The Elias University Hospital Approach: A Visual Guide to Ultrasound-Guided Botulinum Toxin Injection in Spasticity: Part II—Proximal Upper Limb Muscles
Abstract
1. Introduction
2. Proximal Upper Limb Muscles Implicated in Post-Stroke Spasticity
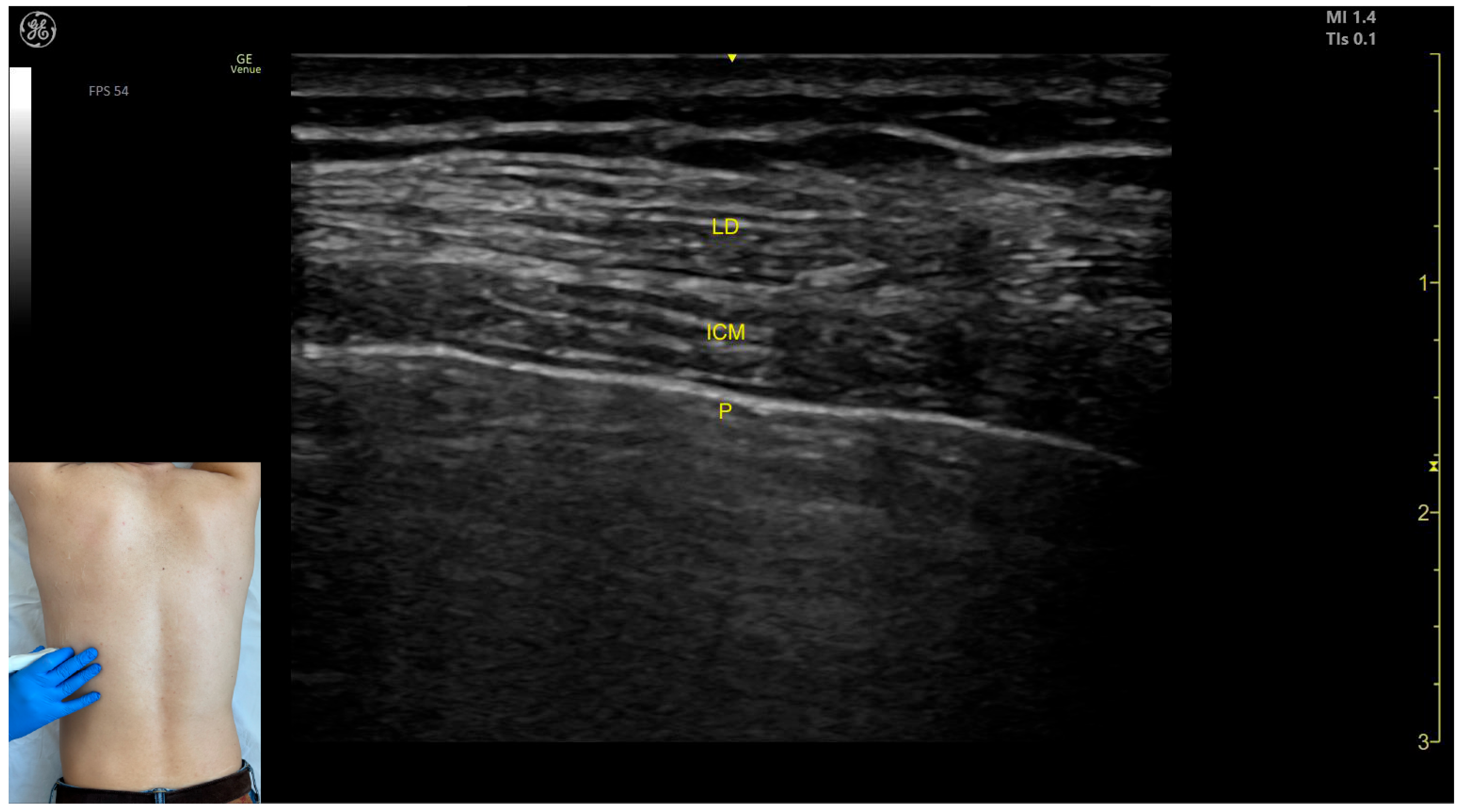
2.1. Latissimus Dorsi (LD)
2.1.1. Overview
2.1.2. Ultrasound Identification
2.1.3. Key Ultrasound Landmarks
- Muscle position: The LD is the most superficial muscle mass on the posterior surface of the trunk at this level.
- Muscle morphology: It has a more pronounced fascia that separates it from the subcutaneous plane than the fascia that separates it from the intercostal muscle (ICM) muscle during BoNT-A injections.
- Dynamic evaluation: Scanning proximally (~5 cm) shows a reduction in the size of the LD and the appearance and enlargement of the serratus anterior (SA) muscle beneath it. Contraction is visible with extension, adduction, and medial rotation maneuvers of the humerus at the shoulder joint [7]. At this level the LD has a more pronounced fascia that separates it from the subcutaneous plane and the SA muscle.
2.1.4. Clinical Implications and Injection Strategy
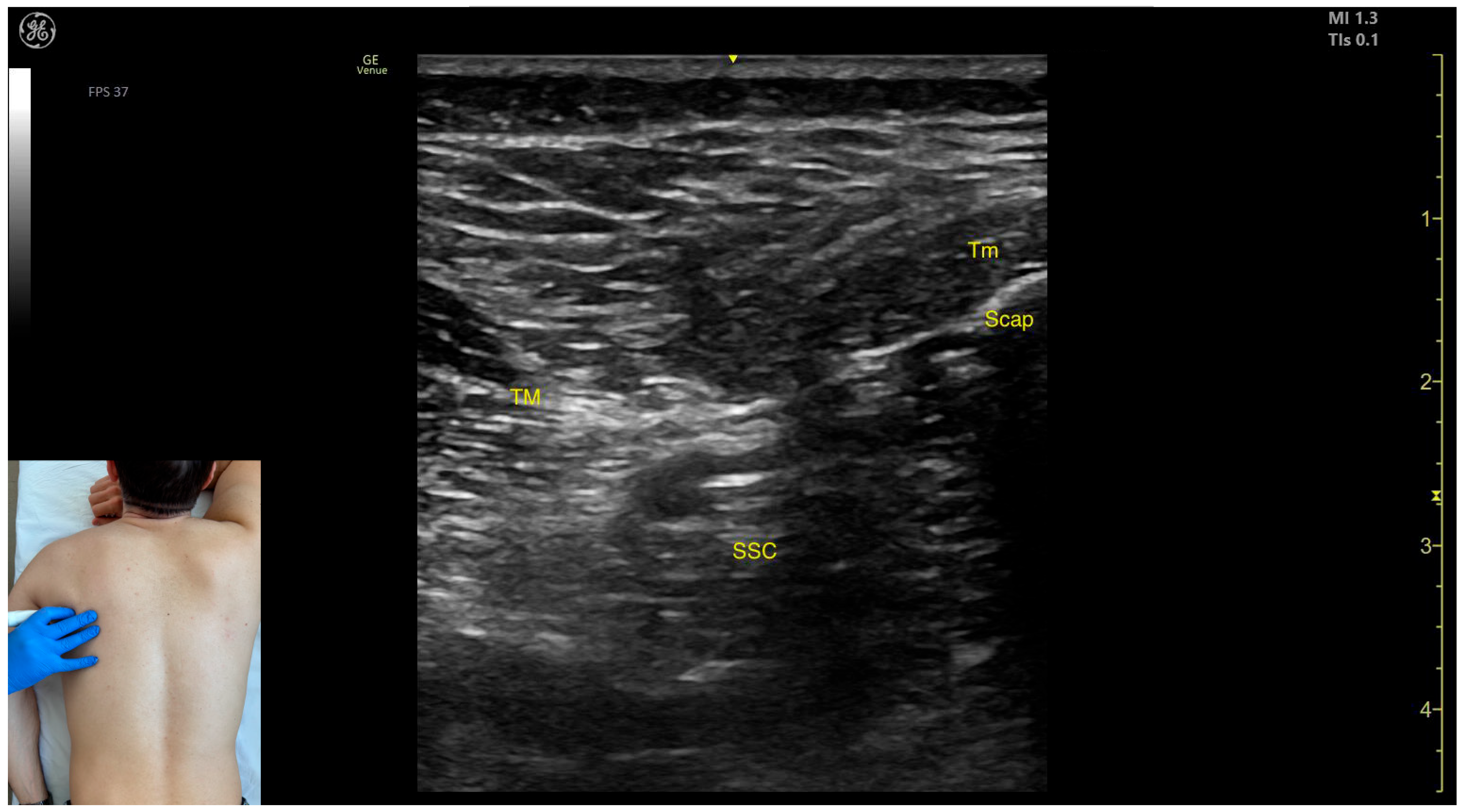
2.2. Teres Major (TM)
2.2.1. Overview
2.2.2. Ultrasound Identification
2.2.3. Key Ultrasound Landmarks
- Muscle morphology: The TM is a large muscle, visible from the depth of the rib cortex and intercostal muscles up to the subcutaneous plane.
- Muscle position: Medially and superficially, the teres minor (Tm) muscle is observed; medially and deeply, the subscapularis muscle and scapular cortex are visible; while laterally and deeply, the LD muscle is identified.
- External fascia: It has a pronounced fascia that separates it from adjacent muscle masses during BoNT-A injections.
- Dynamic evaluation: During dynamic evaluation, scanning proximally toward the axilla shows the transformation of the LD into a tendon, alongside which the TM forms the inferolateral portion of the posterior axillary wall [7]. The contraction of the TM is visible during adduction and internal rotation maneuvers of the humerus at the shoulder joint [12].
2.2.4. Clinical Implications and Injection Strategy
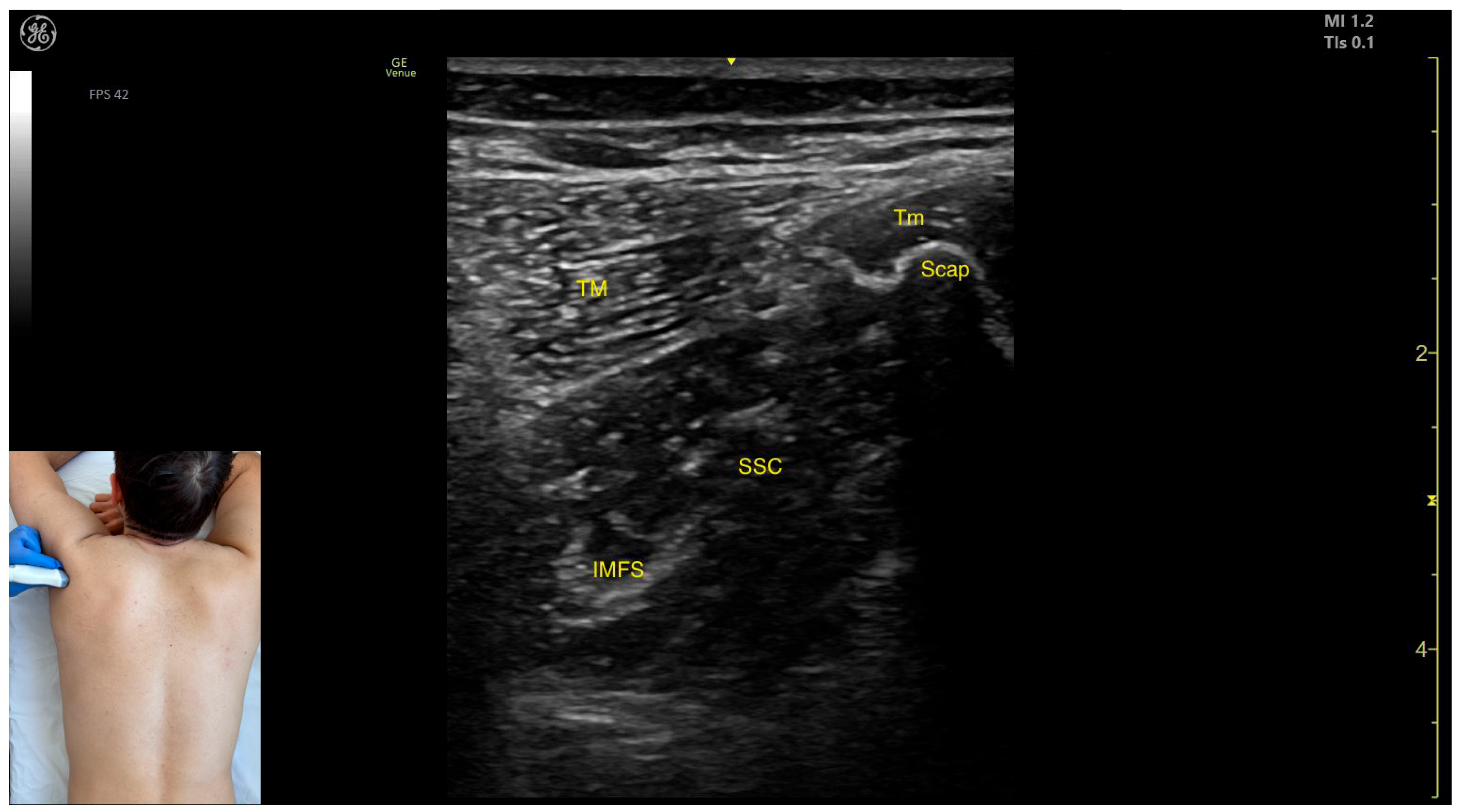
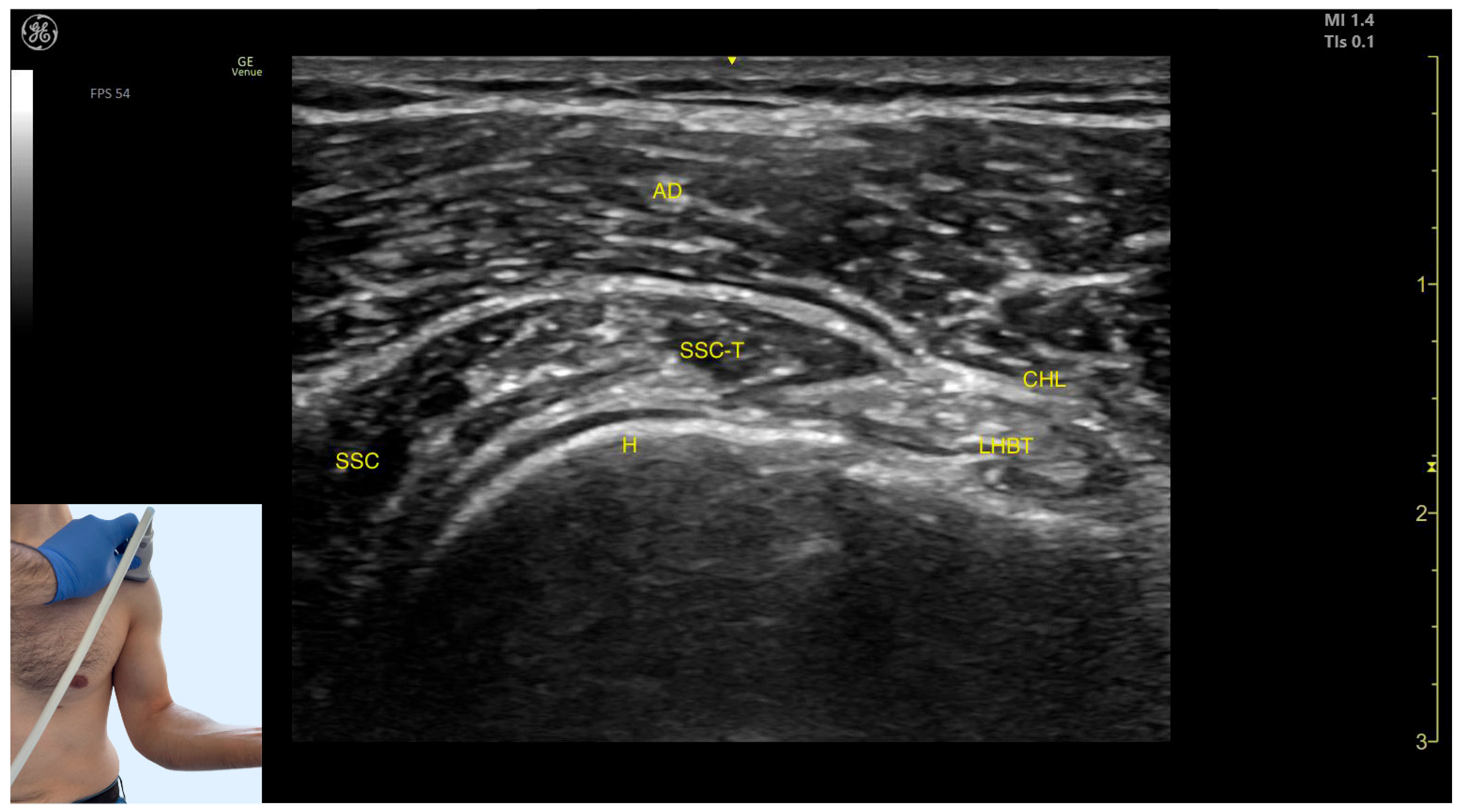
2.3. Subscapularis (SSC)
2.3.1. Overview
2.3.2. Ultrasound Identification
2.3.3. Key Ultrasound Landmarks
- Muscle position: At this level, the SSC represents the first muscle mass located superficial to the ribs and intercostal muscles. Superficial to the SSC, the TM is observed laterally, and the Tm is observed medially.
- Intramuscular fascial septum: It features an intramuscular fascial septum (IMFS) that separates the muscle into superior and inferior compartments, which can be approached individually if needed.
- External fascia: Unlike other muscles, the SSC does not present a pronounced fascial layer that separates it from the TM during BoNT-A injections.
- Superficial to the humeral cortex, the long head tendon of the biceps brachii (LHBT) can be seen, encased by the coracohumeral ligament (CHL).
- Medially, the tendon of the SSC and the muscle itself are visible.
- The anterior deltoid muscle is located superficially over all these structures.
2.3.4. Clinical Implications and Injection Strategy
- The superior compartment contains mainly type II fibers (fast-twitch). Along the muscle length—from the insertion on the lesser tubercle of the humerus to the origin at the subscapular fossa—the highest concentration of type I fibers (slow-twitch) is found around 22% of the distance from the insertion, while the type II fibers peak at approximately 77% of the muscle length, closer to the origin [21,23].
- The inferior compartment is composed predominantly of type I fibers (slow-twitch). The greatest density of type I fibers is located around 81% of the distance from the insertion to the origin, whereas type II fibers are most concentrated near 18% of the muscle length, closer to the insertion [21,23].
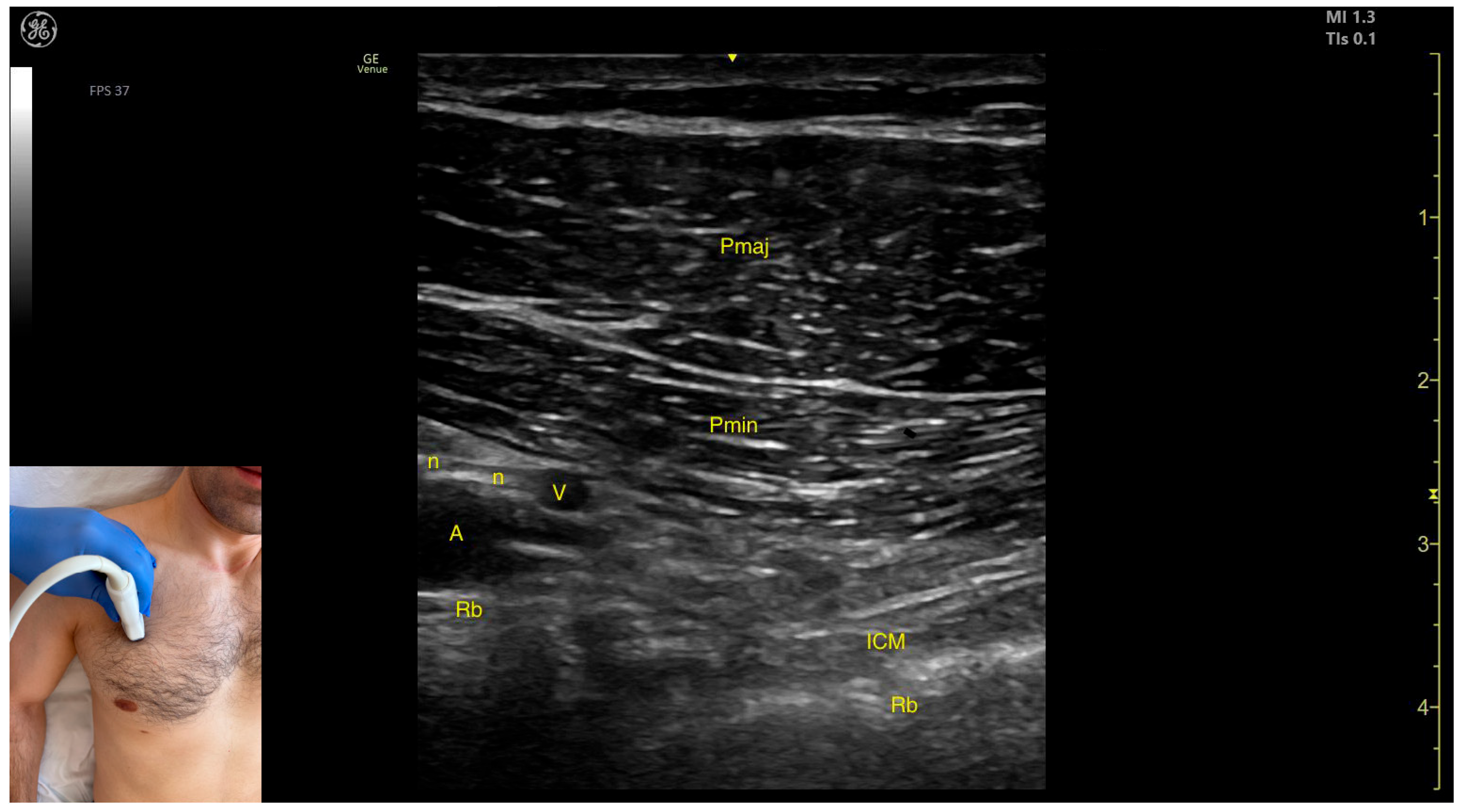
2.4. Pectoralis Major (Pmaj)
2.4.1. Overview
2.4.2. Ultrasound Identification
- The pectoralis minor (Pmin) muscle;
- The pectoralis major (Pmaj) muscle.
2.4.3. Key Ultrasound Landmarks
- Muscle morphology: The muscle has two heads that can be approached individually: the clavicular head (PMC), located laterally, and the sternocostal head (PMS), located medially. An intramuscular fascia (IMF) separates the two heads.
- External fascia: The Pmaj presents a pronounced fascia that clearly separates it from the subcutaneous plane and the Pmin during BoNT-A injections.
- Neurovascular landmark: The lateral pectoral nerve often courses alongside the thoracoacromial artery within the fascia separating P maj and P min. This can be targeted during diagnostic nerve blocks to distinguish reducible from non-reducible shoulder deformities in severe spasticity.
- Dynamic evaluation: During dynamic evaluation, scanning toward the humeral insertion reveals a gradual reduction in muscle thickness for both heads until they transform into tendons. Muscle contraction is visible during adduction and internal rotation maneuvers of the upper limb at the shoulder joint [19].
2.4.4. Clinical Implications and Injection Strategy
- For the clavicular head, the transducer is placed at a 45-degree angle toward the axilla, along the midclavicular line, approximately 2 cm from the clavicle.
- For the sternocostal head, the transducer is positioned longitudinally along the midclavicular line, about 5 cm distal to the clavicle.
2.5. Pectoralis Minor (Pmin)
2.5.1. Overview
2.5.2. Ultrasound Identification
2.5.3. Key Ultrasound Landmarks
- Muscle position: It represents the first muscle mass located superficial to the rib cortex and intercostal muscles at this level. Superficial to the Pmin, the Pmaj muscle is observed. Deep to the Pmin, the axillary artery and vein, along with distal nerve branches from the brachial plexus, are visible.
- External fascia: The Pmin has a pronounced fascia that separates it from the Pmaj during BoNT-A injections.
- Dynamic evaluation: During dynamic evaluation, scanning proximally toward the coracoid process of the scapula shows a decrease in muscle thickness until the muscle transforms into a tendon [37].
2.5.4. Clinical Implications and Injection Strategy
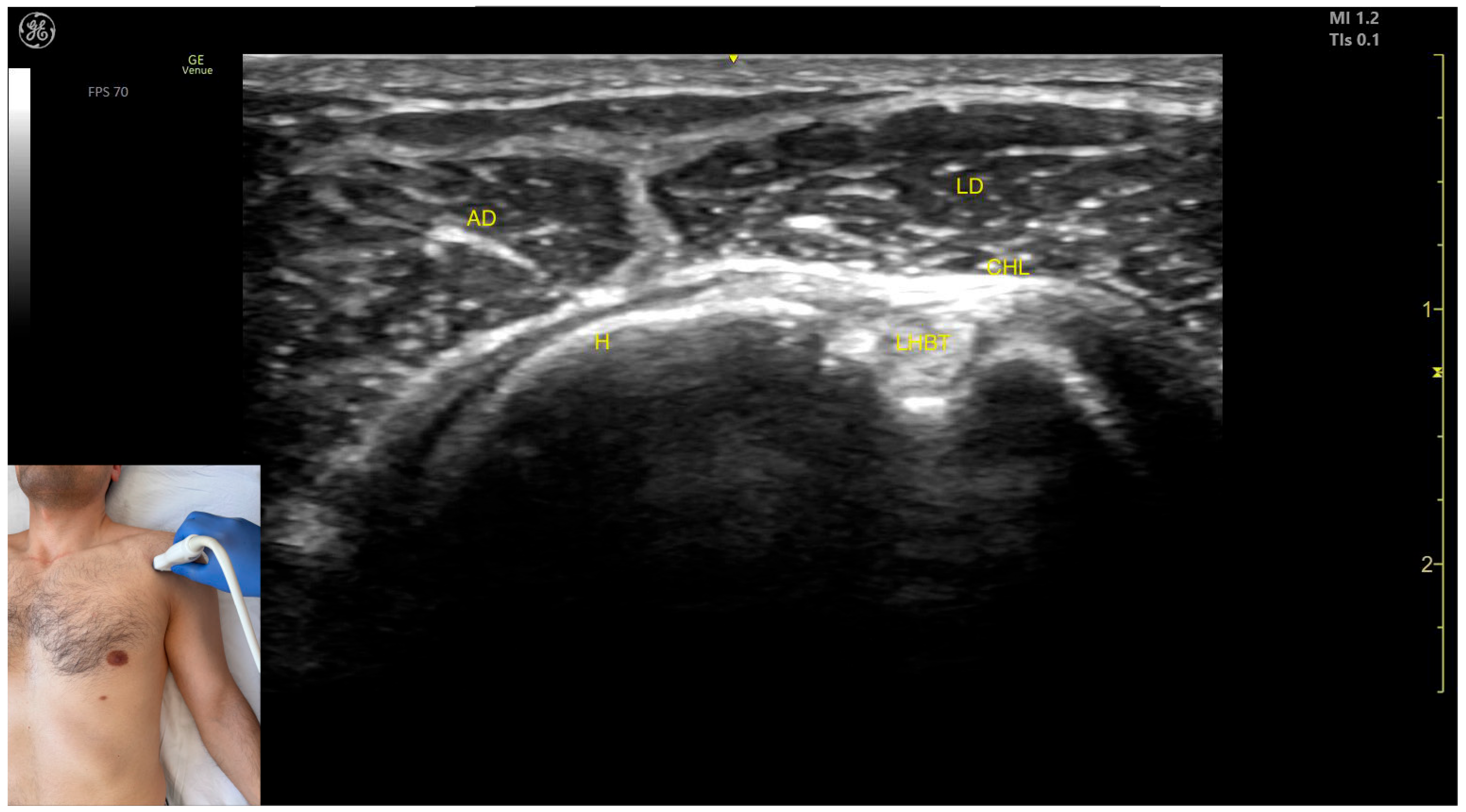
2.6. Deltoid Muscle
2.6.1. Overview
2.6.2. The Clavicular Part of the Deltoid Muscle
Ultrasound Identification
Key Ultrasound Landmarks
- Muscle position: It represents a superficial muscle mass on the anterior surface of the proximal arm. Deep to the anterior deltoid, the LHBT is located in the bicipital groove of the humerus, encased in the superficial portion of the coracohumeral ligament (CHL). Superficial to the LHBT, the lateral deltoid is observed, with the AD positioned anterior to the lateral deltoid.
- External fascia: The AD has a pronounced fascia that separates it from the subscapularis tendon, the lateral deltoid, the clavicular head of the pectoralis major, and the subcutaneous muscular plane during BoNT-A injections.
- Dynamic evaluation: During dynamic evaluation, scanning distally toward the elbow joint shows a decrease in the muscle size of the deltoid, coinciding with an increase in the muscle size of the biceps brachii. Scanning proximally toward the clavicle reveals the origin of the AD on the clavicle, along with the appearance and increased size of the clavicular head of the Pmaj [41]. Contraction of the anterior deltoid is seen during flexion and medial rotation maneuvers of the arm at the shoulder joint [12,39].
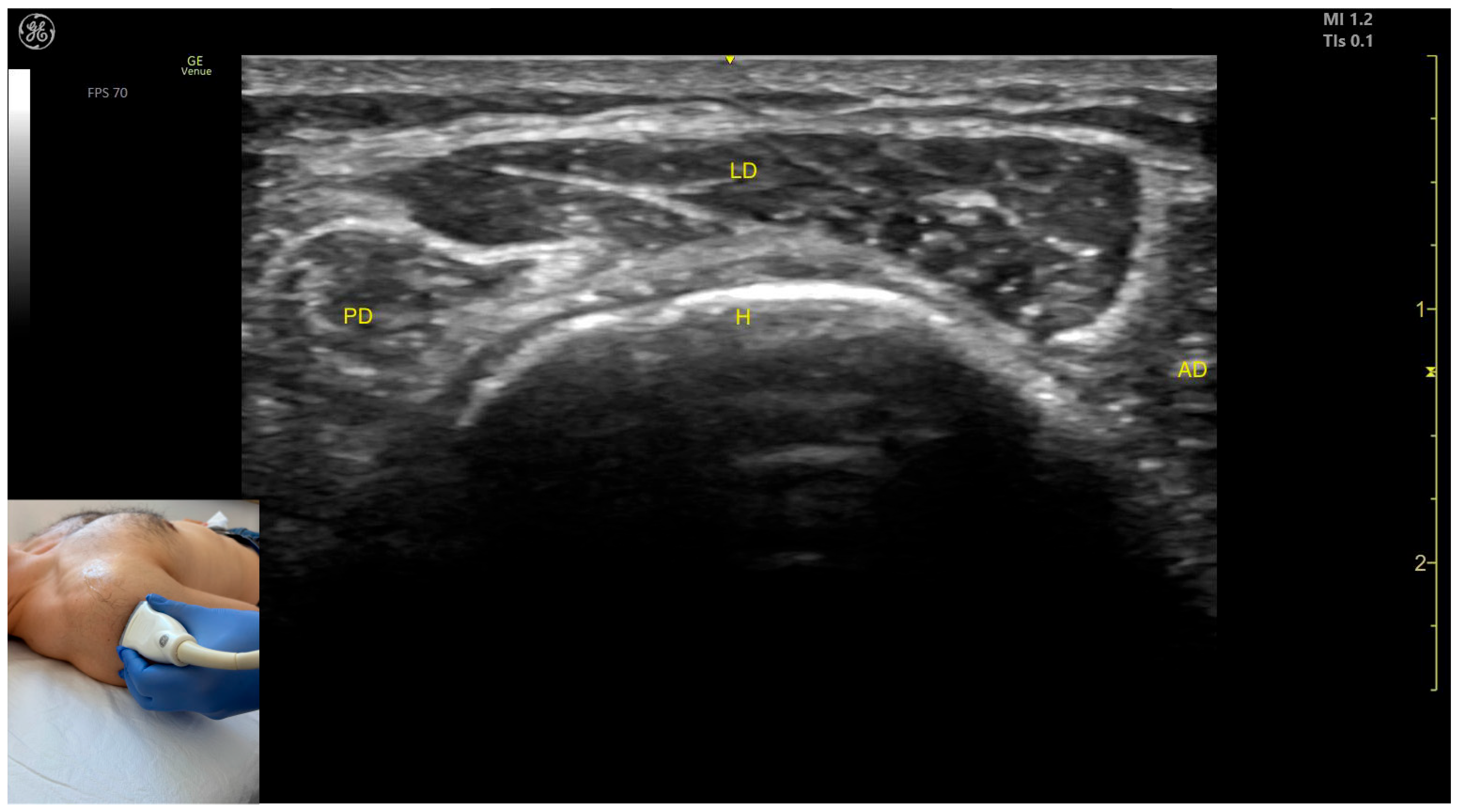
2.6.3. The Acromial Part of the Deltoid Muscle
Ultrasound Identification
Key Ultrasound Landmarks
- Muscle position: It represents a superficial muscle mass on the anterolateral surface of the proximal arm. The AD is observed anterior to the LD, while the posterior deltoid is observed posterior to it.
- External fascia: The LD has a pronounced fascia that separates it from the anterior and posterior deltoid parts, as well as from the subcutaneous plane during BoNT-A injections.
2.6.4. The Spinal Part of the Deltoid Muscle
Ultrasound Identification
Key Ultrasound Landmarks
- Muscle position: It represents a superficial muscle mass on the posterior surface of the proximal arm. Deep to the PD, the infraspinatus muscle and the glenoid labrum are observed.
- External fascia: The PD has a pronounced fascia that separates it from the infraspinatus muscle and the subcutaneous plane during BoNT-A injections.
- Dynamic evaluation: During dynamic evaluation, the origin of the PD at the spine of the scapula is seen when scanning proximally [41]. Contraction of the PD is visible during dynamic assessment when performing maneuvers involving adduction, extension, and lateral rotation of the arm at the shoulder joint [12,39].
2.6.5. Clinical Implications and Injection Strategy
- For the AD, the transducer is placed transversely on the anterior surface of the proximal arm, approximately 2–3 cm distal to the clavicle.
- For the LD, the transducer is positioned transversely on the anterolateral portion of the proximal arm, approximately 2–3 cm distal to the acromion.
- For the PD, the transducer is placed transversely on the posterior surface of the proximal arm, approximately 2 cm distal to the spine of the scapula.
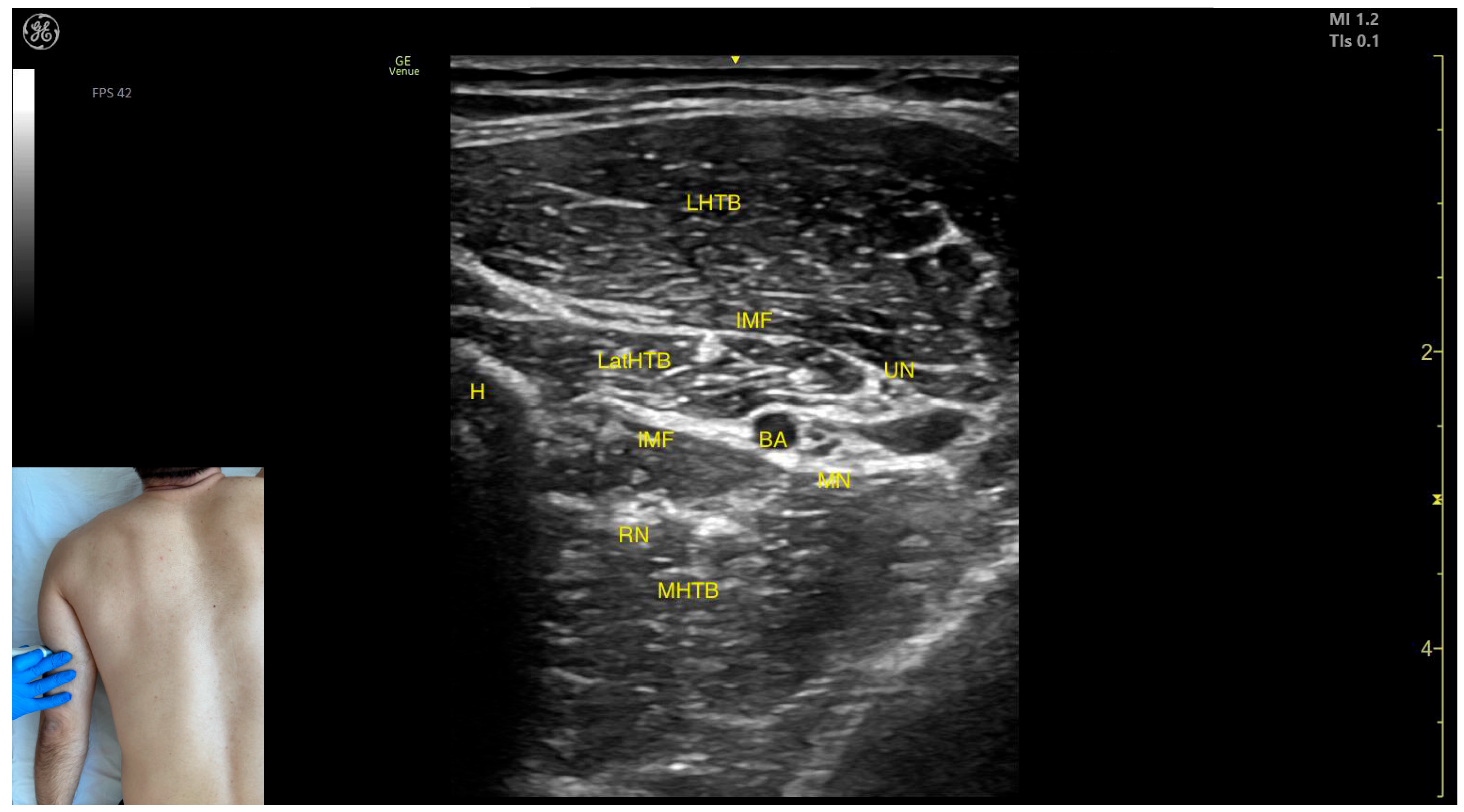
2.7. Triceps Brachii (TB)
2.7.1. Overview
- Flexion of the forearm at the elbow joint (involving all three heads of the muscle);
- Abduction and flexion of the arm at the shoulder joint (specific to the long head).
2.7.2. Ultrasound Identification
2.7.3. Key Ultrasound Landmarks
- Muscle position: At this level, it represents the only muscle mass in the posterior compartment of the arm.
- Muscle morphology: The TB has three heads—long, medial, and lateral—each of which can be approached individually. These three heads are separated by intramuscular fasciae:
- 1.
- Long head (LHTB): Located superficial to the cortical bone of the humerus.
- 2.
- Medial head (MHTB): Found medial to the humeral cortex.
- 3.
- Lateral head (LatHTB): Situated between the long and medial heads.
- Innervation and vascular supply: In the medial portion of the TB, the neurovascular bundle is observed, consisting of three nerves—median, radial, and ulnar—and two vascular structures, namely the brachial artery with its branches and the brachial vein.
- External fascia: The TB features a pronounced fascia that separates it from the superficial plane during BoNT-A injections.
- Dynamic evaluation: During dynamic evaluation, scanning distally toward the elbow joint reveals a decrease in the size of the long and lateral heads, while the medial head shows an increase in size. Muscle contraction is visible during elbow extension maneuvers, confirming its role in this movement [12,50].
2.7.4. Clinical Implications and Injection Strategy
- Long head: Injected at two sites—one at the midpoint of the posterior arm and another 2 cm proximal to this level, closer to the shoulder joint.
- Lateral head: Injected at the midpoint of the posterior arm.
- Medial head: Injected 1 cm distal to the midpoint of the posterior arm.
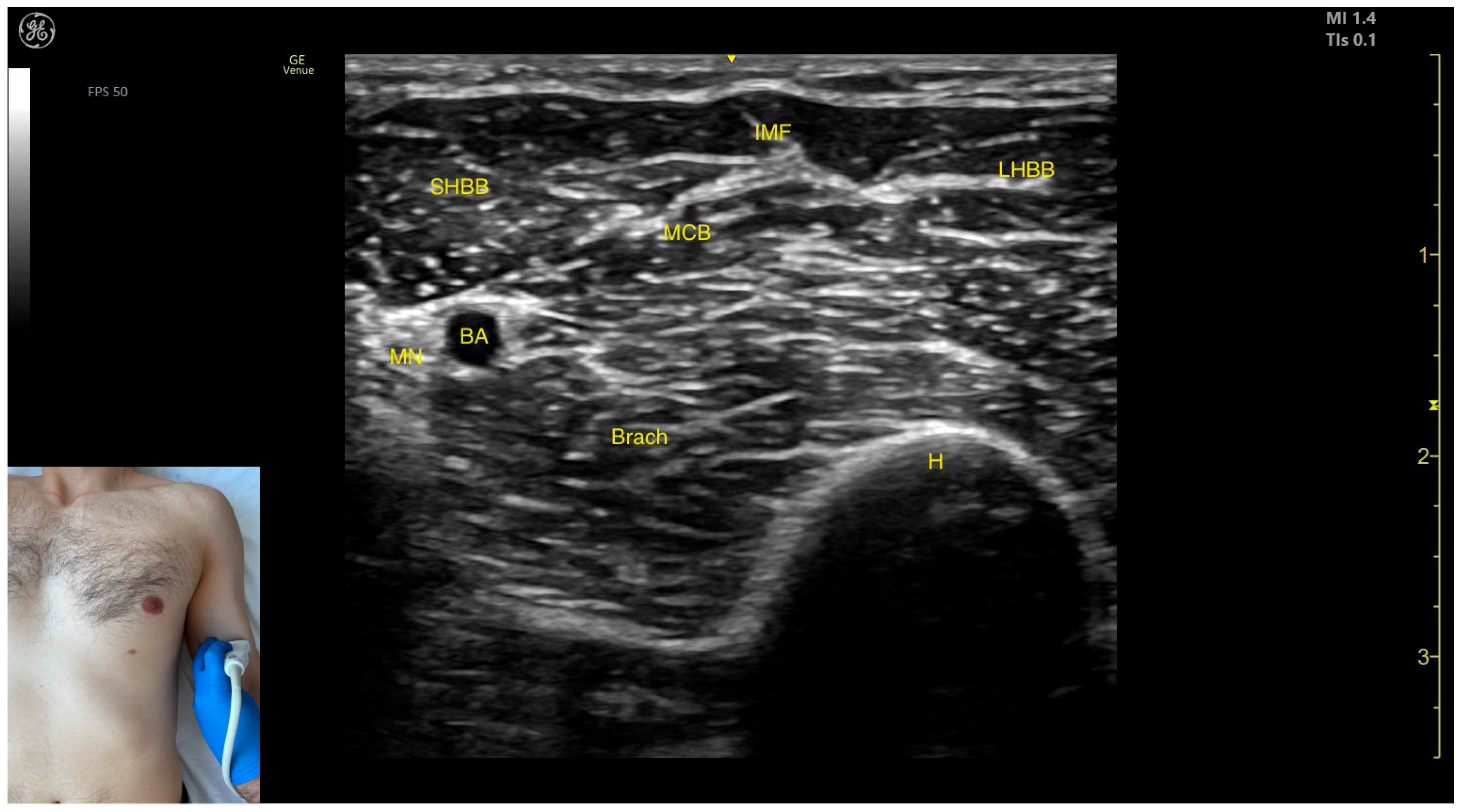
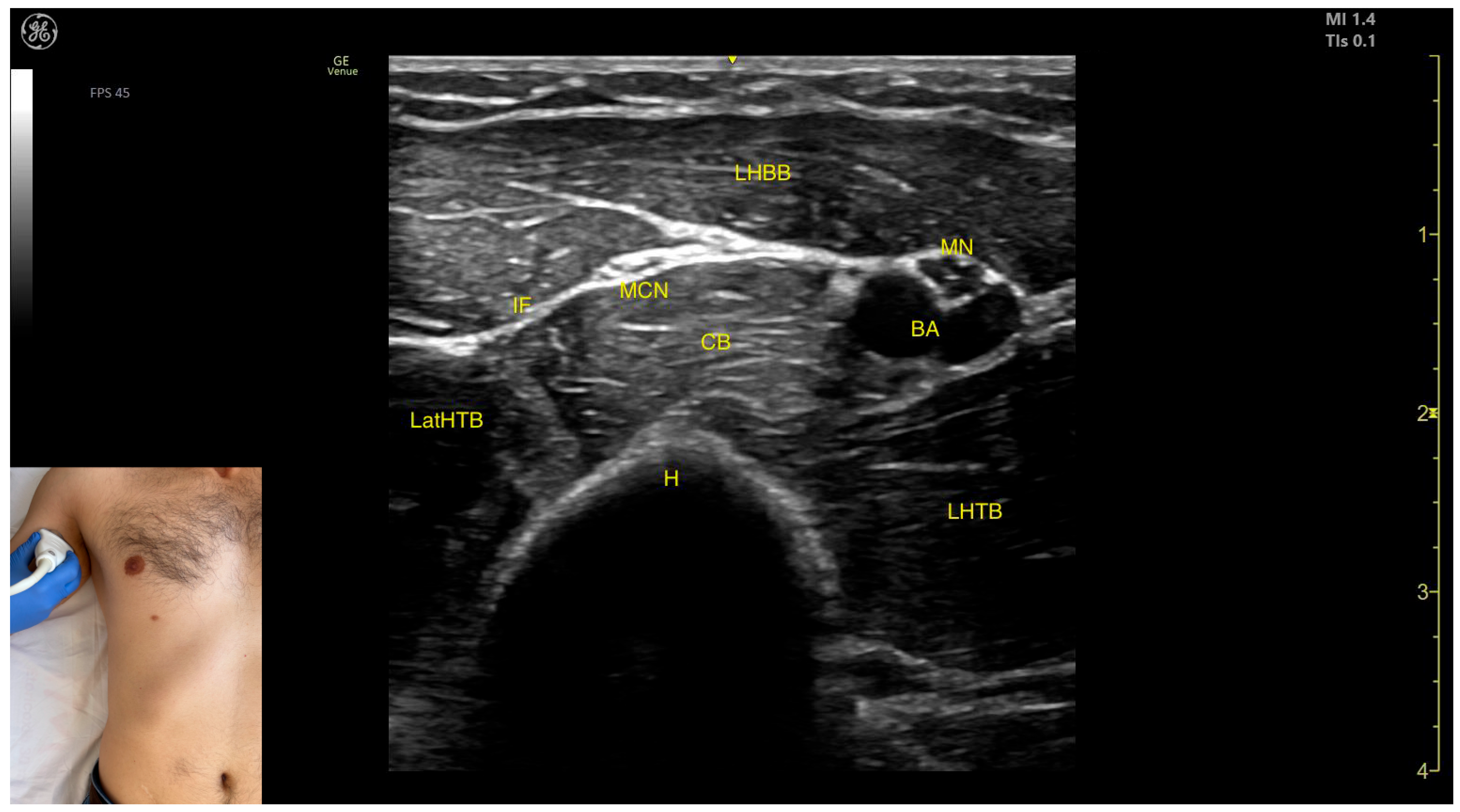
2.8. Biceps Brachii (BB)
2.8.1. Overview
2.8.2. Ultrasound Identification
2.8.3. Key Ultrasound Landmarks
- Muscle position: It represents the most superficial muscle mass of the anterior arm at this level [57].
- Muscle morphology: It has two heads, with the long head (LHBB) located laterally and the short head (SHBB) positioned medially, separated by an intramuscular fascia that allows them to be individually targeted during procedures.
- Innervation and vascular supply: In the depth of the muscle at the level of the bicipital fossa (medial portion), the brachial artery is observed laterally, while the median nerve is located medially to the artery, with the bicipital fossa representing a longitudinal groove bordered anteriorly by the BB and brachialis muscles and posteriorly by the medial head of the triceps brachii.
- External fascia: The BB lacks a pronounced fascia that separates it from the brachialis, which is relevant when performing BoNT-A injections.
- Dynamic evaluation: During dynamic evaluation (Video S1), proximal scanning toward the shoulder joint reveals a decrease in the size of the brachialis muscle until it transforms into a tendon at the mid-arm level, where the insertion of the coracobrachialis (CB) muscle on the humerus can be observed deep to the BB. Scanning further toward the proximal third of the arm shows the inversion of the positions of the median nerve and brachial artery within the bicipital fossa [57]. Continuing the scan proximally toward the shoulder joint reveals an increase in the size of the CB muscle in the medial portion of the arm. The musculocutaneous nerve is usually seen piercing the coracobrachialis before descending in the intermuscular fascia (IF) between the BB and brachialis. The CB muscle is not injected with BoNT-A due to its minor role as a weak flexor of the arm at the shoulder joint. The musculocutaneous nerve is targeted during diagnostic nerve block procedures to differentiate between spasticity and rigidity in cases where severe spasticity restricts elbow flexion or extension. Branches of the musculocutaneous nerve can be visualized via US on the anterior surface of the BB, appearing prominently and potentially being mistaken for intramuscular fascia [58]. The contraction of the BB can be observed during flexion maneuvers of the elbow and shoulder joints. With the elbow flexed at 90 degrees, performing forearm supination shows the BB gliding over the underlying brachialis muscle (Video S2) [12,55].
2.8.4. Clinical Implications and Injection Strategy
- At this level, the short head is injected into the medial portion;
- The long head is injected into the lateral portion.
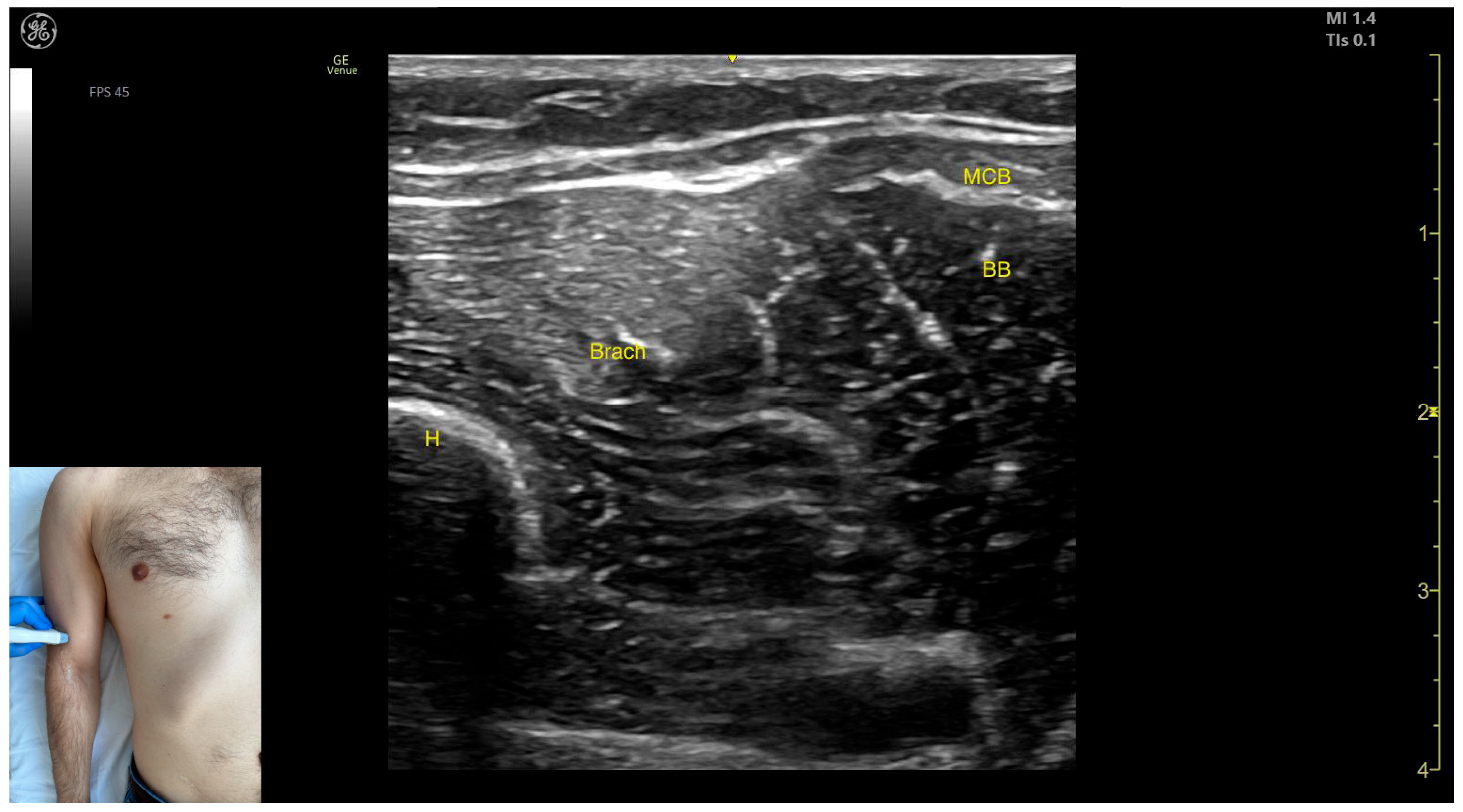
2.9. Brachialis Muscle (Brach)
2.9.1. Overview
2.9.2. Ultrasound Identification
2.9.3. Key Anatomical Landmarks
- Muscle position: It represents the first muscle mass located superficial to the cortical bone of the humerus on the anterior lateral surface of the arm.
- Innervation: The radial nerve is seen superficially in the intermuscular fascia, which separates the Brach from the brachioradialis muscle.
- External fascia: The Brach has a pronounced fascia that separates it from the brachioradialis. A very thin fascia separates it from the BB during BoNT-A injections.
- Dynamic evaluation: During dynamic evaluation, proximal scanning toward the shoulder joint shows the radial nerve moving closer to the cortical bone of the humerus before following an oblique path and disappearing from the view as it enters the posterior compartment of the arm. Medial scanning reveals the BB muscle superficial to the Brach. Muscle contraction of the Brach is visible during forearm flexion at the elbow joint, as it is the only pure flexor of the elbow [7].
2.9.4. Clinical Implications and Injection Strategy
- Medial to the radial nerve, the zone of maximum thickness of the Brach is visualized.
- Medial to the Brach at this level lies the BB, which can be dynamically confirmed by performing a forearm supination maneuver (Video S3).
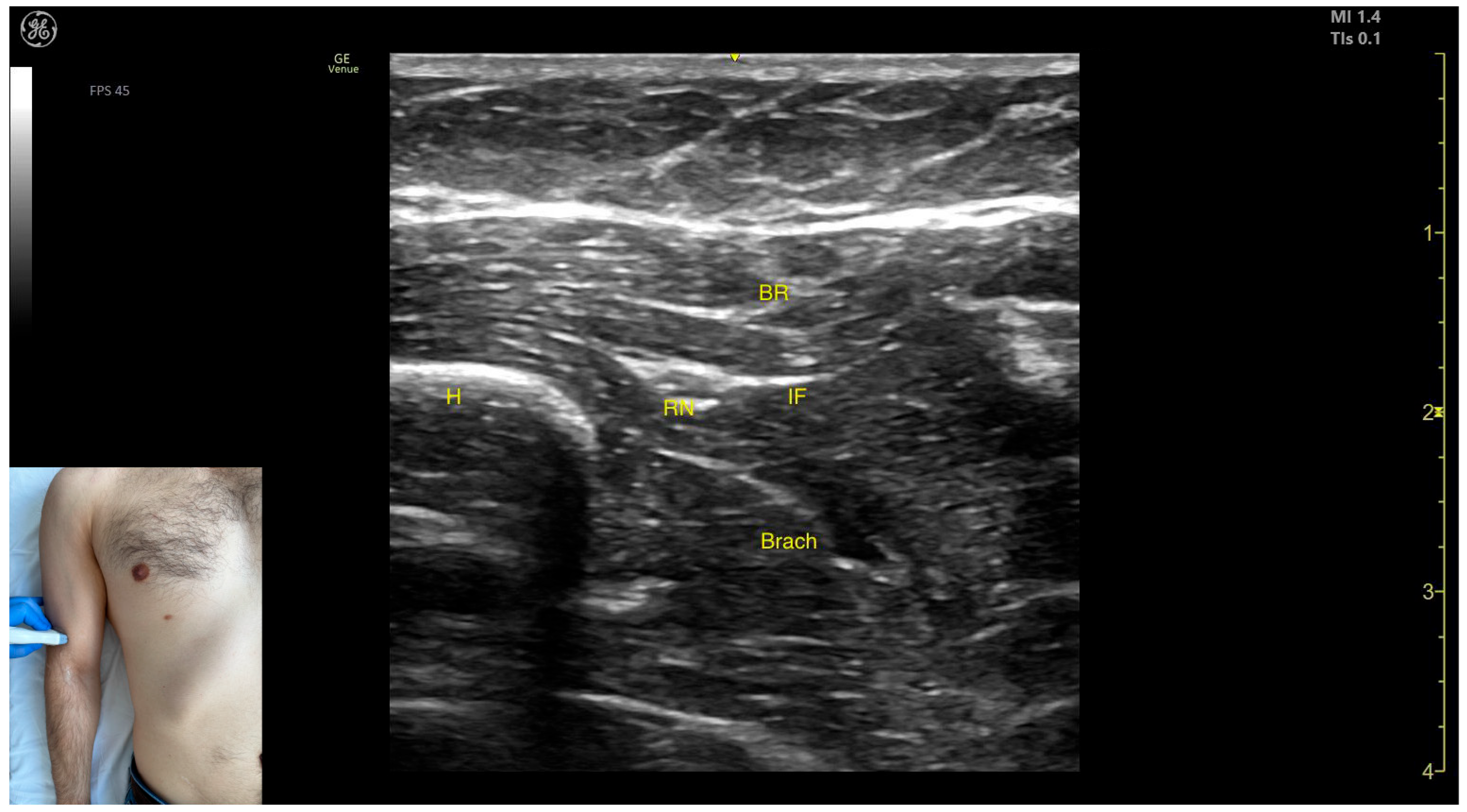
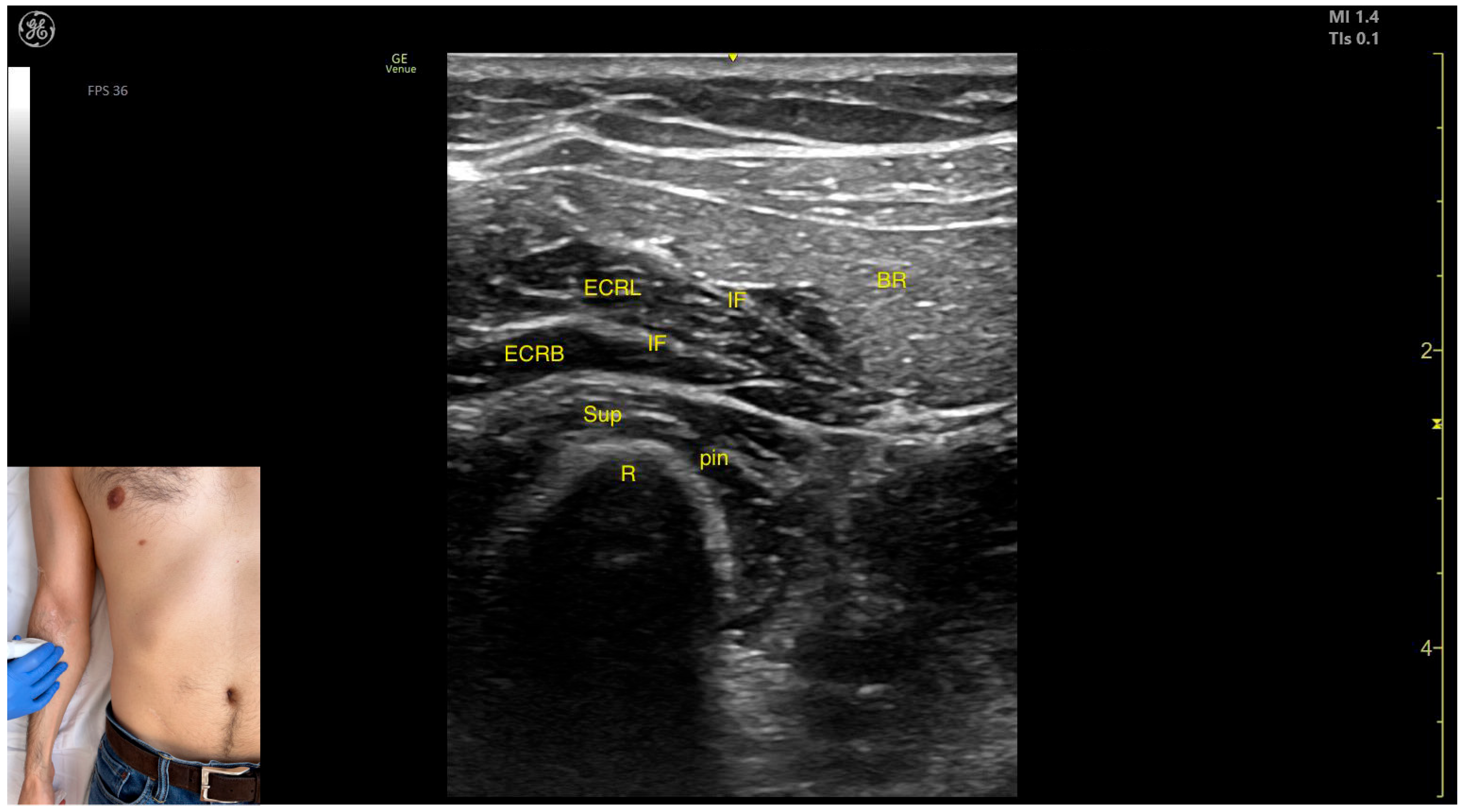
2.10. Brachioradialis (BR)
2.10.1. Overview
2.10.2. The Arm Compartment
Ultrasound Identification
Key Anatomical Landmarks
- Muscle position: It represents the most superficial muscle mass on the anterolateral surface of the arm at this level.
- Innervation: The radial nerve is located deep within the muscle, enclosed in the intermuscular fascia that separates the BR from the Brach muscle.
- External fascia: The BR has a pronounced fascia that separates it from both the Brach and the subcutaneous plane during BoNT-A injections.
- Dynamic evaluation: During dynamic evaluation, distal scanning toward the lateral epicondyle reveals the division of the radial nerve into its superficial branch and deep branch (posterior interosseous nerve), with the deep branch continuing along the anterolateral proximal third of the forearm.
2.10.3. The Forearm Compartment
Ultrasound Identification
Key Anatomical Landmarks
- Muscle position: It represents the most superficial muscle mass at this level.
- External fascia: It has a thin intermuscular fascia that separates it from the extensor carpi radialis longus muscle, located deep to it.
- Dynamic evaluation: During dynamic evaluation, the BR can be differentiated from the extensor carpi radialis longus by performing wrist extension and abduction maneuvers with the forearm in pronation, during which contraction of the extensor carpi radialis longus is observed (Video S4). Contraction is visible both in the arm and forearm during elbow extension maneuvers, with the forearm positioned midway between supination and pronation.
2.10.4. Clinical Implications and Injection Strategy
- The transducer is placed transversely on the anterolateral surface of the arm, approximately 2–3 cm proximal to the lateral epicondyle.
- The transducer is placed transversely on the anterolateral surface of the forearm, approximately 2–3 cm distal to the lateral epicondyle.
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BoNT-A | botulinum toxin type A |
| EUH | Elias University Hospital |
| LD | latissimus dorsi (muscle) |
| SA | serratus anterior (muscle) |
| ICM | intercostal muscle |
| P | pleura |
| Rb | rib |
| TM | teres major (muscle) |
| Tm | teres minor (muscle) |
| SSC | subscapularis (muscle) |
| Scap | scapula |
| US | ultrasound |
| IMFS | intramuscular fascial septum |
| LHBT | long head of the biceps brachii tendon |
| CHL | coracohumeral ligament |
| AD | anterior deltoid (muscle) |
| SSC-T | subscapularis tendon |
| Pmaj | pectoralis major (muscle) |
| Pmin | pectoralis minor (muscle) |
| PMC | clavicular head of the pectoralis major (muscle) |
| PMS | sternocostal head of the pectoralis major (muscle) |
| IMF | intramuscular fascia |
| n | nerve |
| A | artery |
| V | vein |
| ICM | intercostal muscle |
| LD | lateral deltoid (muscle) |
| PD | posterior deltoid (muscle) |
| GL | glenoid labrum |
| TB | triceps brachii (muscle) |
| LHTB | long head of the triceps brachii (muscle) |
| MHTB | medial head of the triceps brachii (muscle) |
| LatHTB | lateral head of the triceps brachii (muscle) |
| BA | brachial artery |
| MN | median nerve |
| RN | radial nerve |
| UN | ulnar nerve |
| BB | biceps brachii (muscle) |
| SHBB | short head of the biceps brachii (muscle) |
| LHBB | long head of the biceps brachii (muscle) |
| CB | coracobrachialis (muscle) |
| MCB | musculocutaneous branch (nerve) |
| Brach | brachialis (muscle) |
| IF | intermuscular fascia |
| EMG | electromyography |
| BR | brachioradialis (muscle) |
| ECRL | extensor carpi radialis longus (muscle) |
| ECRB | extensor carpi radialis brevis (muscle) |
| Sup | supinator (muscle) |
| pin | posterior interosseous nerve |
| H | humerus |
References
- Popescu, M.N.; Petca, R.C.; Beiu, C.; Dumitrascu, M.C.; Petca, A.; Mehedintu, C.; Farcasanu, P.D.; Sandru, F. Efficiency of Different Preparations of Botulinum Toxin Type A, Xeomin and Dysport, in the Management of Spastic Upper Limb After Stroke. Rehabilitation 2019, 70, 3490–3494. [Google Scholar] [CrossRef]
- Specchia, A.; Ferreri, G.; Cassetta, E.; Cerbarano, L.; Bentivoglio, A.R. Practical Handbook for Ultrasound-Guided Botulinum Toxin Injection; Edi.Ermes srl: Millan, Italy, 2020; ISBN 978-88-7051-717-0. [Google Scholar]
- Malfitano, C.; Robecchi Majnardi, A.; Pesaresi, A.; Ricci, V. Ultrasound-Guided Botulinum Toxin Injections for Hand Spasticity: A Technical Guide for the Dorsal Approach. Toxins 2025, 17, 225. [Google Scholar] [CrossRef] [PubMed]
- Alter, K.E.; Karp, B.I. Ultrasound Guidance for Botulinum Neurotoxin Chemodenervation Procedures. Toxins 2018, 10, 18. [Google Scholar] [CrossRef]
- Kara, M.; Kaymak, B.; Ulaşli, A.M.; Tok, F.; Öztürk, G.T.; Chang, K.-V.; Hsiao, M.-Y.; Hung, C.-Y.; Yağiz On, A.; Özçakar, L. Sonographic Guide for Botulinum Toxin Injections of the Upper Limb: EUROMUSCULUS/USPRM Spasticity Approach. Eur. J. Phys. Rehabil. Med. 2018, 54, 469–485. [Google Scholar] [CrossRef]
- Popescu, M.N.; Căpeț, C.; Beiu, C.; Berteanu, M. The Elias University Hospital Approach: A Visual Guide to Ultrasound-Guided Botulinum Toxin Injection in Spasticity: Part I—Distal Upper Limb Muscles. Toxins 2025, 17, 107. [Google Scholar] [CrossRef]
- Drake, R.L.; Vogl, A.W.; Mitchell, A.W.M. Gray’s Anatomy for Students; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Netter, F.H. Atlas of Human Anatomy, 6th ed.; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Pagan-Rosado, R.; Kindle, B.; North, T.; Smith, J. Ultrasound Imaging Protocol for Latissimus Dorsi and Teres Major in Overhead Athletes: A Comprehensive Approach. J. Ultrasound Med. 2025, 44, 945–954. [Google Scholar] [CrossRef] [PubMed]
- Yi, K.-H.; Lee, H.-J.; Seo, K.K.; Kim, H.-J. Intramuscular Neural Arborization of the Latissimus Dorsi Muscle: Application of Botulinum Neurotoxin Injection in Flap Reconstruction. Toxins 2022, 14, 107. [Google Scholar] [CrossRef]
- Jacinto, J.; Camões-Barbosa, A.; Carda, S.; Hoad, D.; Wissel, J. A Practical Guide to Botulinum Neurotoxin Treatment of Shoulder Spasticity 1: Anatomy, Physiology, and Goal Setting. Front. Neurol. 2022, 13, 100629. [Google Scholar] [CrossRef]
- Palastanga, N.; Soames, R. Anatomy and Human Movement Structure and Function, 7th ed.; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Arrillaga, B.; Miguel-Pérez, M.; Möller, I.; Rubio, L.; Blasi, J.; Pérez-Bellmunt, A.; Ortiz-Sagristà, J.C.; Ortiz-Miguel, S.; Martinoli, C. Human Shoulder Anatomy: New Ultrasound, Anatomical, and Microscopic Perspectives. Anat. Sci. Int. 2024, 99, 290–304. [Google Scholar] [CrossRef]
- Ceballos-Laita, L.; Medrano-de-la-Fuente, R.; Estébanez-De-Miguel, E.; Moreno-Cerviño, J.; Mingo-Gómez, M.T.; Hernando-Garijo, I.; Jiménez-Del-Barrio, S. Effects of Dry Needling in Teres Major Muscle in Elite Handball Athletes. A Randomised Controlled Trial. J. Clin. Med. 2021, 10, 4260. [Google Scholar] [CrossRef]
- Struyf, P.; Triccas, L.T.; Schillebeeckx, F.; Struyf, F. The Place of Botulinum Toxin in Spastic Hemiplegic Shoulder Pain after Stroke: A Scoping Review. Int. J. Environ. Res. Public. Health 2023, 20, 2797. [Google Scholar] [CrossRef]
- Bell, K.R.; Williams, F. Use of Botulinum Toxin Type A and Type B for Spasticity in Upper and Lower Limbs. Phys. Med. Rehabil. Clin. N. Am. 2003, 14, 821–835. [Google Scholar] [CrossRef]
- Sheean, G. Botulinum Toxin Treatment of Adult Spasticity. Expert Rev. Neurother. 2003, 3, 773–785. [Google Scholar] [CrossRef]
- Yi, K.-H.; Lee, K.-W.; Hu, H.-W.; Lee, J.-H.; Lee, H.-J. A Practical Guide to Botulinum Neurotoxin Treatment of Teres Major Muscle in Shoulder Spasticity: Intramuscular Neural Distribution of Teres Major Muscle in Cadaver Model. PM&R 2024, 16, 160–164. [Google Scholar] [CrossRef]
- Moore, K.L.; Agur, A.M.R.; Dalley, A.F. Clinically Oriented Anatomy, 8th ed.; Wolters Kluwer: Philadelphia, PA, USA, 2018. [Google Scholar]
- Corazza, A.; Orlandi, D.; Fabbro, E.; Ferrero, G.; Messina, C.; Sartoris, R.; Bernardi, S.P.; Arcidiacono, A.; Silvestri, E.; Sconfienza, L.M. Dynamic High-Resolution Ultrasound of the Shoulder: How We Do It. Eur. J. Radiol. 2015, 84, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, K.; Mudreac, A.; Kiel, J. Anatomy, Shoulder and Upper Limb, Subscapularis Muscle. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Zielinska, N.; Tubbs, R.S.; Borowski, A.; Podgórski, M.; Olewnik, Ł. The Subscapularis Muscle: A Proposed Classification System. BioMed Res. Int. 2021, 2021, 7450000. [Google Scholar] [CrossRef]
- Cho, T.-H.; Hong, J.-E.; Yang, H.-M. Neuromuscular Compartmentation of the Subscapularis Muscle and Its Clinical Implication for Botulinum Neurotoxin Injection. Sci. Rep. 2023, 13, 11167. [Google Scholar] [CrossRef]
- Xue, H.; Bird, S.; Jiang, L.; Jiang, J.; Cui, L. Anchoring Apparatus of Long Head of the Biceps Tendon: Ultrasonographic Anatomy and Pathologic Conditions. Diagnostics 2022, 12, 659. [Google Scholar] [CrossRef]
- Pinho, S.; Camões-Barbosa, A.; Hatia, M.; Moeda, F.; Melo, X.; Tocha, J. Shoulder Spasticity Treatment With Botulinum Toxin: A Nationwide Cross-Sectional Survey of Clinical Practices. Cureus 2023, 15, e48493. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, M.P.; Pinto, D.; Gorayeb, M.; Jacinto, J. Analysis of a 15-Years’ Experience in Including Shoulder Muscles, When Treating Upper-Limb Spasticity Post-Stroke with Botulinum Toxin Type A. Top. Stroke Rehabil. 2018, 25, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.; Jia, L. Ultrasound-Guided BoNT-A (Botulinum Toxin A) Injection Into the Subscapularis for Hemiplegic Shoulder Pain: A Randomized, Double-Blind, Placebo-Controlled Trial. Stroke 2021, 52, 3759–3767. [Google Scholar] [CrossRef]
- Yelnik, A.P.; Colle, F.M.; Bonan, I.V.; Vicaut, E. Treatment of Shoulder Pain in Spastic Hemiplegia by Reducing Spasticity of the Subscapular Muscle: A Randomised, Double Blind, Placebo Controlled Study of Botulinum Toxin A. J. Neurol. Neurosurg. Psychiatry 2007, 78, 845–848. [Google Scholar] [CrossRef]
- Lieber, R.L.; Steinman, S.; Barash, I.A.; Chambers, H. Structural and Functional Changes in Spastic Skeletal Muscle. Muscle Nerve 2004, 29, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Gracies, J.-M. Pathophysiology of Spastic Paresis. I: Paresis and Soft Tissue Changes. Muscle Nerve 2005, 31, 535–551. [Google Scholar] [CrossRef] [PubMed]
- Chiavaras, M.M.; Jacobson, J.A.; Smith, J.; Dahm, D.L. Pectoralis Major Tears: Anatomy, Classification, and Diagnosis with Ultrasound and MR Imaging. Skelet. Radiol. 2015, 44, 157–164. [Google Scholar] [CrossRef]
- Aktas, I.; Kaya, E.; Akpinar, P.; Atici, A.; Ozkan, F.U.; Palamar, D.; Akgun, K. Spasticity-Induced Pectoralis Minor Syndrome: A Case-Report. Top. Stroke Rehabil. 2020, 27, 316–319. [Google Scholar] [CrossRef]
- Howard, M.; Jones, M.; Clarkson, R.; Donaldson, O. Pectoralis Minor Syndrome: Diagnosis with Botulinum Injection and Treatment with Tenotomy—A Prospective Case Series. Shoulder Elb. 2022, 14, 157–161. [Google Scholar] [CrossRef]
- Kassam, F.; Lim, B.; Afroz, S.; Boissonnault, È.; Reebye, R.; Finlayson, H.; Winston, P. Canadian Physicians’ Use of Intramuscular Botulinum Toxin Injections for Shoulder Spasticity: A National Cross-Sectional Survey. Toxins 2023, 15, 58. [Google Scholar] [CrossRef]
- Li, Y.; Wang, M.; Tang, S.; Zhu, X.; Yang, S. Localization of Nerve Entry Points and the Center of Intramuscular Nerve-dense Regions in the Adult Pectoralis Major and Pectoralis Minor and Its Significance in Blocking Muscle Spasticity. J. Anat. 2021, 239, 1123–1133. [Google Scholar] [CrossRef] [PubMed]
- Sucher, B.M. Thoracic Outlet Syndrome-Postural Type: Ultrasound Imaging of Pectoralis Minor and Brachial Plexus Abnormalities. PM&R 2012, 4, 65–72. [Google Scholar] [CrossRef]
- Khan, A.; Anderson, S.; Strakowski, J.; Vallabh, J. Use of Dynamic Ultrasound Imaging in the Evaluation of Neurogenic Pectoralis Minor Syndrome. Am. J. Phys. Med. Rehabil. 2023, 102, e144–e145. [Google Scholar] [CrossRef]
- Lee, J.-H.; Lee, H.-J.; Yi, K.-H.; Lee, K.-W.; Gil, Y.-C.; Kim, H.-J. Ideal Injection Points for Botulinum Neurotoxin for Pectoralis Minor Syndrome: A Cadaveric Study. Toxins 2023, 15, 603. [Google Scholar] [CrossRef] [PubMed]
- Sinnatamby, C.S. Last’s Anatomy: Regional and Applied, 12th ed.; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Singh, J.P. Shoulder Ultrasound: What You Need to Know. Indian J. Radiol. Imaging 2012, 22, 284–292. [Google Scholar] [CrossRef]
- Saladin, K.S. Anatomy Physiology: The Unity of Form and Function, 9th ed.; McGraw Hill: New York City, NY, USA, 2021. [Google Scholar]
- Gómez-Sánchez, S.M.; Gómez-Esquer, F.; Gil-Crujera, A.; Palomar-Gallego, M.A.; Delcán-Giráldez, J.; Díaz-Gil, G. A Variant of the Deltoid Muscle and Its Clinical Implications: A Cadaveric Study. Anatomia 2022, 1, 119–125. [Google Scholar] [CrossRef]
- Pavone, V.; Testa, G.; Restivo, D.A.; Cannavò, L.; Condorelli, G.; Portinaro, N.M.; Sessa, G. Botulinum Toxin Treatment for Limb Spasticity in Childhood Cerebral Palsy. Front. Pharmacol. 2016, 7, 29. [Google Scholar] [CrossRef]
- Sheean, G.; Lannin, N.A.; Turner-Stokes, L.; Rawicki, B.; Snow, B.J. Botulinum Toxin Assessment, Intervention and After-care for Upper Limb Hypertonicity in Adults: International Consensus Statement. Eur. J. Neurol. 2010, 17, 74–93. [Google Scholar] [CrossRef]
- Wissel, J.; Camões-Barbosa, A.; Carda, S.; Hoad, D.; Jacinto, J. A Practical Guide to Botulinum Neurotoxin Treatment of Shoulder Spasticity 2: Injection Techniques, Outcome Measurement Scales, and Case Studies. Front. Neurol. 2022, 13, 1022549. [Google Scholar] [CrossRef]
- Mense, S. Neurobiological Basis for the Use of Botulinum Toxin in Pain Therapy. J. Neurol. 2004, 251, i1–i7. [Google Scholar] [CrossRef]
- Behringer, M.; Franz, A.; McCourt, M.; Mester, J. Motor Point Map of Upper Body Muscles. Eur. J. Appl. Physiol. 2014, 114, 1605–1617. [Google Scholar] [CrossRef]
- Li, L.; Tong, R.K. Combined Ultrasound Imaging and Biomechanical Modeling to Estimate Triceps Brachii Musculotendon Changes in Stroke Survivors. BioMed Res. Int. 2016, 2016, 5275768. [Google Scholar] [CrossRef] [PubMed]
- Downey, R.; Jacobson, J.A.; Fessell, D.P.; Tran, N.; Morag, Y.; Kim, S.M. Sonography of Partial-Thickness Tears of the Distal Triceps Brachii Tendon. J. Ultrasound Med. 2011, 30, 1351–1356. [Google Scholar] [CrossRef]
- Ooi, M.W.X.; Tham, J.-L.; Al-Ani, Z. Role of Dynamic Ultrasound in Assessment of the Snapping Elbow and Distal Biceps Tendon Injury. Ultrasound J. Br. Med. Ultrasound Soc. 2022, 30, 315–321. [Google Scholar] [CrossRef]
- Buchanan, P.J.; Grossman, J.A.I.; Price, A.E.; Reddy, C.; Chopan, M.; Chim, H. The Use of Botulinum Toxin Injection for Brachial Plexus Birth Injuries: A Systematic Review of the Literature. HAND 2019, 14, 150–154. [Google Scholar] [CrossRef]
- Michaud, L.J.; Louden, E.J.; Lippert, W.C.; Allgier, A.J.; Foad, S.L.; Mehlman, C.T. Use of Botulinum Toxin Type A in the Management of Neonatal Brachial Plexus Palsy. PM&R 2014, 6, 1107–1119. [Google Scholar] [CrossRef]
- DeMatteo, C.; Bain, J.R.; Galea, V.; Gjertsen, D. Botulinum Toxin as an Adjunct to Motor Learning Therapy and Surgery for Obstetrical Brachial Plexus Injury. Dev. Med. Child Neurol. 2006, 48, 245–252. [Google Scholar] [CrossRef]
- Yi, K.-H.; Lee, J.-H.; Hur, H.-W.; Lee, H.-J.; Choi, Y.-J.; Kim, H.-J. Distribution of the Intramuscular Innervation of the Triceps Brachii: Clinical Importance in the Treatment of Spasticity with Botulinum Neurotoxin. Clin. Anat. 2023, 36, 964–970. [Google Scholar] [CrossRef]
- Tiwana, M.S.; Charlick, M.; Varacallo, M. Anatomy, Shoulder and Upper Limb, Biceps Muscle. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Gao, J.; He, W.; Du, L.-J.; Chen, J.; Park, D.; Wells, M.; Fowlkes, B.; O’Dell, M. Quantitative Ultrasound Imaging to Assess the Biceps Brachii Muscle in Chronic Post-Stroke Spasticity: Preliminary Observation. Ultrasound Med. Biol. 2018, 44, 1931–1940. [Google Scholar] [CrossRef]
- Bianchi, S.; Martinoli, C. Ultrasound of the Musculoskeletal System; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Al-Sobhi, M.G.; Zaki, A.I.; Hamid, F.A.A.E.; Alshali, R.A.; Mustafa, H.N. The Pattern of Branching and Intercommunications of the Musculocutaneous Nerve for Surgical Issues: Anatomical Study. Folia Morphol. 2023, 82, 79–87. [Google Scholar] [CrossRef]
- Moon, J.-Y.; Hwang, T.-S.; Sim, S.-J.; Chun, S.; Kim, M. Surface Mapping of Motor Points in Biceps Brachii Muscle. Ann. Rehabil. Med. 2012, 36, 187. [Google Scholar] [CrossRef] [PubMed]
- Kong, K.H.; Shuen-Loong, T.; Tay, M.R.J.; Lui, W.L.; Rajeswaran, D.K.; Kim, J. Ultrasound Assessment of Changes in Muscle Architecture of the Brachialis Muscle After Stroke-A Prospective Study. Arch. Rehabil. Res. Clin. Transl. 2022, 4, 100215. [Google Scholar] [CrossRef] [PubMed]
- Bargalló, X.; Carrera, A.; Sala-Blanch, X.; Santamaría, G.; Morro, R.; Llusá, M.; Gilabert, R. Ultrasound-Anatomic Correlation of the Peripheral Nerves of the Upper Limb. Surg. Radiol. Anat. 2010, 32, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Kim, H.-W.; Im, S.; An, X.; Lee, M.-S.; Lee, U.-Y.; Han, S.-H. Localization of Motor Entry Points and Terminal Intramuscular Nerve Endings of the Musculocutaneous Nerve to Biceps and Brachialis Muscles. Surg. Radiol. Anat. 2010, 32, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Tagliafico, A.; Michaud, J.; Perez, M.M.; Martinoli, C. Ultrasound of Distal Brachialis Tendon Attachment: Normal and Abnormal Findings. Br. J. Radiol. 2013, 86, 20130004. [Google Scholar] [CrossRef] [PubMed]
- Glover, N.M.; Black, A.C.; Murphy, P.B. Anatomy, Shoulder and Upper Limb, Radial Nerve. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Yang, S.; Hu, S.; Li, B.; Li, X. Localization of Nerve Entry Point and Intramuscular Nerve-Dense Regions as Targets to Block Brachioradialis Muscle Spasticity. Int. J. Clin. Exp. Med. 2017, 10, 11912–11920. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popescu, M.N.; Căpeț, C.; Beiu, C.; Berteanu, M. The Elias University Hospital Approach: A Visual Guide to Ultrasound-Guided Botulinum Toxin Injection in Spasticity: Part II—Proximal Upper Limb Muscles. Toxins 2025, 17, 276. https://doi.org/10.3390/toxins17060276
Popescu MN, Căpeț C, Beiu C, Berteanu M. The Elias University Hospital Approach: A Visual Guide to Ultrasound-Guided Botulinum Toxin Injection in Spasticity: Part II—Proximal Upper Limb Muscles. Toxins. 2025; 17(6):276. https://doi.org/10.3390/toxins17060276
Chicago/Turabian StylePopescu, Marius Nicolae, Claudiu Căpeț, Cristina Beiu, and Mihai Berteanu. 2025. "The Elias University Hospital Approach: A Visual Guide to Ultrasound-Guided Botulinum Toxin Injection in Spasticity: Part II—Proximal Upper Limb Muscles" Toxins 17, no. 6: 276. https://doi.org/10.3390/toxins17060276
APA StylePopescu, M. N., Căpeț, C., Beiu, C., & Berteanu, M. (2025). The Elias University Hospital Approach: A Visual Guide to Ultrasound-Guided Botulinum Toxin Injection in Spasticity: Part II—Proximal Upper Limb Muscles. Toxins, 17(6), 276. https://doi.org/10.3390/toxins17060276






