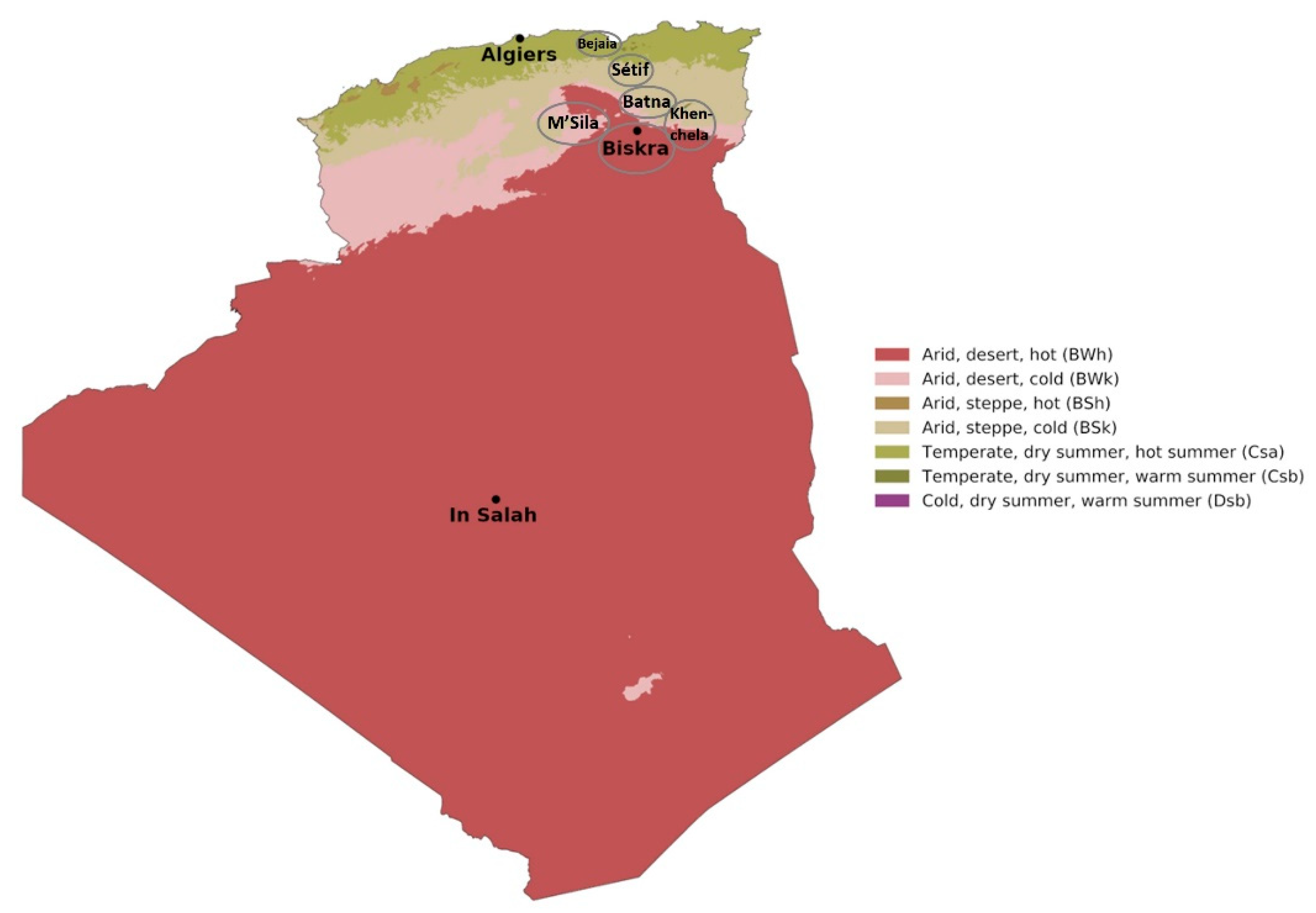
| Region | Climate Conditions | No. of Total Samples | Wheat Variety | No. of Samples | Wheat Sample |
|---|---|---|---|---|---|
| Bejaia | Temperate, dry and warm summer | 1 | Boussalem | 1 | V30 |
| Sètif | Temperate, dry and hot summer | 28 | Boussalem | 11 | V8, V10, V12, V14, V21, V23, V25, V26, V27, V36, V42 |
| GTA Dur | 1 | V9 | |||
| Mohamed Ben Bachir | 1 | V32 | |||
| Oued El Bared | 15 | V4, V15, V16, V17, V18, V19, V20, V22, V28, V31, V39, V43, V46, V47, V48 | |||
| Batna | Arid, steppe, cold | 5 | Oued El Bared | 2 | V29, V35 |
| Simeto | 3 | V24, V33, V34 | |||
| M’Sila | Arid, desert, cold | 5 | Vitron | 5 | V1, V2, V3, V7, V11 |
| Khenchela | Arid, desert, cold | 2 | Vitron | 2 | V41, V44 |
| Biskra | Arid, desert, hot | 7 | Oued El Bared | 2 | V38, V40 |
| Vitron | 5 | V5, V6, V13, V37, V45 |
| Phylogenetic Clade | AME | AOH | TeA | ATX-I | TEN | ALT | ||
|---|---|---|---|---|---|---|---|---|
| Alternaria section | Sub-clade A1 (27 strains) | Producer strains (%) | 67 | 70 | 85 | 41 | 15 | 55 |
| Mean (mg kg−1) | 211 | 980 | 2915 | 27 | 63 | 85 | ||
| Range (mg-kg−1) | 18–564 | 9–15949 | 16–9032 | 1–87 | 8–136 | 1–395 | ||
| Sub-clade A2 (3 strains) | Producer strains (%) | 100 | 67 | 67 | 0 | 0 | 100 | |
| Mean (mg kg−1) | 449 | 574 | 542 | 0 | 0 | 344 | ||
| Range (mg-kg−1) | 192–878 | 423–724 | 413–670 | 0 | 0 | 212–423 | ||
| Sub-clade A3 (2 strains) | Producer strains (%) | 100 | 100 | 100 | 100 | 50 | 100 | |
| Mean (mg kg−1) | 109 | 34 | 804 | 111 | 10 | 206 | ||
| Range (mg-kg−1) | 17–200 | 21–48 | 507–1100 | 109–114 | 10 | 99–313 | ||
| Sub-clade A4 (11 strains) | Producer strains (%) | 100 | 100 | 91 | 73 | 18 | 36 | |
| Mean (mg kg−1) | 272 | 203 | 6794 | 34 | 19 | 71 | ||
| Range (mg-kg−1) | 2–1019 | 20–508 | 90–13,165 | 5–76 | 8–30 | 7–192 | ||
| Eureka section | Clade C (1 strain) | Producer strains (%) | 0 | 0 | 0 | 100 | 0 | 100 |
| Mean (mg kg−1) | 0 | 0 | 0 | 5 | 0 | 1 | ||
| Range (mg-kg−1) | 0 | 0 | 0 | 5 | 0 | 1 | ||
| Infectoriae section | Clade E (17 strains) | Producer strains (%) | 0 | 0 | 6 | 18 | 12 | 6 |
| Mean (mg kg−1) | 0 | 0 | 291 | 11 | 74 | 15 | ||
| Range (mg-kg−1) | 0 | 0 | 291 | 4–21 | 12–135 | 15 |
| Mycotoxin | Contaminated Samples (%) | Mycotoxin Content (µg kg−1) | |
|---|---|---|---|
| Mean Value | Range | ||
| AME | 75 | 314 | 50–980 |
| AOH | 69 | 44 | 25–425 |
| TeA | 35 | 177 | 381–705 |
| ATX-I | 8 | 43 | 58–1758 |
| TEN | 21 | 38 | 52–321 |
| Region | No. of Samples | AME | AOH | TeA | ATX-I | TEN | |
|---|---|---|---|---|---|---|---|
| Bejaia | 1 | Contaminated samples (%) | 0 | 100 | 100 | 0 | 0 |
| Mean value (µg kg−1) | 0 | 35 | 489 | 0 | 0 | ||
| Range (µg kg−1) | 0 | 35 | 489 | 0 | 0 | ||
| Sètif | 28 | Contaminated samples (%) | 82 | 93 | 39 | 11 | 32 |
| Mean value (µg kg−1) | 348 | 18 | 210 | 71 | 57 | ||
| Range (µg kg−1) | 50–820 | 25–45 | 381–705 | 82–1758 | 52–321 | ||
| Batna | 5 | Contaminated samples (%) | 40 | 40 | 60 | 0 | 0 |
| Mean value (µg kg−1) | 370 | 13 | 261 | 0 | 0 | ||
| Range (µg kg−1) | 870–980 | 25–40 | 407–471 | 0 | 0 | ||
| M’Sila | 5 | Contaminated samples (%) | 60 | 60 | 0 | 0 | 0 |
| Mean value (µg kg−1) | 224 | 203 | 0 | 0 | 0 | ||
| Range (µg kg−1) | 205–365 | 275–425 | 0 | 0 | 0 | ||
| Khenchela | 2 | Contaminated samples (%) | 100 | 100 | 0 | 0 | 0 |
| Mean value (µg kg−1) | 415 | 40 | 0 | 0 | 0 | ||
| Range (µg kg−1) | 370–460 | 40 | 0 | 0 | 0 | ||
| Biskra | 7 | Contaminated samples (%) | 71 | 86 | 29 | 14 | 14 |
| Mean value (µg kg−1) | 218 | 15 | 120 | 8 | 31 | ||
| Range (µg kg−1) | 180–380 | 25 | 394–444 | 58 | 216 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daichi, M.B.; Masiello, M.; Haidukowski, M.; De Girolamo, A.; Moretti, A.; Bencheikh, A.; Rouag, N.; Somma, S. Assessing Alternaria Species and Related Mycotoxin Contamination in Wheat in Algeria: A Food Safety Risk. Toxins 2025, 17, 309. https://doi.org/10.3390/toxins17060309
Daichi MB, Masiello M, Haidukowski M, De Girolamo A, Moretti A, Bencheikh A, Rouag N, Somma S. Assessing Alternaria Species and Related Mycotoxin Contamination in Wheat in Algeria: A Food Safety Risk. Toxins. 2025; 17(6):309. https://doi.org/10.3390/toxins17060309
Chicago/Turabian StyleDaichi, Meriem Barkahoum, Mario Masiello, Miriam Haidukowski, Annalisa De Girolamo, Antonio Moretti, Amor Bencheikh, Noureddine Rouag, and Stefania Somma. 2025. "Assessing Alternaria Species and Related Mycotoxin Contamination in Wheat in Algeria: A Food Safety Risk" Toxins 17, no. 6: 309. https://doi.org/10.3390/toxins17060309
APA StyleDaichi, M. B., Masiello, M., Haidukowski, M., De Girolamo, A., Moretti, A., Bencheikh, A., Rouag, N., & Somma, S. (2025). Assessing Alternaria Species and Related Mycotoxin Contamination in Wheat in Algeria: A Food Safety Risk. Toxins, 17(6), 309. https://doi.org/10.3390/toxins17060309








