Insecticidal Activities of Diterpene Alkaloids in Plants of the Genera Aconitum and Delphinium
Abstract
1. Introduction
2. Diterpenoid Alkaloids (DAs) and Pest Control
2.1. Classification and Structure of DAs
2.2. Insecticidal and Antifeedant Activities of DAs
2.2.1. Insecticidal and Inhibiting Activities of C19-Ditepenoid Alkaloids
2.2.2. Insecticidal and Inhibiting Activities of C20-Ditepenoid Alkaloids
2.2.3. Insecticidal and Inhibiting Activities of C18-Ditepenoid Alkaloids
3. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aronson, A.I.; Dunn, P.E. Biological Pesticide. U.S. Patent 5,055,293, 8 November 1991. [Google Scholar]
- Zhang, J.F.; Wang, W.; Lu, X.H.; Xu, Y.Q.; Zhang, X.H. The stability and degradation of a new biological pesticide, pyoluteorin. Pest Manag. Sci. 2010, 66, 248–252. [Google Scholar] [CrossRef]
- David, P.; Rajinder, P. Integrated Pest Management; Springer: Dordrecht, The Netherlands, 1995; pp. 1–474. [Google Scholar]
- Makkar, H.P.S.; Siddhuraju, P.; Becker, K. Plant Secondary Metabolites. In Methods in Molecular Biology; Clifton, N.J., Ed.; Humana Press: Totowa, NJ, USA, 2007; pp. 1–222. [Google Scholar]
- Wang, G.Q.; Ji, L.Z.; Zhang, H.; Wang, X.W. Current Progress in Research of Botanical Insecticides in China. Sci. Agric. Sin. 2006, 39, 510–517. [Google Scholar]
- Wang, F.P. Retrospection on studies of diterpenoid alkaloidal chemistry. NPRD 2021, 33, 1427–1443. [Google Scholar]
- Shen, Y.; Liang, W.J.; Shi, Y.N.; Kennelly, E.J.; Zhao, D.K. Structural diversity, bioactivities, and biosynthesis of natural diterpenoid alkaloids. Nat. Prod. Rep. 2020, 37, 763–796. [Google Scholar] [CrossRef]
- Wang, F.P. Modern Chemistry of Natural Products; Science Press: Beijing, China, 2009; pp. 1–1125. [Google Scholar]
- Manske, R.H.F.; Rodrigo, R.G.A. The Alkaloids; Academic Press Inc.: San Diego, CA, USA, 1979; Volume XVII, p. 46. [Google Scholar]
- Jennings, K.R.; Brown, D.G.; Wright, D.P. Methyllycaconitine, a naturally occurring insecticide with a high affinity for the insect cholinergic receptor. Cell. Mol. Life Sci. 1986, 42, 611–613. [Google Scholar] [CrossRef]
- Macallan, D.R.E.; Lunt, G.G.; Wonnacott, S.; Swanson, K.L.; Rapoport, H.; Albuquerque, E.X. Methyllycaconitine and (+)-anatoxin-a differentiate between nicotinic receptors in vertebrate and invertebrate nervous systems. FEBS Lett. 1988, 226, 357–363. [Google Scholar] [CrossRef]
- Kukel, C.F.; Jennings, K.R. Delphinium alkaloids as inhibitors of α-bungarotoxin binding to rat and insect neural membranes. Can. J. Physiol. Pharmacol. 1994, 72, 104–107. [Google Scholar] [CrossRef]
- Ward, J.M.; Cockcroft, V.B.; Lunt, G.G.; Smillie, F.S.; Wonnacott, S. Methyllycaconitine: A selective probe for neuronal α-bungarotoxin binding sites. FEBS Lett. 1990, 270, 45–48. [Google Scholar] [CrossRef]
- Ulubelen, A.; Meriçli, A.H.; Meriçli, F.; Kilinçer, N.; Ferizli, A.G.; Emekci, M.; Pelletier, S.W. Insect repellent activity of diterpenoid alkaloids. Phytother. Res. 2001, 15, 170–171. [Google Scholar] [CrossRef]
- Coloma, A.G.; Reina, M.; Medinaveitia, A.; Guadaño, A.; Santana, O.; Martínez-Díaz, R.; Ruiz-Mesía, L.; Alva, A.; Grandez, M.; Díaz, R.; et al. Structural diversity and defensive properties of norditerpenoid alkaloids. J. Chem. Ecol. 2004, 30, 1393–1408. [Google Scholar] [CrossRef]
- Reina, M.; Coloma, A.G. Structural diversity and defensive properties of diterpenoid alkaloids. Phytochem. Rev. 2007, 6, 81–95. [Google Scholar] [CrossRef]
- Ameri, A. The effects of Aconitum alkaloids on the central nervous system. Prog. Neurobiol. 1998, 56, 211–235. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.L.; Cao, J.Z.; Hai, M.; Lin, L.L.; Liu, H.J.; Du, S.S.; Zhou, L.; Deng, Z.W. Feeding deterrents from Aconitum episcopale roots against the red flour beetle, Tribolium castaneum. J. Agric. Food Chem. 2011, 59, 3701–3706. [Google Scholar] [CrossRef]
- Chen, L. Studies on Akaloid Constituents of Four Medicinal Plants and Antifeedant Activities of Spodoptera exigua Hiibner. Ph.D. Thesis, Southwest Jiaotong University, Chengdu, China, 2017. [Google Scholar]
- Zhang, J.F.; Chen, L.; Huang, S.; Shan, L.H.; Gao, F.; Zhou, X.L. Diterpenoid Alkaloids from Two Aconitum Species with Antifeedant Activity against Spodoptera exigua. J. Nat. Prod. 2017, 80, 3136–3142. [Google Scholar] [CrossRef]
- Shan, L.H.; Chen, L.; Gao, F.; Zhou, X.L. Diterpenoid alkaloids from Delphinium naviculare var. lasiocarpum with their antifeedant activity on Spodoptera exigua. Nat. Prod. Res. 2019, 33, 3254–3259. [Google Scholar] [CrossRef]
- Shan, L.H.; Chen, L.; Zhou, X.L. Diterpenoid alkaloids from Aconitum karakolicum Rapaics. Nat. Prod. Res. 2019, 31, 1573–1579. [Google Scholar]
- Ren, J.L. Diterpenoid Alkaloids from Aconitum rockii and Their Antifeedant Activity. Ph. D. Thesis, Southwest Jiaotong University, Chengdu, China, 2021. [Google Scholar]
- Wang, J. Isolationand Biologicalactivities Screening Ofdiiterpenealkaloids from Aconitum leucostomum Worosch. Ph. D. Thesis, Southwest Jiaotong University, Chengdu, China, 2021. [Google Scholar]
- González-Coloma, A.; Guadaño, A.; Gutiérrez, C.; Cabrera, R.; de la Peña, E.; de la Fuente, G.; Reina, M. Antifeedant delphinium diterpenoid alkaloids. Structure-activity relationships. J. Agric. Food Chem. 1998, 46, 286–290. [Google Scholar] [CrossRef]
- González-Coloma, A.; Reina, M.; Guadaño, A.; Martínez-Díaz, R.; Díaz, J.G.; García-Rodriguez, J.; Alva, A.; Grandez, M. Antifeedant C20 diterpene alkaloids. Chem. Biodivers. 2004, 1, 1327–1335. [Google Scholar] [CrossRef] [PubMed]
- Reina, M.; Mancha, R.; Gonzalez-Coloma, A.; Bailen, M.; Rodriguez, M.L.; Martinez-Diaz, R.A. Diterpenoid alkaloids from Delphinium gracile. Nat. Prod. Res. 2007, 21, 1048–1055. [Google Scholar] [CrossRef]
- González, P.; Marín, C.; Rodríguez-González, I.; Hitos, A.B.; Rosales, M.J.; Reina, M.; Díaz, J.G.; González-Coloma, A.; Sánchez-Moreno, M. In vitro activity of C20-diterpenoid alkaloid derivatives in promastigotes and intracellular amastigotes of Leishmania infantum. Int. J. Antimicrob. Agents 2005, 25, 136–141. [Google Scholar] [CrossRef]
- Yuan, C.L.; Wang, X.L. Isolation of active substances and bioactivity of Aconitum sinomontanum Nakai. Nat. Prod. Res. 2012, 26, 2099–2102. [Google Scholar] [PubMed]
- Fan, D.L.; Yang, L.L.; Zeng, D.Q.; Tang, W.X. Insecticidal alkaloids constituents from Aconitum anthoroideum DC. J. Environ. Entom. 2020, 42, 1250–1256. [Google Scholar]
- Song, S.Y.; Lan, X.M.; Xu, J.; Cui, Y.F.; Zhou, H.Y.; Zheng, J.; Dai, S.Y.; Zhang, J.Y. Systematic analysis and identification of diterpenoid alkaloids from Aconitum carmichaeli Debx. by UHPLC-Q-Exactive Orbitrap MS. J. Pharm. Anal. 2023, 43, 918–929. [Google Scholar]
- Wang, X.; Cao, X.Q.; Guo, D.M.; Xu, F.R.; Ma, X.H. Cloning and Expression Analysis of Acetyl-CoA Acetyltransferase Gene from Aconitum vilmorinianum. Chin. Med. Mat. 2024, 47, 2198–2202. [Google Scholar]
- Li, C.; Zhang, C.; Liu, X.Y.; Qin, Y. Recent Progress in the Total Synthesis of Diterpenoid Alkaloids. Chin. J. Org. Chem. 2024, 45, 881. [Google Scholar] [CrossRef]

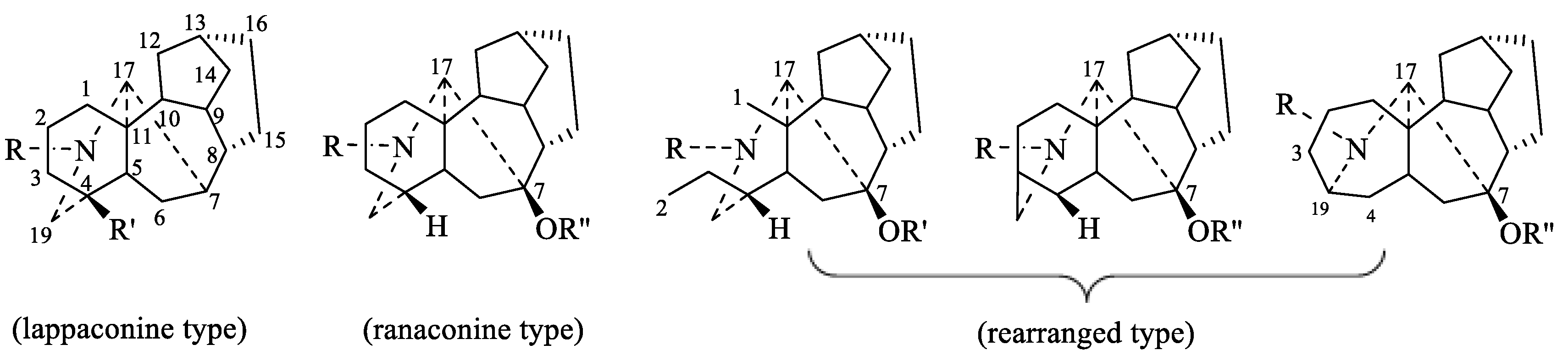
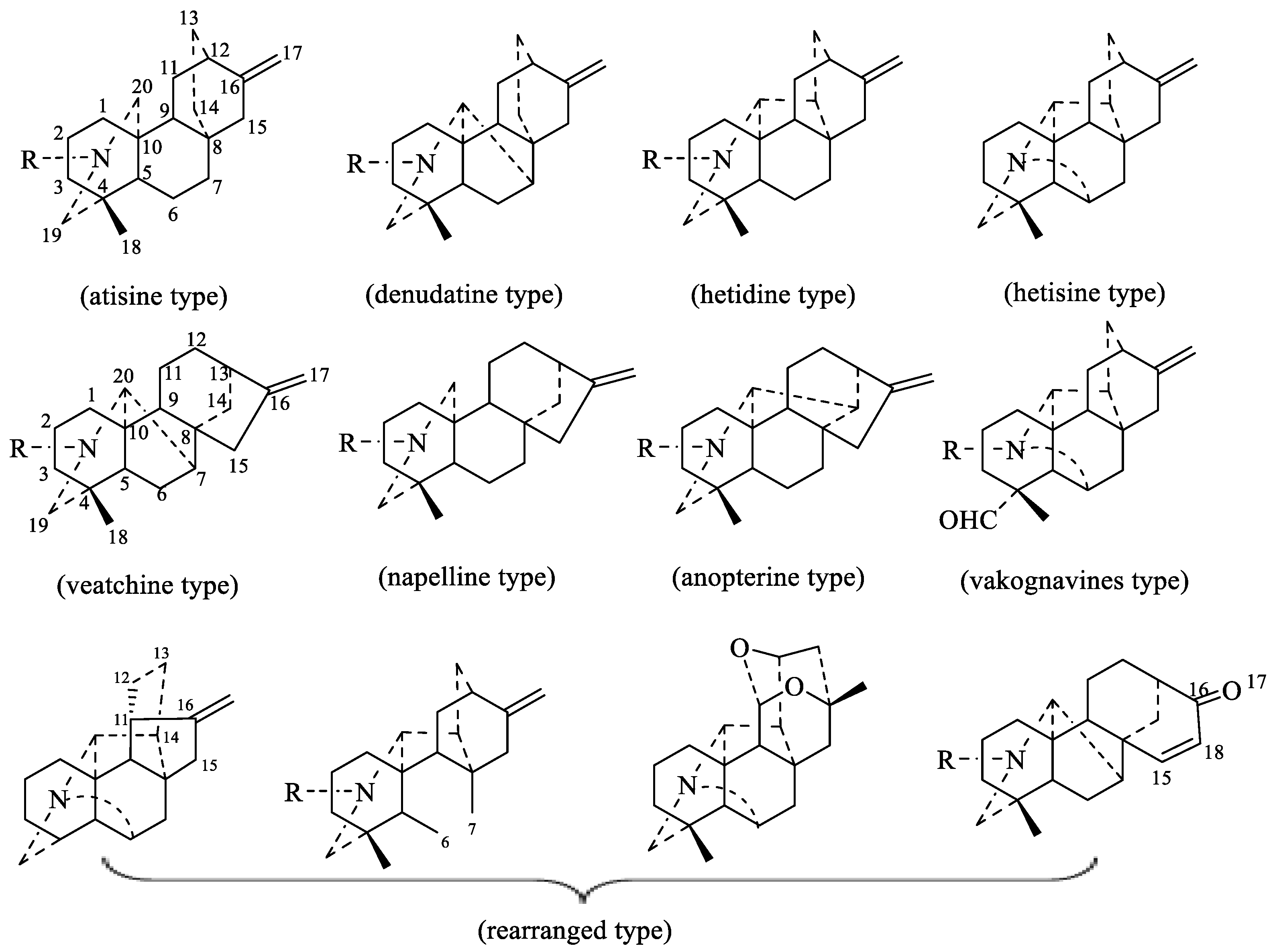

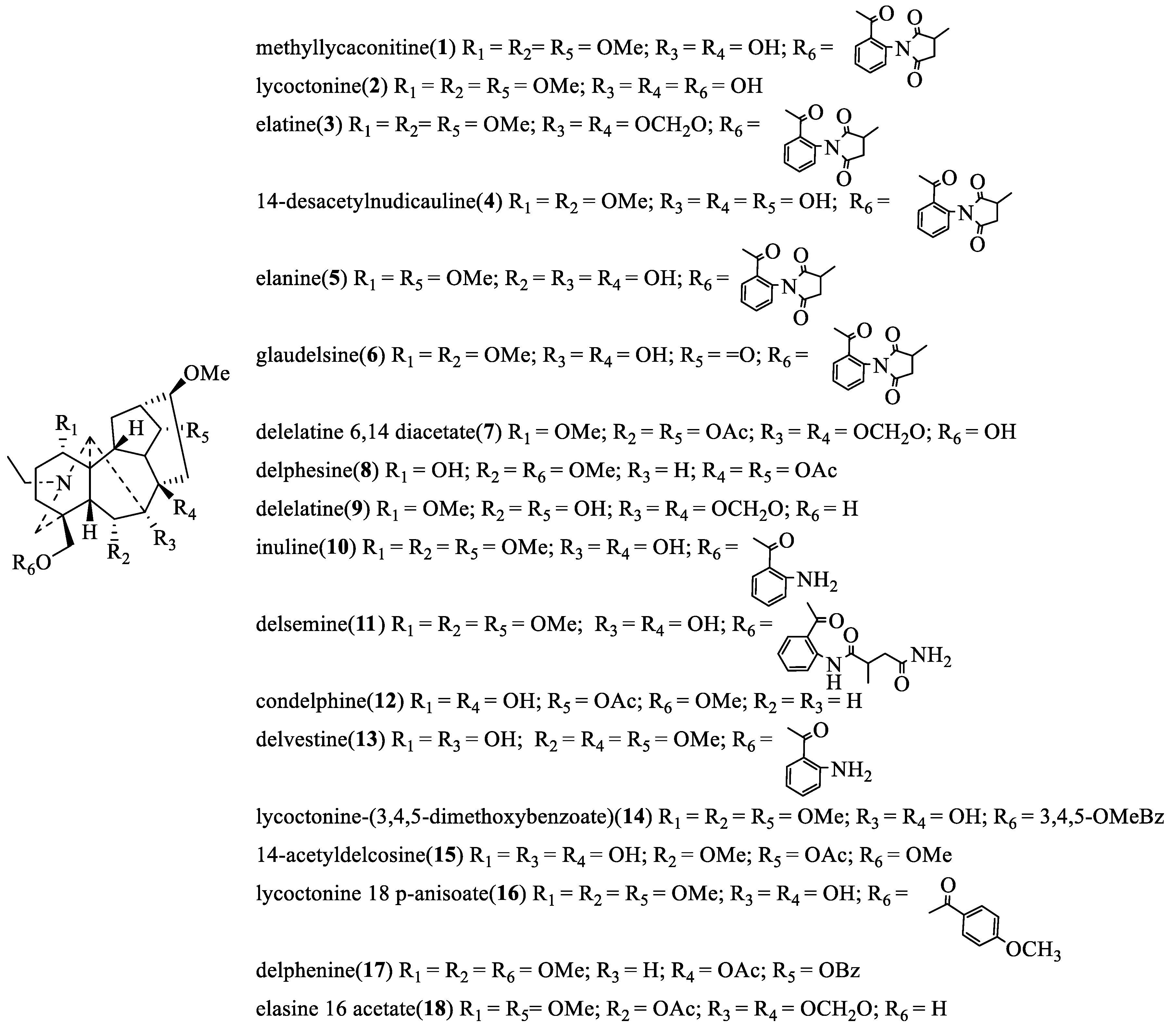



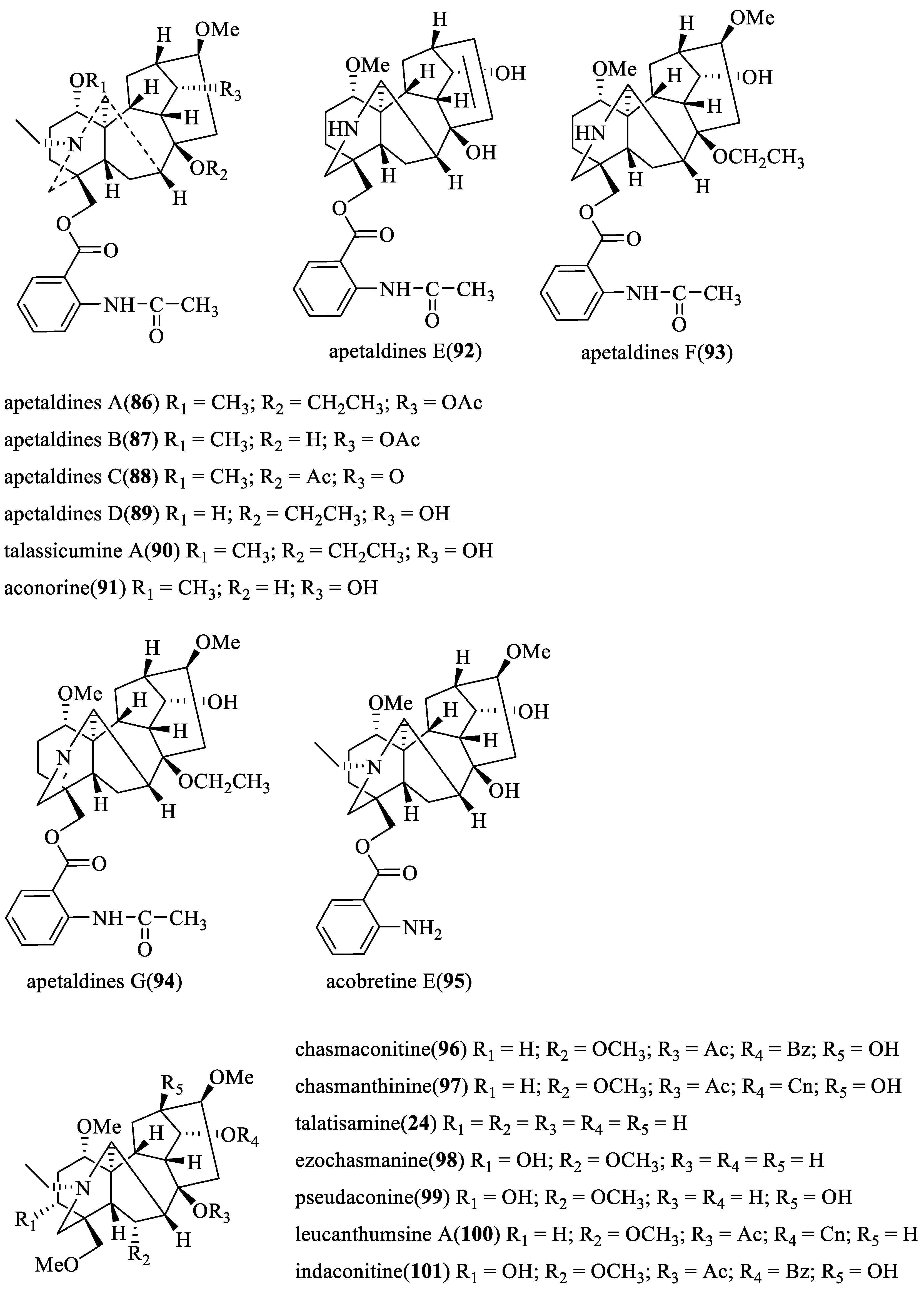


| Substance | Average Repellency(%) | Mean Repellency Class a |
|---|---|---|
| condelphine | 40.62 | III |
| 14-acetylneoline | 53.12 | III |
| peregrine | 53.12 | III |
| delsoline | 37.50 | II |
| karakoline | 37.50 | II |
| peregrine alcohol | 37.50 | II |
| talatisamine | 34.37 | III |
| 14-acetylvirescenine | 43.75 | III |
| lycoctonine | 46.87 | III |
| 14-acetyltalatisamine | 46.87 | III |
| 3-hydroxytalatisamine | 53.12 | III |
| browniine | 46.87 | III |
| Substance | Average Repellency(%) | Mean Repellency Class a |
|---|---|---|
| benzoyldavisinol | 46.87 | III |
| hetisinone | 37.50 | II |
| venulol | 31.25 | II |
| ajaconine | 53.12 | III |
| venudelphine | 40.62 | III |
| venuluson | 56.25 | III |
| hetisine | 59.12 | III |
| orientinine | 46.87 | III |
| Compounds | Insect Species/Cells | Feeding * | Activity | Relevant Data | Ref. |
|---|---|---|---|---|---|
| methyllycaconitine (1) | Spodoptera eridania | C | Antifeedant activity | LC50 = 308 ppm | [10] |
| feeding damage to the leaf was less than 5% at 100 ppm | |||||
| Musca domesticcs (house fly) | Insecticidal activity | Active denotes significant (50% +) mortality at a screening rate of 1000 ppm | |||
| Musca domesticcs | Inhibition of α-Bungarotoxin | IC50 = 6.4 × 10−10 M | [12] | ||
| Rat brain | IC50 = 1.7 × 10−9 M | ||||
| -- | Inhibition of 3H α-Bungarotoxin | Kinh = 0.25 ± 0.05 nM | [10] | ||
| aconitine (30) | -- | - | Kinh = 2.7 ± 0.8 × 10−4 M | ||
| lycoctonine (2) | -- | - | Kinh = 3.8 ± 0.6 × 10−7 M | ||
| elatine (3) | Rat brain | - | Inhibition of α-Bungarotoxin | IC50 = 4.3 ± 0.4 × 10−9 mol/L | [12] |
| Musca domestics (house fly) | O | IC50 = 2.9 ± 0.1 × 10−10 mol/L | |||
| 14-desacetylnudicauline (4) | Rat brain | - | IC50 = 1.0 ± 0.1 × 10−8 mol/L | ||
| Musca domesticcs | O | IC50 = 8.8 × 10−10 mol/L | |||
| elanine (5) | Rat brain | - | IC50 = 1.2 ± 0.4 × 10−8 mol/L | ||
| Musca domesticcs | O | IC50 = 1.1 ± 0.1 × 10−8 mol/L | |||
| glaudelsine (6) | Rat brain | - | IC50 = 1.6 ± 0.7 × 10−8 mol/L | ||
| Musca domesticcs | O | IC50 = 4.2 ± 0.1 × 10−11 mol/L | |||
| delelatine 6,14 diacetate (7) | Rat brain | - | IC50 = 8.9 ± 1.0 × 10−8 mol/L | ||
| Musca domesticcs | O | IC50 = 9.8 ± 0.1 × 10−8 mol/L | |||
| delphesine (8) | Rat brain | - | IC50 = 1.4 ± 0.4 × 10−7 mol/L | ||
| Musca domesticcs | O | IC50 = 1.0 ± 0.1 × 10−7 mol/L | |||
| delelatine (9) | Rat brain | - | IC50 = 2.9 ± 0.1 × 10−7 mol/L | ||
| Musca domesticcs | O | IC50 = 9.0 ± 0.1 × 10−8 mol/L | |||
| anthranoyllycoctonine (inuline) (10) | Rat brain | - | IC50 = 3.4 ± 0.5 × 10−7 mol/L | ||
| Musca domesticcs | O | IC50 = 3.4 ± 0.1 × 10−8 mol/L | |||
| delsemine (11) | Rat brain | - | IC50 = 3.6 ± 0.3 × 10−7 mol/L | ||
| Musca domesticcs | O | IC50 = 5.9 ± 0.5 × 10−9 mol/L | |||
| condelphine (12) | Tribolium casteneum | S | Repellent activity | Average repellency = 40.63% | [14] |
| Rat brain | - | Inhibition of α-Bungarotoxin | IC50 = 8.0 ± 1.1 × 10−7 mol/L | [12] | |
| Musca domesticcs | O | IC50 = 3.10 ± 0.01 × 10−8 mol/L | |||
| delvestine (13) | Rat brain | - | IC50 = 1.6 ± 0.3 × 10−6 mol/L | ||
| Musca domesticcs | O | IC50 = 2.6 ± 0.4 × 10−8 mol/L | |||
| lycoctonine-18-(3,4,5-dimethoxybenzoate (14) | Rat brain | - | IC50 = 2.8 ± 0.6 × 10−6 mol/L | ||
| Musca domesticcs | O | IC50 = 9.4 ± 0.6 × 10−9 mol/L | |||
| 14-acetyldelcosine (15) | Rat brain | - | IC50 = 4.9 ± 1.0 × 10−6 mol/L | ||
| Musca domesticcs | O | IC50 = 7.1 ± 0.4 × 10−9 mol/L | |||
| lycoctonine 18 p-anisoate (16) | Rat brain | - | IC50 = 1.8 ± 0.3 × 10−5 mol/L | ||
| Musca domesticcs | O | IC50 = 3.0 ± 0.1 × 10−8 mol/L | |||
| delphenine (17) | IC50 = 3.2 ± 0.1 × 10−8 mol/L | ||||
| elasine 16 acetate (18) | Musca domesticcs | O | Inhibition of α-Bungarotoxin | IC50 = 7.9 ± 0.2 × 10−8 mol/L | [12] |
| 14-acetylneoline (19) | Tribolium casteneum | S | Repellent activity | Average repellency = 53.12% | [14] |
| peregrine (20) | Average repellency = 53.12% | ||||
| delsoline (21) | Average repellency = 37.50% | ||||
| karakoline (22) | Leptinotarsa decemlineata | C | Antifeedant activity | EC50 = 0.44 µg/cm2 | [15] |
| Tribolium castaneum | S | Antifeedant activity | EC50 = 395.3 ppm | [14] | |
| Repellent activity | Average repellency = 37.50% | ||||
| peregrine alcohol (23) | Average repellency = 37.50% | ||||
| talatisamine (24) | Average repellency = 34.37% | ||||
| 14-acetylvirescenine (25) | Average repellency = 43.75% | ||||
| browniine (55) | Average repellency = 46.87% | ||||
| delsemine b (143) | Average repellency = 37.50% | ||||
| 14-acetyltalatisamine (28) | Average repellency = 46.87% | ||||
| 3-hydroxytalatisamine (29) | Average repellency = 53.12% | ||||
| N-deacetyllappaconitine (144) | Average repellency = 50.00% | ||||
| lappaconitine (145) | Average repellency = 34.37% | ||||
| lycoctonine (27) | Average repellency = 46.87% | ||||
| SW480 | - | Cytotoxicity | % V = 7 ± 2 | [15] | |
| aconitine (30) | Spodoptera littoralis | C | Insecticidal toxicity. A covariance analysis (ANCOV A1) of food consumption (ΔI) and biomass gains (ΔB) with initial larval weight as covariate, using oral injection. | ΔB = 34, ΔI = 67 | |
| Antifeedant activity | EC50 = 0.02 mg/cm2 | ||||
| neoline (34) | SW480 | - | Cytotoxicity | % V = 5 ± 0 | |
| 8-O-methylcolumbianine (35) | Leptinotarsa decemlineata | C | Antifeedant activity | EC50 = 0.99 µg/cm2 | |
| Spodoptera littoralis | C | EC50 > 50 µg/cm2 | |||
| cardiopetaline (36) | Leptinotarsa decemlineata | C | Antifeedant activity | EC50 = 0.42 µg/cm2 | |
| Insecticidal toxicity | % M = 4 | ||||
| Spodoptera littoralis | C | Insecticidal toxicity. A covariance analysis (ANCOV A1) of food consumption (ΔI) and biomass gains (ΔB) with initial larval weight as covariate, using oral injection. | ΔB = 26, ΔI = 70 | ||
| 1,14-diactylcardiopetalina (37) | Leptinotarsa decemlineata | C | Antifeedant activity | EC50 = 0.11 µg/cm2 | |
| cardiopetadine(39) | Leptinotarsa decemlineata | C | Insecticidal toxicity | % M = 61 | |
| 1,14-O-acetylcardiopetalidina(40) | Spodoptera littoralis | C | Insecticidal toxicity. A covariance analysis (ANCOV A1) of food consumption (ΔI) and biomass gains (ΔB) with initial larval weight as covariate, using oral injection. | ΔB = 69, ΔI = 112 | [15] |
| 8-O-methylconsolarine (41) | Leptinotarsa decemlineata | C | Antifeedant activity | EC50 = 0.23 µg/cm2 | |
| Spodoptera littoralis | C | Insecticidal toxicity. A covariance analysis (ANCOV A1) of food consumption (ΔI) and biomass gains (ΔB) with initial larval weight as covariate, using oral injection. | ΔB = 79, ΔI = 94 | ||
| 18-O-demethylpubescenine (42) | Leptinotarsa decemlineata | C | Antifeedant activity | EC50 = 0.60 µg/cm2 | |
| SF9 cell | - | Cytotoxicity | LD50 = 29.17 µg/ml | ||
| 14-deacetyl-pubescenine (43) | Leptinotarsa decemlineata | C | Insecticidal toxicity | % M = 47 | |
| Spodoptera littoralis | C | Insecticidal toxicity. A covariance analysis (ANCOV A1) of food consumption (ΔI) and biomass gains (ΔB) with initial larval weight as covariate, using oral injection. | ΔB = 78, ΔI = 95 | ||
| SF9 cell | - | Cytotoxicity | LD50 = 0.38 µg/ml | ||
| pubescenine (44) | SW480 | - | Cytotoxicity (determined with MTT method) | % V = 10 ± 0 | |
| 18-O-benzoyl-18-O-demethyl-14-O-demethylpubescenine (46) | Leptinotarsa decemlineata | C | Insecticidal toxicity | % M = 11 | |
| 14-O-acetyldeltatsine (47) | Leptinotarsa decemlineata | C | Antifeedant activity | EC50 = 0.54 µg/cm2 | |
| Spodoptera littoralis | C | EC50 = 0.84 µg/cm2 | |||
| 14-O-acetyl-delcosine (48) | Spodoptera littoralis | C | Antifeedant activity | EC50 = 1.51 µg/cm2 | |
| Leptinotarsa decemlineata | C | Insecticidal toxicity | % M = 41 | ||
| SF9 cell | - | Cytotoxicity | LD50 = 14.88 µg/ml | ||
| takaosamine (49) | Leptinotarsa decemlineata | C | Antifeedant activity | EC50 = 0.66 µg/cm2 | |
| delcosine (51) | SF9 cell | - | Cytotoxicity | LD50 = 32.37 µg/ml | |
| ajadine (52) | Leptinotarsa decemlineata | C | Antifeedant activity | EC50 = 0.84 µg/cm2 | |
| 14-deacetylajadine (53) | Leptinotarsa decemlineata | C | Insecticidal toxicity | % M = 47 | |
| SW480 | - | Cytotoxicity (determined with MTT method) | Not enough compound available | ||
| 14-O-acetydelectinine (54) | Leptinotarsa decemlineata | C | Antifeedant activity | EC50 = 0.29 µg/cm2 | |
| delphatine (56) | Spodoptera littoralis | C | Antifeedant activity | EC50 = 2.72 µg/cm2 | |
| methyllycaconitine (1) | Leptinotarsa decemlineata | C | Insecticidal toxicity | % M = 47 | [15] |
| 18-hydroxy-14-O-methlygadesine (59) | Antifeedant activity | EC50 = 0.13 µg/cm2 | |||
| dehydrotakaosamine (60) | SW480 | - | Cytotoxicity (determined with MTT method) | % V = 5 ± 0 | |
| dehydrodelsoline (62) | SF9 cell | - | Cytotoxicity | LD50 = 18.89 µg/ml | |
| ajadelphinine (63) | SW480 | - | Cytotoxicity (determined with MTT method) | % V = 4 ± 0 | |
| tuguaconitine (64) | SF9 cell | - | Cytotoxicity | LD50 = 1.83 µg/ml | |
| 14-demethyldelboxine (66) | SF9 cell | - | Cytotoxicity | LD50 = 6.27 µg/ml | |
| 1,18-O-diacetyl-19-oxo-gigactonine (67) | Spodoptera littoralis | C | Insecticidal toxicity | EC50 > 50 µg/ml | |
| SF9 cell | - | Cytotoxicity | LD50 = 29.45 µg/ml | ||
| olivimine (68) | Spodoptera littoralis | C | Insecticidal toxicity | EC50 > 50 µg/cm2 | |
| yunaconitine (69) | Tribolium castaneum | S | Antifeedant activity | EC50 = 653.4 ppm | [18] |
| crassicauline a (70) | EC50 = 1134.5 ppm | ||||
| chasmanine (71) | EC50 = 297.0 ppm | ||||
| talatisamnine (72) | EC50 = 342.8 ppm | ||||
| sachaconitine (73) | EC50 = 427.8 ppm | ||||
| demethylenedelcorine (74) | mythimna separata | C | Antifeedant activity, 72 h | % IR = 100 | |
| Insecticidal toxicity, 72 h | % M = 40.2 | ||||
| 18-O-methylgigactonine (75) | mythimna separata | C | Antifeedant activity, 72 h | % IR = 70.1 | |
| Insecticidal toxicity, 72 h | % M = 29.2 | ||||
| pubescensine (76) | Spodoptera littoralis | C | Antifeedant activity | EC50 = 0.03 mg/cm2 | [19] |
| 3-deoxyaconitine (77) | EC50 = 0.05 mg/cm2 | ||||
| 15-α-hydroxyneoline (78) | EC50 = 0.47 mg/cm2 | ||||
| taurenine (79) | EC50 = 0.66 mg/cm2 | ||||
| bullatine b (80) | EC50 = 0.41 mg/cm2 | ||||
| anthranoyllycoctonine (81) | EC50 = 0.73 mg/cm2 | ||||
| avadharidine (82) | EC50 = 0.84 mg/cm2 | ||||
| N-acetylsepaconitine (83) | EC50 = 1.21 mg/cm2 | ||||
| finaconitine (84) | EC50 = 1.44 mg/cm2 | ||||
| N-deacetylappaconitine (85) | EC50 = 1.88 mg/cm2 | ||||
| apetaldines a (86) | Spodoptera littoralis | C | Antifeedant activity | EC50 = 0.45 mg/cm2 | [20] |
| apetaldines b (87) | EC50 = 0.94 mg/cm2 | ||||
| apetaldines c (88) | EC50 = 1.18 mg/cm2 | ||||
| apetaldines d (89) | EC50 = 0.64 mg/cm2 | ||||
| apetaldines e (92) | EC50 = 0.28 mg/cm2 | ||||
| apetaldines f (93) | EC50 = 0.68 mg/cm2 | ||||
| apetaldines g (94) | EC50 = 9.23 mg/cm2 | ||||
| talassicumine a (90) | EC50 = 0.76 mg/cm2 | ||||
| aconorine (91) | EC50 = 5.65 mg/cm2 | ||||
| aacobretine e (95) | EC50 = 1.75 mg/cm2 | ||||
| taurenine (79) | Spodoptera littoralis | C | Antifeedant activity | EC50 = 0.66 mg/cm2 | [20] |
| songorine (145) | EC50 = 60 mg/cm2 | ||||
| chasmaconitine (96) | EC50 = 0.2 mg/cm2 | ||||
| chasmanthinine (97) | EC50 = 0.07 mg/cm2 | ||||
| talatisamine (24) | EC50 = 50 mg/cm2 | ||||
| ezochasmanine (98) | EC50 = 2.09 mg/cm2 | ||||
| pseudaconine (99) | EC50 = 1.79 mg/cm2 | ||||
| leucanthumsine a (100) | EC50 = 0.18 mg/cm2 | ||||
| indaconitine (101) | EC50 = 0.41 mg/cm2 | ||||
| leueandine (57) | EC50 = 3.32 mg/cm2 | ||||
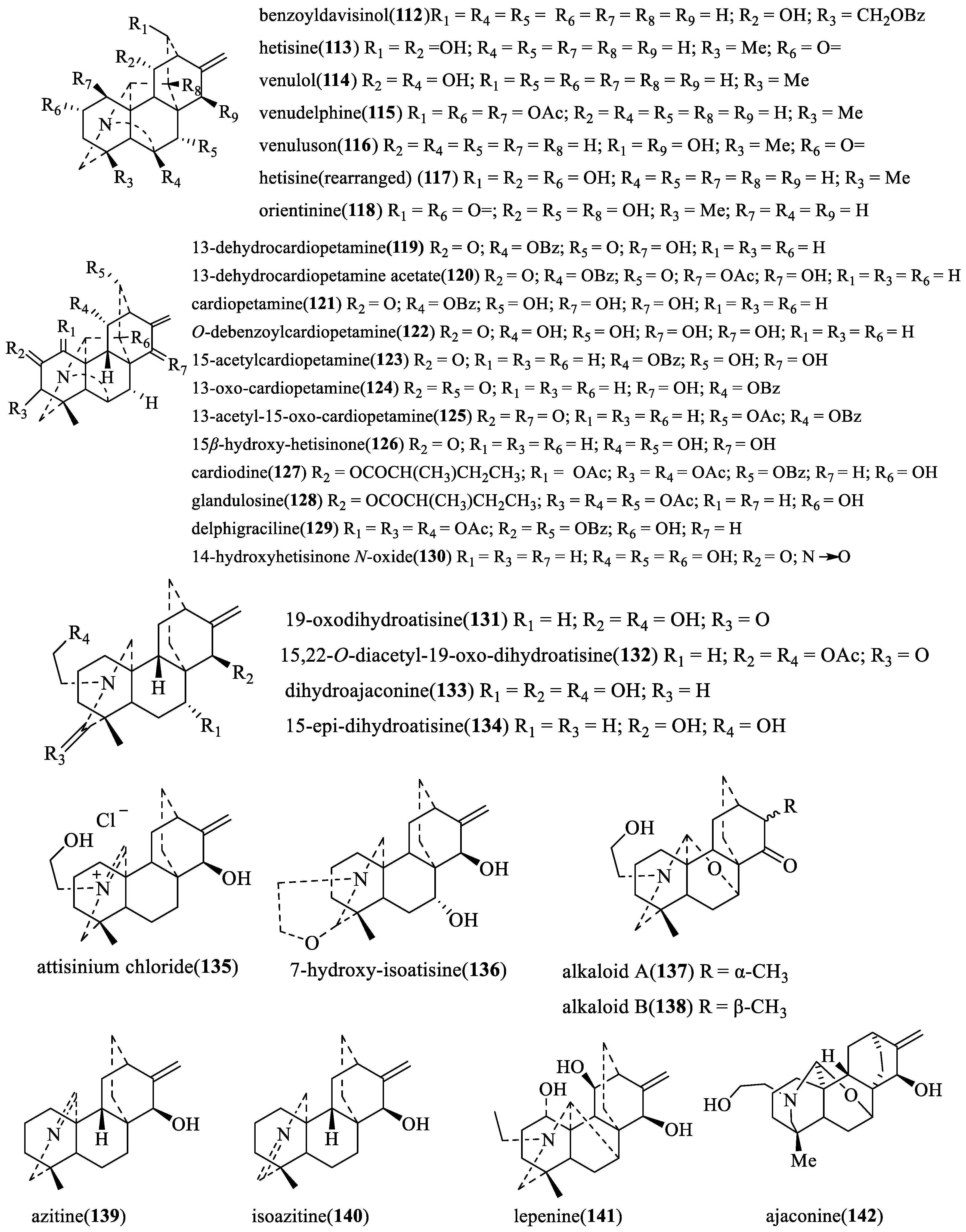
| benzoyldavisinol (112) | Tribolium casteneum | S | Repellent activity | Average repellency = 46.87% | [14] |
| hetisine (113) | Spodoptera littoralis | C | Antifeedant activity | EC50 > 50 µg/cm2 | [26] |
| Insecticidal toxicity. A covariance analysis (ANCOV A1) of food consumption (ΔI) and biomass gains (ΔB) with initial larval weight as covariate, using oral injection. | ΔB = 93.2, ΔI = 110.7 | ||||
| Leptinotarsa decemlineata | C | Antifeedant activity | EC50 = 13.1 µg/cm2 | ||
| Tribolium casteneum | S | Repellent activity | Average repellency = 37.50% | [14] | |
| venulol (114) | Tribolium casteneum | S | Repellent activity | Average repellency = 31.25% | |
| ajaconine (142) | Spodoptera littoralis | C | Antifeedant activity | EC50 = 8.2 µg/cm2 | [26] |
| Insecticidal toxicity. A covariance analysis (ANCOV A1) of food consumption (ΔI) and biomass gains (ΔB) with initial larval weight as covariate, using oral injection. | ΔB = 80.4, ΔI = 104.5 | ||||
| Leptinotarsa decemlineata | C | Antifeedant activity | EC50 = 5.1 µg/cm2 | ||
| Tribolium casteneum | S | Repellent activity | Average repellency = 53.12% | [14] | |
| venudelphine (115) | Average repellency = 40.62% | ||||
| venuluson (116) | Average repellency = 56.25% | ||||
| hetisine(rearranged) (117) | Spodoptera littoralis | C | Antifeedant activity | EC50 > 50 µg/cm2 | [26] |
| Insecticidal toxicity. A covariance analysis (ANCOV A1) of food consumption (ΔI) and biomass gains (ΔB) with initial larval weight as covariate, using oral injection. | ΔB = 89.5, ΔI = 121.9 | ||||
| Leptinotarsa decemlineata | C | Antifeedant activity | EC50 = 1.73 µg/cm2 | ||
| Tribolium casteneum | S | Repellent activity | Average repellency = 59.12% | [14] | |
| orientinine (118) | Average repellency = 46.87% | ||||
| 15-acetylcardiopetamine (123) | Leptinotarsa decemlineata | C | Antifeedant activity | EC50 = 12.86 nmol/cm2 | [25] |
| Spodoptera littoralis | C | Antifeedant activity | EC50 > 100 nmol/cm2 | ||
| cardiopetamine (121) | Spodoptera littoralis | C | Antifeedant activity | EC50 = 5.5 µg/cm2 | [25] |
| Insecticidal toxicity. A covariance analysis (ANCOV A1) of food consumption (ΔI) and biomass gains (ΔB) with initial larval weight as covariate, using oral injection. | ΔB = 110.3, ΔI = 103.3 | ||||
| Leptinotarsa decemlineata | C | Antifeedant activity | EC50 = 22.5 µg/cm2 | ||
| 13-oxo-cardiopetamine (124) | Spodoptera littoralis | C | Antifeedant activity | EC50 > 100 µg/cm2 | [26] |
| Insecticidal toxicity. A covariance analysis (ANCOV A1) of food consumption (ΔI) and biomass gains (ΔB) with initial larval weight as covariate, using oral injection. | ΔB = 105.8, ΔI = 97.2 | ||||
| Leptinotarsa decemlineata | C | Antifeedant activity | Not enough compound available | ||
| 13-acetyl-15-oxo-cardiopetamine (125) | Spodoptera littoralis | C | Antifeedant activity | EC50 > 100 µg/cm2 | |
| Insecticidal toxicity. A covariance analysis (ANCOV A1) of food consumption (ΔI) and biomass gains (ΔB) with initial larval weight as covariate, using oral injection. | ΔB = 87.9, ΔI = 113.9 | ||||
| Leptinotarsa decemlineata | C | Antifeedant activity | EC50 = 27.2 µg/cm2 | ||
| 15β-hydorxy-hetisinone (126) | Spodoptera littoralis | C | Antifeedant activity | EC50 = 23.7 µg/cm2 | |
| Insecticidal toxicity. A covariance analysis (ANCOV A1) of food consumption (ΔI) and biomass gains (ΔB) with initial larval weight as covariate, using oral injection. | ΔB = 104.5, ΔI = 106.5 | ||||
| cardiodine (127) | Spodoptera littoralis | C | Antifeedant activity | EC50 = 4.4 µg/cm2 | |
| Insecticidal toxicity. A covariance analysis (ANCOV A1) of food consumption (ΔI) and biomass gains (ΔB) with initial larval weight as covariate, using oral injection. | ΔB = 97.6, ΔI = 119.7 | ||||
| Leptinotarsa decemlineata | C | Insecticidal toxicity. A covariance analysis (ANCOV A1) of food consumption (ΔI) and biomass gains (ΔB) with initial larval weight as covariate, using oral injection. | EC50 = 2.2 µg/cm2 | ||
| glandulosine (128) | Spodoptera littoralis | C | Antifeedant activity | EC50 > 50 µg/cm2 | |
| Insecticidal toxicity. A covariance analysis (ANCOV A1) of food consumption (ΔI) and biomass gains (ΔB) with initial larval weight as covariate, using oral injection. | ΔB = 82.93, ΔI = 73.48 | ||||
| glandulosine (128) | Leptinotarsa decemlineata | C | Antifeedant activity | EC50 = 4.0 µg/cm2 | [26] |
| delphigraciline (129) | Leptinotarsa decemlineata | C | Antifeedant activity | EC50 = 12.2 µg/cm2 | [27] |
| Trypanosoma cruzi | - | Insecticidal toxicity | IC50 = 7.3 mg/ml | ||
| 19-oxodihydroatisine (131) | Spodoptera littoralis | C | Antifeedant activity | EC50 = 0.1 µg/cm2 | [26] |
| Spodoptera littoralis | C | Insecticidal toxicity. A covariance analysis (ANCOV A1) of food consumption (ΔI) and biomass gains (ΔB) with initial larval weight as covariate, using oral injection. | ΔB = 90, ΔI = 92 | ||
| Leptinotarsa decemlineata | C | Antifeedant activity | EC50 > 50 µg/cm2 | ||
| 15,22-O-diacetyl-19-oxo-dihydroatisine (132) | Spodoptera littoralis | C | Antifeedant activity | EC50 = 6.1 µg/cm2 | |
| - | Insecticidal toxicity. A covariance analysis (ANCOV A1) of food consumption (ΔI) and biomass gains (ΔB) with initial larval weight as covariate, using oral injection. | ΔB = 91, ΔI = 85 | |||
| Leptinotarsa decemlineata | C | Antifeedant activity | EC50 > 50 µg/cm2 | ||
| dihydroajaconine (133) | Spodoptera littoralis | C | Antifeedant activity | EC50 > 50 µg/cm2 | |
| Insecticidal toxicity. A covariance analysis (ANCOV A1) of food consumption (ΔI) and biomass gains (ΔB) with initial larval weight as covariate, using oral injection. | ΔB = 80.5, ΔI = 97.2 | ||||
| Leptinotarsa decemlineata | C | Antifeedant activity | EC50 = 5.0 µg/cm2 | ||
| 15-epi-dihydroatisine (134) | Spodoptera littoralis | C | Antifeedant activity | EC50 > 50 µg/cm2 | |
| Insecticidal toxicity. A covariance analysis (ANCOV A1) of food consumption (ΔI) and biomass gains (ΔB) with initial larval weight as covariate, using oral injection. | ΔB = 98.10, ΔI = 96.37 | ||||
| Leptinotarsa decemlineata | C | Antifeedant activity | EC50 = 2.9 µg/cm2 | ||
| attisinium chloride (135) | Spodoptera littoralis | S | Antifeedant activity | EC50 = 2.4 µg/cm2 | |
| Insecticidal toxicity. A covariance analysis (ANCOV A1) of food consumption (ΔI) and biomass gains (ΔB) with initial larval weight as covariate, using oral injection. | ΔB = 123, ΔI = 103 | ||||
| Leptinotarsa decemlineata | C | Antifeedant activity | EC50 = 3.4 µg/cm2 | ||
| 7-hydroxy-isoatisine (136) | Spodoptera littoralis | C | Antifeedant activity | EC50 > 50 µg/cm2 | [26] |
| Insecticidal toxicity. A covariance analysis (ANCOV A1) of food consumption (ΔI) and biomass gains (ΔB) with initial larval weight as covariate, using oral injection. | ΔB = 93.6, ΔI = 119.2 | ||||
| Leptinotarsa decemlineata | C | Antifeedant activity | EC50 = 3.4 µg/cm2 | ||
| alkaloid a (137) | Spodoptera littoralis | C | Antifeedant activity | EC50 = 50 µg/cm2 | |
| Insecticidal toxicity. A covariance analysis (ANCOV A1) of food consumption (ΔI) and biomass gains (ΔB) with initial larval weight as covariate, using oral injection. | ΔB = 112.5, ΔI = 115.4 | ||||
| Leptinotarsa decemlineata | C | Antifeedant activity | EC50 = 5.4 µg/cm2 | ||
| alkaloid b (138) | Spodoptera littoralis | C | Antifeedant activity | EC50 > 50 µg/cm2 | |
| Insecticidal toxicity. A covariance analysis (ANCOV A1) of food consumption (ΔI) and biomass gains (ΔB) with initial larval weight as covariate, using oral injection. | ΔB = 101.0, ΔI = 119.9 | ||||
| Leptinotarsa decemlineata | C | Antifeedant activity | EC50 = 3.6 µg/cm2 | ||
| azitine (139) | Spodoptera littoralis | C | Antifeedant activity | EC50 = 1.1 µg/cm2 | |
| Insecticidal toxicity. A covariance analysis (ANCOV A1) of food consumption (ΔI) and biomass gains (ΔB) with initial larval weight as covariate, using oral injection. | ΔB = 109, ΔI = 99 | ||||
| Leptinotarsa decemlineata | C | Antifeedant activity | EC50 > 50 µg/cm2 | ||
| isozitine (140) | Spodoptera littoralis | C | Antifeedant activity | EC50 = 4.1 µg/cm2 | |
| Insecticidal toxicity. A covariance analysis (ANCOV A1) of food consumption (ΔI) and biomass gains (ΔB) with initial larval weight as covariate, using oral injection. | ΔB = 115, ΔI = 100 | ||||
| Leptinotarsa decemlineata | C | Antifeedant activity | EC50 = 6.9 µg/cm2 | ||
| beiwudine (102) | Spodoptera littoralis | C | Antifeedant activity | EC50 = 1.81 mg/cm2 | [22] |
| spicatine a (103) | EC50 = 8.18 mg/cm2 | ||||
| rockidine b (104) | EC50 = 0.32 mg/cm2 | [23] | |||
| ludaconitine (105) | EC50 = 0.77 mg/cm2 | ||||
| vilmorrianine c (107) | EC50 = 0.68 mg/cm2 | ||||
| transconitine b (106) | EC50 = 0.29 mg/cm2 | ||||
| geniculatine a (108) | EC50 = 0.35 mg/cm2 | ||||
| 4-hydroxynicotinic acid methyl ester (149) | Nilaparvata lugens | C | Contact toxicity | LD50 = 0.33 ± 0.05 μg/insect | [30] |
| Sogatella furcifera | C | LD50 = 0.26 ± 0.03 μg/insect | |||
| ranaconitine (146) | Nilaparvata lugens | C | LD50 = 0.26 ± 0.03 μg/insect | ||
| Sogatella furcifera | C | LD50 = 0.25 ± 0.02 μg/insect | |||
| shawurensine (109) | Spodoptera littoralis | C | Antifeedant activity | EC50 = 0.45 mg/cm2 | [21] |
| EC50 = 0.81 mg/cm2 | |||||
| leucostosineb (110) | EC50 = 1.54 mg/cm2 | [24] | |||
| delvestidine (111) | EC50 = 2.82 mg/cm2 | ||||
| 13-hydroxylappaconitine (148) | Nilaparvata lugens | C | Contact toxicity | LD50 = 0.38 ± 0.05 μg/insect | [27] |
| Sogatella furcifera | C | LD50 = 0.33 ± 0.02 μg/insect |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Zheng, L.; Huang, W.; Li, L.; Yuan, J.; Chen, L. Insecticidal Activities of Diterpene Alkaloids in Plants of the Genera Aconitum and Delphinium. Toxins 2025, 17, 254. https://doi.org/10.3390/toxins17050254
Wang J, Zheng L, Huang W, Li L, Yuan J, Chen L. Insecticidal Activities of Diterpene Alkaloids in Plants of the Genera Aconitum and Delphinium. Toxins. 2025; 17(5):254. https://doi.org/10.3390/toxins17050254
Chicago/Turabian StyleWang, Jinqiu, Luchuan Zheng, Wenxi Huang, Linxuan Li, Jialian Yuan, and Lin Chen. 2025. "Insecticidal Activities of Diterpene Alkaloids in Plants of the Genera Aconitum and Delphinium" Toxins 17, no. 5: 254. https://doi.org/10.3390/toxins17050254
APA StyleWang, J., Zheng, L., Huang, W., Li, L., Yuan, J., & Chen, L. (2025). Insecticidal Activities of Diterpene Alkaloids in Plants of the Genera Aconitum and Delphinium. Toxins, 17(5), 254. https://doi.org/10.3390/toxins17050254





