Serum Indoxyl Sulfate as a Potential Biomarker of Peripheral Arterial Stiffness in Patients with Non-Dialysis Chronic Kidney Disease Stages 3 to 5
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics
2.2. Identification of Clinical Factors Associated with PAS
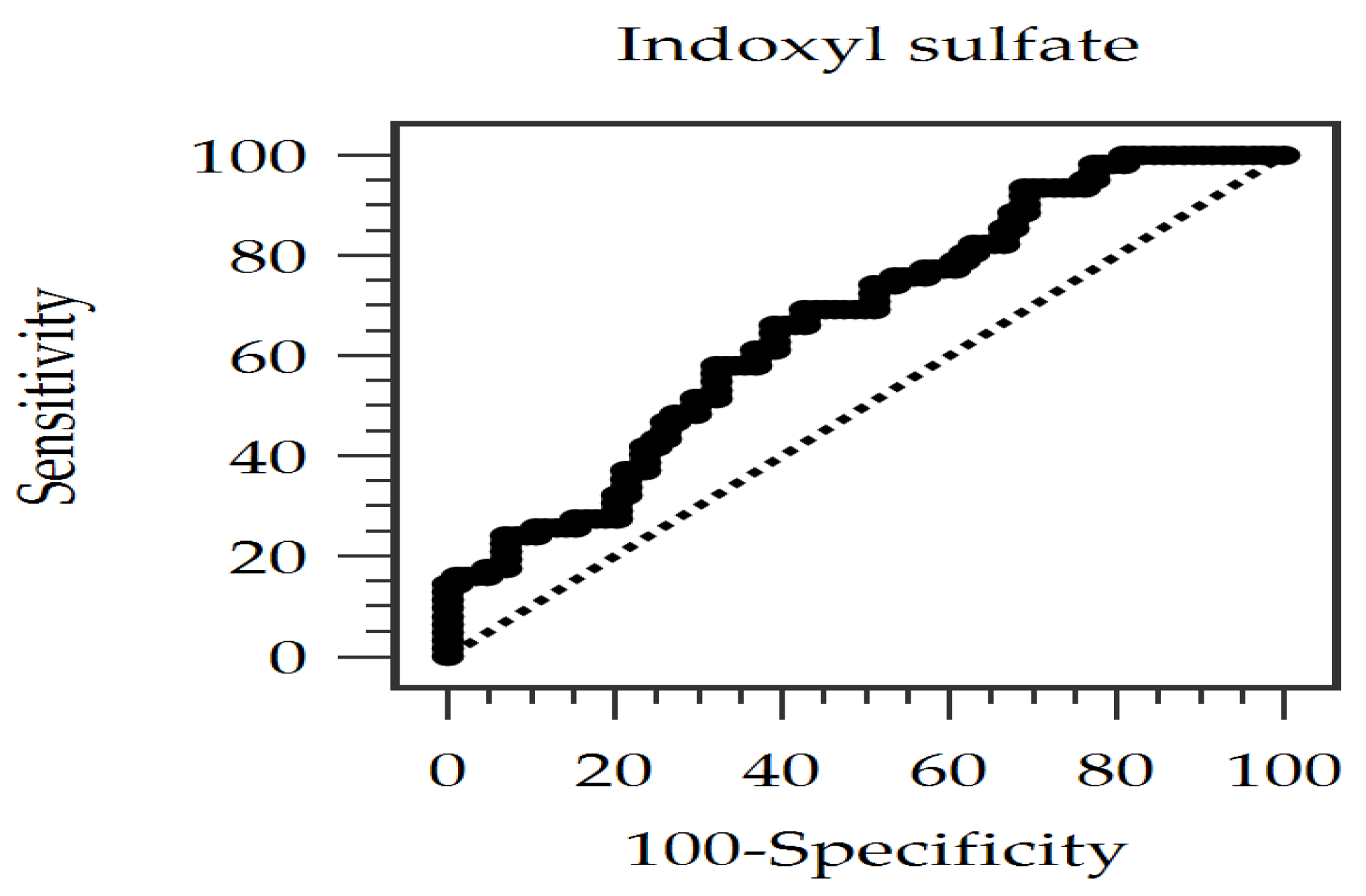
2.3. Evaluating the Diagnostic Power of Serum IS for PAS
2.4. Analyses of the Correlation
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Enrollment of Patients and Collection of Data
5.2. Anthropometric Details and Serum Samples Assessment (Biochemical and Total IS Determination)
5.3. Using baPWV for the Detection of PAS
5.4. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ARICs | Atherosclerosis risk in communities |
| AS | Arterial stiffness |
| AUC | Area under the ROC curve |
| BMI | Body mass index |
| CKD | Chronic kidney disease |
| DM | Diabetes mellitus |
| IS | Indoxyl sulfate |
| KDIGOs | Kidney Disease Improving Global Outcomes |
| PAS | Peripheral arterial stiffness |
| PCS | P-cresyl sulfate |
| PWV | Pulse wave velocity |
| RBCs | Red blood cells |
| ROC | Receiver operating characteristic |
| UPCR | Urine protein-to-creatinine ratio |
References
- Hill, N.R.; Fatoba, S.T.; Oke, J.L.; Hirst, J.A.; O’Callaghan, C.A.; Lasserson, D.S.; Hobbs, F.D. Global prevalence of chronic kidney disease—A systematic review and meta-analysis. PLoS ONE 2016, 11, e0158765. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.H.; Hsu, C.Y.; Lin, M.Y.; Yen, M.F.; Chen, H.H.; Chiu, Y.H.; Hwang, S.J. Incidence, prevalence, and duration of chronic kidney disease in Taiwan: Results from a community-based screening program of 106,094 individuals. Nephron 2018, 140, 175–184. [Google Scholar] [CrossRef]
- Bansal, N. Evolution of cardiovascular disease during the transition to end-stage renal disease. Semin. Nephrol. 2017, 37, 120–131. [Google Scholar] [CrossRef]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2024 Clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. 2024, 105, S117–S314. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Oh, B.H. Aging and arterial stiffness. Circ. J. 2010, 74, 2257–2262. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.P.; Hsu, B.G. Arterial stiffness: A brief review. Tzu Chi Med. J. 2020, 33, 115–121. [Google Scholar] [CrossRef]
- Townsend, R.R. Arterial stiffness in CKD: A review. Am. J. Kidney Dis. 2019, 73, 240–247. [Google Scholar] [CrossRef]
- Tanaka, A.; Tomiyama, H.; Maruhashi, T.; Matsuzawa, Y.; Miyoshi, T.; Kabutoya, T.; Kario, K.; Sugiyama, S.; Munakata, M.; Ito, H.; et al. Physiological diagnosis criteria for vascular failure committee physiological diagnostic criteria for vascular failure. Hypertension 2018, 72, 1060–1071. [Google Scholar] [CrossRef]
- Vlachopoulos, C.; Aznaouridis, K.; Stefanadis, C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: A systematic review and meta-analysis. J. Am. Coll. Cardiol. 2010, 55, 1318–1327. [Google Scholar] [CrossRef]
- Vlachopoulos, C.; Aznaouridis, K.; Terentes-Printzios, D.; Ioakeimidis, N.; Stefanadis, C. Prediction of cardiovascular events and all-cause mortality with brachial-ankle elasticity index: A systematic review and meta-analysis. Hypertension 2012, 60, 556–562. [Google Scholar] [CrossRef]
- Kim, E.D.; Tanaka, H.; Ballew, S.H.; Sang, Y.; Heiss, G.; Coresh, J.; Matsushita, K. Associations between kidney disease measures and regional pulse wave velocity in a large community-based cohort: The atherosclerosis risk in communities (ARIC) study. Am. J. Kidney Dis. 2018, 72, 682–690. [Google Scholar] [CrossRef] [PubMed]
- Ohkuma, T.; Ninomiya, T.; Tomiyama, H.; Kario, K.; Hoshide, S.; Kita, Y.; Inoguchi, T.; Maeda, Y.; Kohara, K.; Tabara, Y.; et al. Brachial-ankle pulse wave velocity and the risk prediction of cardiovascular disease: An individual participant data meta-analysis. Hypertension 2017, 69, 1045–1052. [Google Scholar] [CrossRef]
- Lin, C.C.; Li, C.I.; Liu, C.S.; Lin, C.H.; Yang, S.Y.; Li, T.C. Prediction of all-cause and cardiovascular mortality using ankle-brachial index and brachial-ankle pulse wave velocity in patients with type 2 diabetes. Sci. Rep. 2022, 12, 11053. [Google Scholar] [CrossRef]
- Kim, J.; Song, T.J.; Song, D.; Lee, K.; Kim, E.H.; Lee, H.S.; Nam, C.M.; Nam, H.S.; Kim, Y.D.; Heo, J.H. Brachial-ankle pulse wave velocity is a strong predictor for mortality in patients with acute stroke. Hypertension 2014, 64, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, Y.; Duan, X.; Wang, Q.; Zhang, H. Research progress on the relationship between IS and kidney disease and its complications. Int. Urol. Nephrol. 2022, 54, 2881–2890. [Google Scholar] [CrossRef]
- Lin, C.J.; Wu, V.; Wu, P.C.; Wu, C.J. Meta-analysis of the associations of p-cresyl sulfate (PCS) and indoxyl sulfate (IS) with cardiovascular events and all-cause mortality in patients with chronic renal failure. PLoS ONE 2015, 10, e0132589. [Google Scholar] [CrossRef]
- Takkavatakarn, K.; Phannajit, J.; Udomkarnjananun, S.; Tangchitthavorngul, S.; Chariyavilaskul, P.; Sitticharoenchai, P.; Praditpornsilpa, K.; Eiam-Ong, S.; Susantitaphong, P. Association between indoxyl sulfate and dialysis initiation and cardiac outcomes in chronic kidney disease patients. Int. J. Nephrol. Renovasc. Dis. 2022, 15, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.H.; Lai, Y.H.; Kuo, C.H.; Lin, Y.L.; Tsai, J.P.; Hsu, B.G. Association between serum indoxyl sulfate levels and endothelial function in non-dialysis chronic kidney disease. Toxins 2019, 11, 589. [Google Scholar] [CrossRef]
- Harlacher, E.; Wollenhaupt, J.; Baaten, C.C.F.M.J.; Noels, H. Impact of uremic toxins on endothelial dysfunction in chronic kidney disease: A systematic review. Int. J. Mol. Sci. 2022, 23, 531. [Google Scholar] [CrossRef]
- Gao, Y.; Zhao, C.J.; Liu, Q.; Li, C.C.; Li, Z.; Li, J.; Wang, Q.; Zhang, L. Relationship between serum indoxyl sulfate and klotho protein and vascular calcification in patients with chronic kidney disease stages 3–5. Int. J. Endocrinol. 2024, 2024, 8229604. [Google Scholar] [CrossRef]
- Lin, T.J.; Hsu, B.G.; Wang, J.H.; Lai, Y.H.; Dongoran, R.A.; Liu, C.H. Serum indoxyl sulfate as a potential biomarker of aortic arterial stiffness in coronary artery disease. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 2320–2327. [Google Scholar] [CrossRef] [PubMed]
- Kamiński, T.W.; Pawlak, K.; Karbowska, M.; Myśliwiec, M.; Pawlak, D. Indoxyl sulfate—The uremic toxin linking hemostatic system disturbances with the prevalence of cardiovascular disease in patients with chronic kidney disease. BMC Nephrol. 2017, 18, 35. [Google Scholar] [CrossRef]
- Wang, S.C.; Lai, Y.H.; Liu, C.H.; Wang, C.H.; Hsu, B.G.; Tsai, J.P. Association between serum indoxyl sulfate levels with carotid-femoral pulse wave velocity in patients with chronic kidney disease. Ren. Fail. 2021, 43, 796–802. [Google Scholar] [CrossRef]
- Beros, A.L.; Sluyter, J.D.; Hughes, A.D.; Hametner, B.; Wassertheurer, S.; Scragg, R. Positive association of oscillometrically estimated baseline arterial stiffness with incident diabetes and prediabetes: A large population-based cohort study. Prim. Care Diabetes 2025, 19, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Loutradis, C.; Schoina, M.; Dimitroulas, T.; Doumas, M.; Garyfallos, A.; Karagiannis, A.; Papagianni, A.; Sarafidis, P. Comparison of ambulatory central hemodynamics and arterial stiffness in patients with diabetic and non-diabetic CKD. J. Clin. Hypertens. 2020, 22, 2239–2249. [Google Scholar] [CrossRef]
- Dubsky, M.; Veleba, J.; Sojakova, D.; Marhefkova, N.; Fejfarova, V.; Jude, E.B. Endothelial dysfunction in diabetes Mellitus: New insights. Int. J. Mol. Sci. 2023, 24, 10705. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, N.J.; Fluck, R.J.; McIntyre, C.W.; Fakis, A.; Taal, M.W. Determinants of arterial stiffness in chronic kidney disease stage 3. PLoS ONE 2013, 8, e55444. [Google Scholar] [CrossRef]
- McIntyre, N.J.; Shardlow, A.; Fluck, R.J.; McIntyre, C.W.; Taal, M.W. Determinants of change in arterial stiffness over 5 years in early chronic kidney disease. Nephrol. Dial. Transplant. 2021, 36, 281–288. [Google Scholar] [CrossRef]
- Xing, C.; Xu, L.; Li, F.; Xie, X.; Guan, X.; Zhan, X.; Chen, W.; Yang, H.; Wang, X.; Wang, Y.; et al. Structural and load-dependent arterial stiffness across the adult life span. J. Hypertens. 2025, 43, 615–622. [Google Scholar] [CrossRef]
- Ahmed, B.; Rahman, A.A.; Lee, S.; Malhotra, R. The implications of aging on vascular Health. Int. J. Mol. Sci. 2024, 25, 11188. [Google Scholar] [CrossRef]
- Nagayama, D.; Watanabe, Y.; Fujishiro, K.; Suzuki, K.; Ohira, M.; Shirai, K.; Saiki, A. Age-related arterial stiffening is associated with a body shape index (ABSI) and lean body mass index—A retrospective cohort study in healthy Japanese population. Obes. Facts 2025, 18, 248–259. [Google Scholar] [CrossRef] [PubMed]
- Lo Cicero, L.; Lentini, P.; Sessa, C.; Castellino, N.; D’Anca, A.; Torrisi, I.; Marcantoni, C.; Castellino, P.; Santoro, D.; Zanoli, L. Inflammation and arterial stiffness as drivers of cardiovascular risk in kidney disease. Cardiorenal. Med. 2025, 15, 29–40. [Google Scholar] [CrossRef]
- Vervloet, M.G. Can we reverse arterial stiffness by intervening on CKD-MBD biomarkers? Clin. Kidney J. 2023, 16, 1766–1775. [Google Scholar] [CrossRef] [PubMed]
- London, G.M. Arterial stiffness in chronic kidney disease and end-stage renal disease. Blood Purif. 2018, 45, 154–158. [Google Scholar] [CrossRef]
- Barzilay, J.I.; Farag, Y.M.K.; Durthaler, J. Albuminuria: An underappreciated risk factor for cardiovascular disease. J. Am. Heart Assoc. 2024, 13, e030131. [Google Scholar] [CrossRef]
- He, X.; Jiang, H.; Gao, F.; Liang, S.; Wei, M.; Chen, L. Indoxyl sulfate-induced calcification of vascular smooth muscle cells via the PI3K/Akt/NF-κB signaling pathway. Microsc. Res. Tech. 2019, 82, 2000–2006. [Google Scholar] [CrossRef] [PubMed]
- Ellis, R.J.; Small, D.M.; Ng, K.L.; Vesey, D.A.; Vitetta, L.; Francis, R.S.; Gobe, G.C.; Morais, C. Indoxyl sulfate induces apoptosis and hypertrophy in human kidney proximal tubular cells. Toxicol. Pathol. 2018, 46, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Pieniazek, A.; Kopera, M.; Gwozdzinski, L.; Gwozdzinski, K. Indoxyl sulfate induces oxidative changes in plasma and hemolysate. Molecules 2022, 27, 3848. [Google Scholar] [CrossRef]
- Mert, M.; Dinc, U.; Çerï, M.; Dursun, B.; Özban, M.; Aslan, H.S.; Avcı, E.; Odabaşı, Y. Relationship between arterial stiffness, measured by cardio-ankle vascular index, and uremic toxins, vascular calcification, and inflammation markers after kidney donation. Exp. Clin. Transplant. 2024, 22, 613–621. [Google Scholar]
- Viaene, L.; Annaert, P.; de Loor, H.; Poesen, R.; Evenepoel, P.; Meijers, B. Albumin is the main plasma binding protein for indoxyl sulfate and p-cresyl sulfate. Biopharm. Drug Dispos. 2013, 34, 165–175. [Google Scholar] [CrossRef]
- van der Made, T.K.; Fedecostante, M.; Scotcher, D.; Rostami-Hodjegan, A.; Sastre Toraño, J.; Middel, I.; Koster, A.S.; Gerritsen, K.G.; Jankowski, V.; Jankowski, J.; et al. Quantitative translation of microfluidic transporter in vitro data to in vivo reveals impaired albumin-facilitated indoxyl sulfate secretion in chronic kidney disease. Mol. Pharm. 2019, 16, 4551–4562. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.F.; Hou, J.S.; Wang, C.H.; Lin, Y.L.; Lai, Y.H.; Kuo, C.H.; Liou, H.H.; Tsai, J.P.; Hsu, B.G. Serum sclerostin but not dkk-1 correlated with central arterial stiffness in end stage renal disease patients. Int. J. Environ. Res. Public Health 2020, 17, 1230. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.C.; Lin, Y.L.; Lai, Y.H.; Wang, C.H.; Hsu, B.G. Serum p-cresyl sulfate level is an independent marker of peripheral arterial stiffness as assessed using brachial-ankle pulse wave velocity in patients with non-dialysis chronic kidney disease stage 3 to 5. Toxins 2022, 14, 287. [Google Scholar] [CrossRef]
- Wu, H.C.; Lee, L.C.; Wang, W.J. Associations among serum beta 2 microglobulin, malnutrition, inflammation, and advanced cardiovascular event in patients with chronic kidney disease. J. Clin. Lab. Anal. 2017, 31, e22056. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.H.; Wang, C.H.; Hsu, B.G.; Tsai, J.P. Serum osteopontin level is positively associated with aortic stiffness in patients with peritoneal dialysis. Life 2022, 12, 397. [Google Scholar] [CrossRef]
- Liu, W.N.; Hsu, Y.C.; Lu, C.W.; Lin, S.C.; Wu, T.J.; Lin, G.M. Serum malondialdehyde-modified low-density lipoprotein as a risk marker for peripheral arterial stiffness in maintenance hemodialysis patients. Medicina 2024, 60, 697. [Google Scholar] [CrossRef]
- Lin, W.; Wu, T.J.; Wang, C.H.; Hsieh, Y.J.; Hsu, B.G. Association between serum soluble urokinase-type plasminogen activator receptor level and arterial stiffness in chronic hemodialysis patients. J. Pers. Med. 2023, 13, 470. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, X.Y.; Yang, S.Y.; Hu, Y.C.; Duan, K. Effects of resistant starch supplementation on renal function and inflammatory markers in patients with chronic kidney disease: A meta-analysis of randomized controlled trials. Ren. Fail. 2024, 46, 2416609. [Google Scholar] [CrossRef]
- Ohkuma, T.; Tomiyama, H.; Ninomiya, T.; Kario, K.; Hoshide, S.; Kita, Y.; Inoguchi, T.; Maeda, Y.; Kohara, K.; Tabara, Y.; et al. Proposed cutoff value of brachial-ankle pulse wave velocity for the management of hypertension. Circ. J. 2017, 81, 1540–1542. [Google Scholar] [CrossRef]
| Aspect | Carotid–Femoral PWV (cfPWV) | Brachial–Ankle PWV (baPWV) |
|---|---|---|
| Measurement Technique | Using tonometric devices, focusing on central arteries (elastic arteries). | Using oscillometric devices, assessing both central and peripheral arteries (elastic and muscular arteries). |
| Reproducibility | Highly operator-dependent but reliable after proper training. | Highly reproducible due to the automated nature of the measurement. |
| Clinical Relevance | Gold standard for central AS but time-consuming; associated with renal and CV outcomes. | Simpler and faster to perform; correlates with renal and CV outcomes. |
| Patient Considerations | Less affected by PAD; preferred for assessment of aortic stiffness. | Less reliable in PAD; useful for overall vascular health assessment. |
| Parameters | All Patients (n = 146) | Non-PAS Group (n = 84) | PAS Group (n = 62) | p Value |
|---|---|---|---|---|
| Age (years) | 68.42 ± 12.64 | 64.74 ± 11.89 | 73.42 ± 11.97 | <0.001 * |
| Height (cm) | 159.49 ± 8.99 | 159.92 ± 9.73 | 158.90 ± 7.91 | 0.496 |
| Body weight (kg) | 66.55 ± 12.45 | 68.08 ± 14.12 | 64.47 ± 9.47 | 0.084 |
| Body mass index (kg per m2) | 26.06 ± 3.80 | 26.48 ± 4.34 | 25.49 ± 2.85 | 0.120 |
| Left baPWV (m/s) | 17.31 ± 3.68 | 14.85 ± 1.54 | 20.66 ± 3.05 | <0.001 * |
| Right baPWV (m/s) | 17.27 ± 3.47 | 14.94 ± 1.97 | 20.42 ± 2.41 | <0.001 * |
| SBP (mmHg) | 149.61 ± 26.00 | 142.07 ± 22.57 | 159.82 ± 27.02 | <0.001 * |
| DBP (mmHg) | 83.97 ± 13.75 | 81.55 ± 12.13 | 87.24 ± 15.16 | 0.013 * |
| Total cholesterol (mg/dL) | 162.29 ± 43.73 | 162.48 ± 47.26 | 162.03 ± 38.81 | 0.952 |
| Triglyceride (mg/dL) | 120.50 (86.00–168.25) | 114.50 (83.00–167.75) | 132.50 (86.75–171.75) | 0.360 |
| LDL-C (mg/dL) | 91.46 ± 35.99 | 92.89 ± 39.72 | 89.52 ± 30.41 | 0.577 |
| Fasting glucose (mg/dL) | 111.00 (97.75–138.25) | 109.50 (97.00–123.50) | 117.00 (100.50–144.00) | 0.132 |
| Blood urea nitrogen (mg/dL) | 32.00 (24.75–44.00) | 29.50 (24.00–43.00) | 34.50 (26.00–50.75) | 0.154 |
| Creatinine (mg/dL) | 1.80 (1.40–2.63) | 1.70 (1.40–2.50) | 2.05 (1.60–3.13) | 0.044 * |
| eGFR (mL/min) | 32.03 ± 15.28 | 35.20 ± 14.90 | 27.72 ± 14.84 | 0.003 * |
| Spot UPCR (g/g) | 0.47 (0.17–1.20) | 0.35 (0.14–0.96) | 0.61 (0.21–1.92) | 0.016 * |
| Albumin (g/dL) | 4.07 ± 0.39 | 4.14 ± 0.38 | 3.98 ± 0.39 | 0.013 * |
| Hemoglobin (g/dL) | 11.66 ± 2.64 | 11.74 ± 2.07 | 11.55 ± 3.28 | 0.682 |
| Indoxyl sulfate (μg/mL) | 1.05 (0.55–2.32) | 0.80 (0.41–1.74) | 1.45 (0.70–3.62) | 0.001 * |
| Female, n (%) | 68 (46.6) | 37 (44.0) | 31 (50.0) | 0.476 |
| Diabetes mellitus, n (%) | 58 (39.7) | 27 (32.1) | 31 (50.0) | 0.029 * |
| Hypertension, n (%) | 88 (60.3) | 42 (50.0) | 46 (74.2) | 0.003 * |
| Glomerulonephritis, n (%) | 34 (23.3) | 21 (25.0) | 13 (21.0) | 0.569 |
| ARB use, n (%) | 76 (52.1) | 40 (47.6) | 36 (58.1) | 0.212 |
| β-blocker use, n (%) | 31 (21.2) | 17 (20.2) | 14 (22.6) | 0.732 |
| CCB use, n (%) | 60 (41.1) | 31 (36.9) | 29 (46.8) | 0.231 |
| α-adrenergic blocker, n (%) | 22 (15.1) | 10 (11.9) | 12 (19.4) | 0.214 |
| Statin use, n (%) | 67 (45.9) | 36 (42.9) | 31 (50.0) | 0.392 |
| Fibrate use, n (%) | 26 (17.8) | 16 (19.0) | 10 (16.1) | 0.649 |
| CKD stage 3, n (%) | 76 (52.1) | 51 (60.7) | 25 (40.3) | 0.037 * |
| CKD stage 4, n (%) | 41 (28.1) | 21 (25.0) | 20 (32.3) | |
| CKD stage 5, n (%) | 29 (19.8) | 12 (14.3) | 17 (27.4) |
| Determinants | Odds Ratio | 95% Confidence Interval | p Value |
|---|---|---|---|
| Indoxyl sulfate, 1 μg/mL | 1.389 | 1.086–1.775 | 0.009 * |
| Age, 1 year | 1.125 | 1.060–1.188 | <0.001 * |
| Presence of diabetes mellitus | 5.111 | 1.612–16.201 | 0.006 * |
| Diastolic blood pressure, 1 mmHg | 1.091 | 1.022–1.1665 | 0.009 * |
| Systolic blood pressure, 1 mmHg | 0.985 | 0.946–1.026 | 0.468 |
| Hypertension, present | 1.341 | 0.383–4.696 | 0.646 |
| Albumin, 1 g/dL | 0.911 | 0.215–3.859 | 0.899 |
| Creatinine, 1 mg/dL | 0.503 | 0.241–1.050 | 0.067 |
| eGFR, 1 mL/min | 0.968 | 0.917–1.022 | 0.245 |
| Spot UPCR, 1 g/g | 1.467 | 0.863–2.492 | 0.157 |
| Fasting glucose, 1 mg/dL | 0.990 | 0.978–1.002 | 0.102 |
| Total cholesterol, 1 mg/dL | 1.019 | 0.995–1.044 | 0.115 |
| Triglyceride, 1 mg/dL | 1.000 | 0.996–1.003 | 0.781 |
| LDL-C (mg/dL) | 0.976 | 0.949–1.004 | 0.090 |
| Variables | Mean baPWV (m/s) | Log-IS (μg/mL) | ||
|---|---|---|---|---|
| Spearman’s Correlation Coefficient | p Value | Spearman’s Correlation Coefficient | p Value | |
| Age (years) | 0.379 | <0.001 * | 0.076 | 0.363 |
| Body mass index (kg/m2) | −0.093 | 0.265 | −0.076 | 0.363 |
| Mean baPWV (m/s) | — | — | 0.213 | 0.010 * |
| Log-IS (μg/mL) | 0.213 | 0.010 * | — | — |
| Systolic BP (mmHg) | 0.371 | <0.001 * | 0.310 | <0.001 * |
| Diastolic BP (mmHg) | 0.295 | <0.001 * | 0.125 | 0.133 |
| Total cholesterol (mg/dL) | 0.030 | 0.717 | −0.140 | 0.092 |
| Log-Triglyceride (mg/dL) | 0.017 | 0.838 | 0.001 | 0.995 |
| LDL-C (mg/dL) | 0.056 | 0.501 | −0.131 | 0.114 |
| Log-Glucose (mg/dL) | 0.061 | 0.465 | 0.134 | 0.106 |
| Albumin (mg/dL) | −0.132 | 0.112 | −0.239 | 0.004 * |
| Hemoglobin (g/dL) | 0.085 | 0.307 | −0.241 | 0.003 * |
| Log-BUN (mg/dL) | 0.096 | 0.249 | 0.534 | <0.001 * |
| Log-Creatinine (mg/dL) | 0.155 | 0.062 | 0.607 | <0.001 * |
| eGFR (mL/min) | −0.201 | 0.015 * | −0.548 | <0.001 * |
| Log-UPCR (g/g) | 0.202 | 0.014 * | 0.385 | <0.001 * |
| Marker | Population Studied | AS/Outcome Measured | Reported AUC | Sensitivity/Specificity (at Optimal Cutoff, If Available) | Citation |
|---|---|---|---|---|---|
| IS | Non-dialysis CKD stages 3–5 | PAS (baPWV > 18 m/s) | 0.667 | 66.13%/60.71% (at 0.996 µg/mL) | Current Study |
| Sclerostin | ESRD | Aortic stiffness (cfPWV > 10 m/s) | 0.673 | 35.14%/91.67% (at 208.64 pmol/L) | [42] |
| PCS | Non-dialysis CKD stages 3–5 | PAS (baPWV > 18 m/s) | 0.628 | 53.7%/70.9% (at 20.49 mg/L) | [43] |
| B2M | CKD stage ≥ 3, including dialysis | CV event | 0.684 | 85.1%/52.1% (at 4.21 mg/dL | [44] |
| Cr | CKD stage ≥ 3, including dialysis | CV event | 0.563 | Not reported | [44] |
| OPN | PD | Aortic stiffness (cfPWV > 10 m/s) | 0.903 | 86.36%/91.67% (at 39.67 ng/mL | [45] |
| MDA-LDL | HD | PAS (baPWV > 18 m/s) | 0.717 | 79.25%/59.57% (at 80.91 mg/dL | [46] |
| suPAR | HD | Aortic stiffness (cfPWV > 10 m/s) | 0.81 | 60.0%/88.7% (at 4.85 pg/mL) | [47] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chern, Y.-B.; Tsai, J.-P.; Liu, C.-H.; Lin, Y.-L.; Wang, C.-H.; Hsu, B.-G. Serum Indoxyl Sulfate as a Potential Biomarker of Peripheral Arterial Stiffness in Patients with Non-Dialysis Chronic Kidney Disease Stages 3 to 5. Toxins 2025, 17, 283. https://doi.org/10.3390/toxins17060283
Chern Y-B, Tsai J-P, Liu C-H, Lin Y-L, Wang C-H, Hsu B-G. Serum Indoxyl Sulfate as a Potential Biomarker of Peripheral Arterial Stiffness in Patients with Non-Dialysis Chronic Kidney Disease Stages 3 to 5. Toxins. 2025; 17(6):283. https://doi.org/10.3390/toxins17060283
Chicago/Turabian StyleChern, Yahn-Bor, Jen-Pi Tsai, Chin-Hung Liu, Yu-Li Lin, Chih-Hsien Wang, and Bang-Gee Hsu. 2025. "Serum Indoxyl Sulfate as a Potential Biomarker of Peripheral Arterial Stiffness in Patients with Non-Dialysis Chronic Kidney Disease Stages 3 to 5" Toxins 17, no. 6: 283. https://doi.org/10.3390/toxins17060283
APA StyleChern, Y.-B., Tsai, J.-P., Liu, C.-H., Lin, Y.-L., Wang, C.-H., & Hsu, B.-G. (2025). Serum Indoxyl Sulfate as a Potential Biomarker of Peripheral Arterial Stiffness in Patients with Non-Dialysis Chronic Kidney Disease Stages 3 to 5. Toxins, 17(6), 283. https://doi.org/10.3390/toxins17060283







