Heterologous Overexpression of Cytochrome P450BM3 from Bacillus megaterium and Its Role in Gossypol Reduction
Abstract
1. Introduction
2. Results
2.1. Heterologous Expression of Cytochrome P450BM3 and Optimization of Gossypol Degradation Conditions
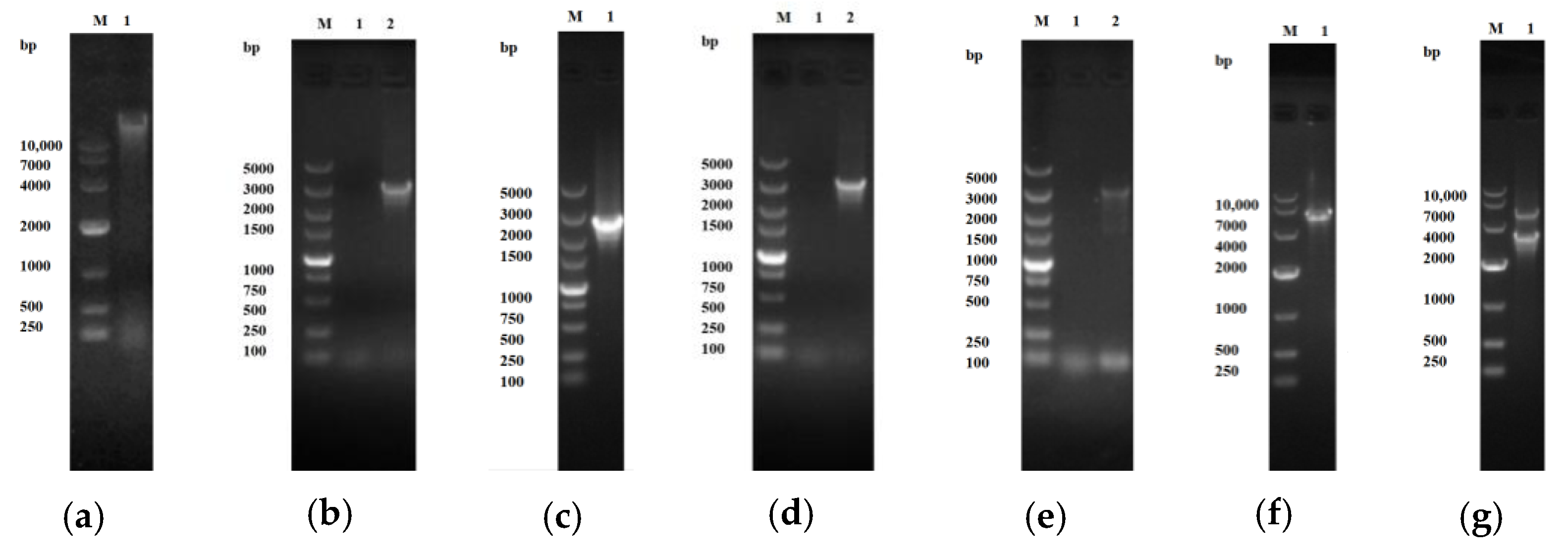
2.1.1. Construction of Cloning and Expression Vectors
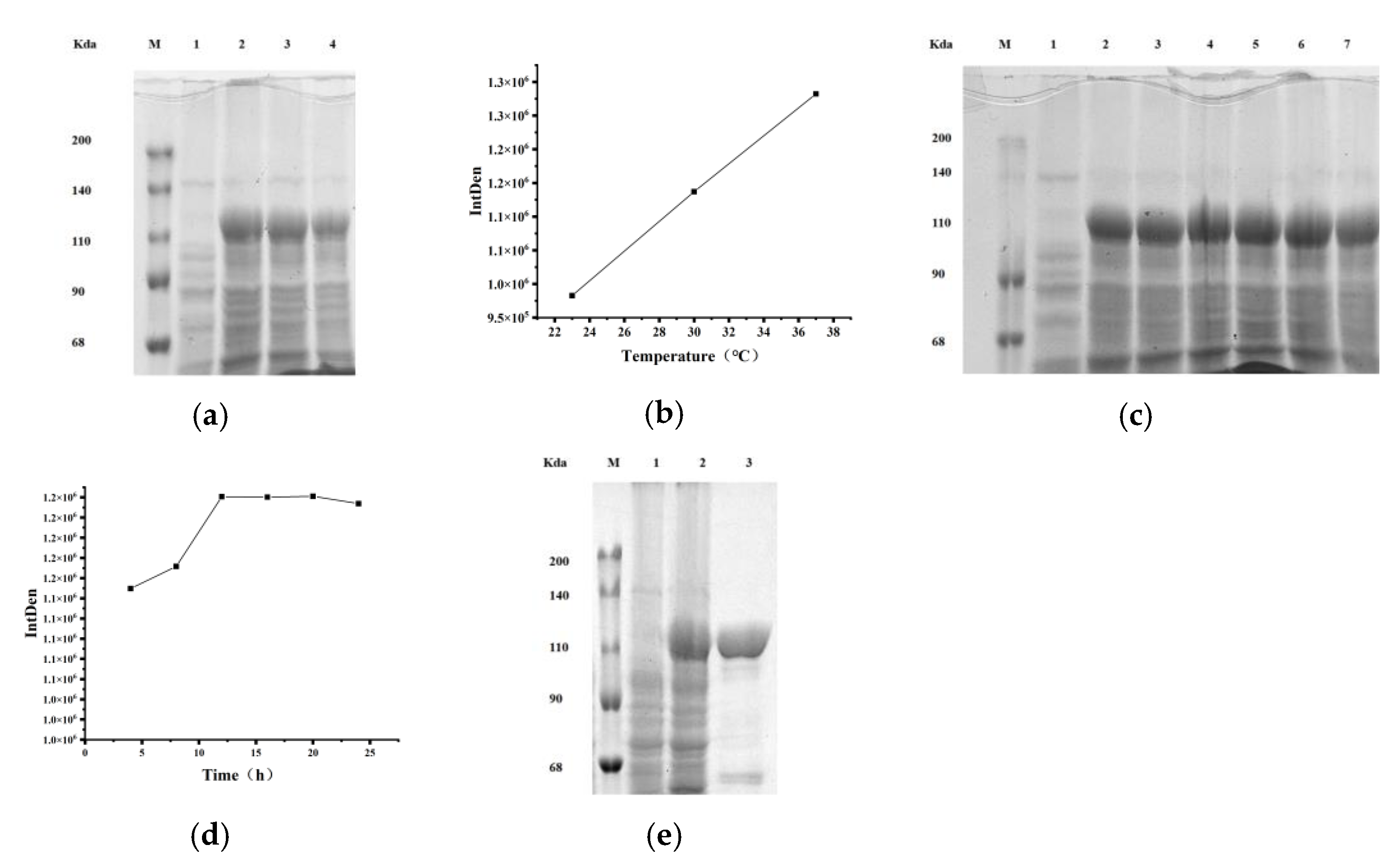
2.1.2. Optimization of Cytochrome P450BM3 Enzyme Expression
2.1.3. Optimization of Cytochrome P450BM3 Catalytic Conditions for Gossypol Degradation
2.2. Site-Directed Mutagenesis of Cytochrome P450BM3 and Comparative Analysis of Gossypol Degradation
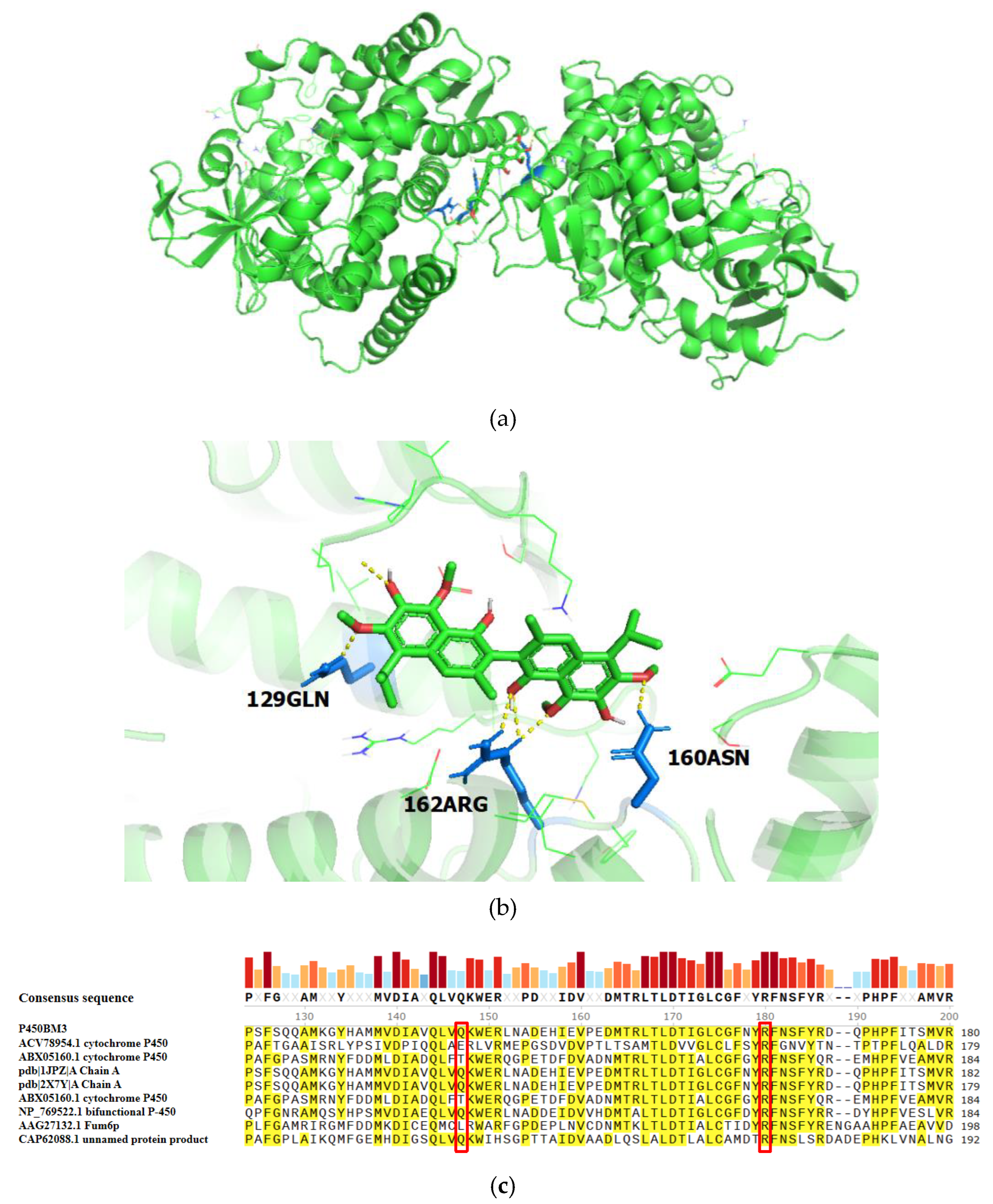
2.2.1. Prediction of Catalytic Active Sites in Cytochrome P450BM3 for Gossypol Reduction
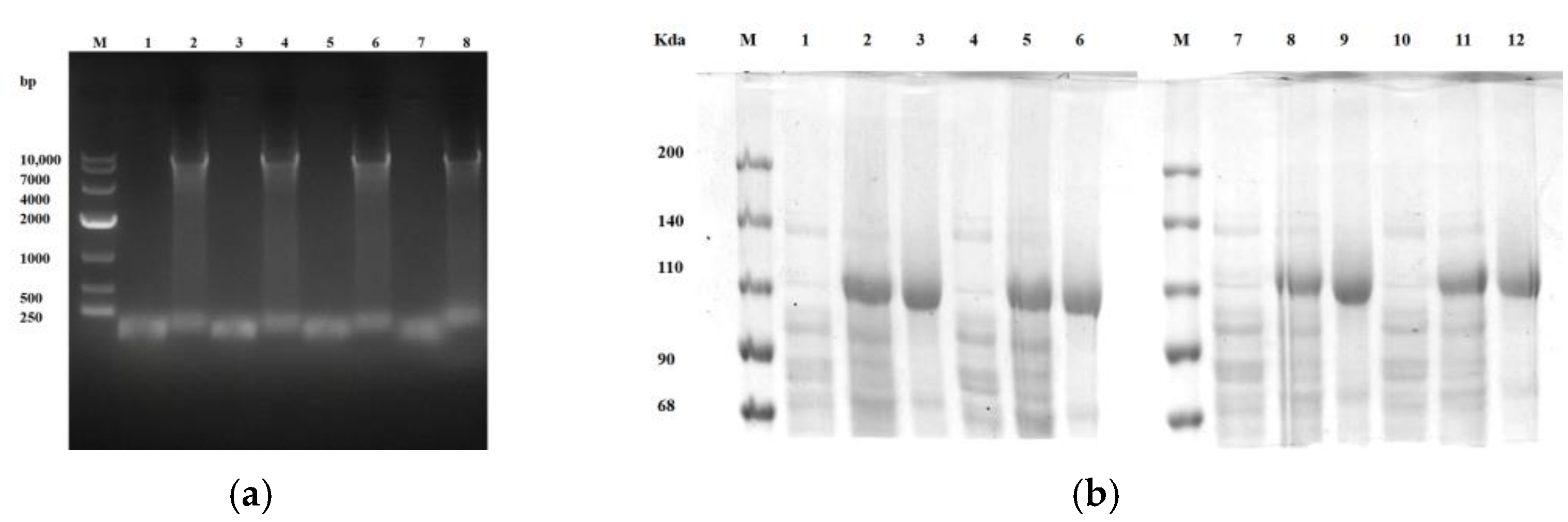
2.2.2. Site-Directed Mutagenesis of Cytochrome P450BM3
2.2.3. Comparison of Gossypol Degradation and Enzymatic Activity Between Cytochrome P450BM3 and Its Mutants
2.3. Changes in Gossypol Metabolites Catalyzed by Cytochrome P450BM3 and Its Mutants
Screening of Differential Metabolites
3. Discussion
3.1. Construction and Expression of Cytochrome P450BM3 in E. coli
3.2. Optimization of Cytochrome P450BM3-Catalyzed Gossypol Degradation Conditions
3.3. Comparison of Catalytic Efficiency Between Cytochrome P450BM3 and Its Mutants
3.4. Degradation Products and Pathways of Gossypol Catalyzed by Cytochrome P450BM3 and Its Mutants
4. Conclusions
5. Materials and Methods
5.1. General
5.2. Bacterial Strains, Plasmids, and DNA Techniques
5.3. Protein Expression and Purification
5.4. Cytochrome P450BM3 Enzyme Activity Measurements
5.5. Examination of the Catalytic Effect of Cytochrome P450BM3 on Gossypol
5.6. HPLC Determination of Gossypol
5.7. Site-Directed Mutagenesis of Cytochrome P450BM3
5.8. Comparative Analysis of Catalytic Effect on Gossypol and Metabolite Detection by Cytochrome P450BM3 Enzyme and Its Mutants
5.9. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| FG | Free gossypol |
| BG | Bound gossypol |
| CYP | Cytochrome P450 |
| UPLC-Q-TOF/MS | Ultra-performance liquid chromatography–quadrupole time-of-flight mass spectrometry |
References
- Adams, R.; Geissman, T.A.; Edwards, J.D. Gossypol, a pigment of cottonseed. Chem. Rev. 1960, 60, 555–574. [Google Scholar] [CrossRef] [PubMed]
- Matlin, S.A.; Zhou, R. Resolution of gossypol: Analytical and preparative HPLC. J. High Resolut. Chromatogr. 1984, 7, 629–631. [Google Scholar] [CrossRef]
- Matlin, S.A.; Zhou, R.H.; Belenguer, A.; Tyson, R.; Brookes, A. Large-scale resolution of gossypol enantiomers for biological evaluation. Contraception 1988, 37, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Radloff, R.J.; Deck, L.M.; Royer, R.E.; Jagt, D.L.V. Antiviral activities of gossypol and its derivatives against herpes simplex virus type II. Pharmacol. Res. Commun. 1986, 18, 1063–1073. [Google Scholar] [CrossRef] [PubMed]
- Montamat, E.E.; Burgos, C.; de Burgos, N.M.G.; Rovai, L.E.; Blanco, A.; Segura, E.L. Inhibitory action of gossypol on enzymes and growth of Trypanosoma cruzi. Science 1982, 218, 288–289. [Google Scholar] [CrossRef]
- Jaroszewski, J.W.; Kaplan, O.; Cohen, J.S. Action of gossypol and rhodamine 123 on wild type and multidrug-resistant MCF-7 human breast cancer cells: 31P nuclear magnetic resonance and toxicity studies. Cancer Res. 1990, 50, 6936–6943. [Google Scholar]
- Yildirim-Aksoy, M.; Lim, C.; Wan, P.; Klesius, P. Effect of natural free gossypol and gossypol-acetic acid on growth performance and resistance of channel catfish (Ictalurus punctatus) to Edwardsiella ictaluri challenge. Aquac. Nutr. 2004, 10, 153–165. [Google Scholar] [CrossRef]
- Chenoweth, P.J.; Chase, C.C., Jr.; Risco, C.A.; Larsen, R.E. Characterization of gossypol-induced sperm abnormalities in bulls. Theriogenology 2000, 53, 1193–1203. [Google Scholar] [CrossRef]
- Brocas, C.; Rivera, R.M.; Paula-Lopes, F.F.; McDowell, L.R.; Calhoun, M.C.; Staples, C.R.; Wilkinson, N.S.; Boning, A.J.; Chenoweth, P.J.; Hansen, P.J. Deleterious actions of gossypol on bovine spermatozoa, oocytes, and embryos. Biol. Reprod. 1997, 57, 901–907. [Google Scholar] [CrossRef][Green Version]
- Reiser, R.; Fu, H.C. The mechanism of gossypol detoxification by ruminant animals. J. Nutr. 1962, 76, 215–218. [Google Scholar] [CrossRef]
- Wang, L.; Chen, M.; Luo, X.; Fan, Y.; Zheng, Z.; He, Z.; Yin, R.; Meng, T.; Xu, S.; Pan, Y.; et al. Intramolecular annulation of gossypol by laccase to produce safe cottonseed protein. Front. Chem. 2020, 8, 583176. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhang, Y.; Pi, W.; Yang, W.; Nie, C.; Liang, J.; Ma, X.; Zhang, W.J. Optimization of the process parameters for reduction of gossypol levels in cottonseed meal by functional recombinant NADPH-cytochrome P450 reductase and cytochrome P450 CYP9A12 of Helicoverpa armigera. AMB Express 2019, 9, 98. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, X.; Huang, R.; Nie, C.; Niu, J.; Chen, C.; Zhang, W. Biodegradation of free gossypol by Helicoverpa armigera carboxylesterase expressed in Pichia pastoris. Toxins 2022, 14, 816. [Google Scholar] [CrossRef] [PubMed]
- Krempl, C.; Sporer, T.; Reichelt, M.; Ahn, S.J.; Heidel-Fischer, H.; Vogel, H.; Heckel, D.G.; Joußen, N. Potential detoxification of gossypol by UDP-glycosyltransferases in the two Heliothine moth species Helicoverpa armigera and Heliothis virescens. Insect Biochem. Mol. Biol. 2016, 71, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Ortiz de Montellano, P.R. Cytochrome P450: Structure, Mechanism, and Biochemistry, 3rd ed.; Plenum Press: New York, NY, USA, 2005. [Google Scholar]
- Narhi, L.O.; Fulco, A.J. Identification and characterization of two functional domains in cytochrome P-450BM-3, a catalytically self-sufficient monooxygenase induced by barbiturates in Bacillus megaterium. J. Biol. Chem. 1987, 262, 6683–6690. [Google Scholar] [CrossRef]
- Boddupalli, S.; Oster, T.; Estabrook, R.; Peterson, J. Reconstitution of the fatty acid hydroxylation function of cytochrome P-450BM-3 utilizing its individual recombinant hemo-and flavoprotein domains. J. Biol. Chem. 1992, 267, 10375–10380. [Google Scholar] [CrossRef]
- Hannemann, F.; Bichet, A.; Ewen, K.M.; Bernhardt, R. Cytochrome P450 systems—Biological variations of electron transport chains. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2007, 1770, 330–344. [Google Scholar] [CrossRef]
- Sideri, A.; Goyal, A.; Di Nardo, G.; Tsotsou, G.E.; Gilardi, G. Hydroxylation of non-substituted polycyclic aromatic hydrocarbons by cytochrome P450 BM3 engineered by directed evolution. J. Inorg. Biochem. 2013, 120, 1–7. [Google Scholar] [CrossRef]
- Sulistyaningdyah, W.T.; Ogawa, J.; Li, Q.S.; Maeda, C.; Yano, Y.; Schmid, R.D.; Shimizu, S. Hydroxylation activity of P450 BM-3 mutant F87V towards aromatic compounds and its application to the synthesis of hydroquinone derivatives from phenolic compounds. Appl. Microbiol. Biotechnol. 2005, 67, 556–562. [Google Scholar] [CrossRef]
- Ma, X. Preliminary Study on the Degradation of Free Gossypol in Cottonseed Meal by Bacillus megaterium and Its Mechanism. Master’s Thesis, Xinjiang Agricultural University, Xinjiang, China, 2024. [Google Scholar]
- Li, C.; Hou, X.; Guo, B.; Rao, Y. Expression and characterization of a novel cytochrome P450 enzyme from Variovorax paradoxus S110. Sheng Wu Gong Cheng Xue Bao=Chin. J. Biotechnol. 2020, 36, 1346–1355. [Google Scholar]
- Yıldırım, D.; Ozic, C.; Ensari, Y. Expression and Characterization of a New Self-Sufficient P450 Monooxygenase (P450 AZC1) from Azorhizobium caulinodans. ChemBioChem 2023, 24, e202300065. [Google Scholar] [CrossRef] [PubMed]
- De Groot, N.S.; Ventura, S. Effect of temperature on protein quality in bacterial inclusion bodies. FEBS Lett. 2006, 580, 6471–6476. [Google Scholar] [CrossRef]
- Lu, Y.; Mei, L. Optimization of fermentation conditions for P450 BM-3 monooxygenase production by hybrid design methodology. J. Zhejiang Univ. Sci. B 2007, 8, 27–32. [Google Scholar] [CrossRef]
- Li, Z. Cloning, Optimized Expression, and Enzymatic Characterization of CYP102A16, a Cytochrome P450 from Bacillus sp. C3. Ph.D. Dissertation, Zhejiang University, Hangzhou, China, 2012. [Google Scholar]
- Fu, P.; Barford, J.P. Optimal induction timing strategy for recombinant protein production in E. coli. IFAC Proc. Vol. 1993, 26, 237–240. [Google Scholar] [CrossRef]
- Dietrich, M.; Eiben, S.; Asta, C.; Do, T.A.; Pleiss, J.; Urlacher, V.B. Cloning, expression and characterisation of CYP102A7, a self-sufficient P450 monooxygenase from Bacillus licheniformis. Appl. Microbiol. Biotechnol. 2008, 79, 931–940. [Google Scholar] [CrossRef]
- Wei, T.L.; Miao, H.B.; Wu, Q.; Huang, Z.X. Heterologous Expression, Enzymatic Characterization of Laccase BmLac and Degradation of Gossypol by It. Biotechnol. Bull 2023, 39, 320. [Google Scholar]
- Rabe, K.S.; Kiko, K.; Niemeyer, C.M. Characterization of the peroxidase activity of CYP119, a thermostable P450 from Sulfolobus acidocaldarius. ChemBioChem 2008, 9, 420–425. [Google Scholar] [CrossRef] [PubMed]
- Cui, H. Molecular Engineering of P450DA and Its Application in the Synthesis of Chiral β-Halohydrins. Ph.D. Dissertation, Zunyi Medical University, Zunyi, China, 2019. [Google Scholar]
- Furukawa, M.; Kawakami, N.; Tomizawa, A.; Miyamoto, K. Efficient degradation of poly (ethylene terephthalate) with Thermobifida fusca cutinase exhibiting improved catalytic activity generated using mutagenesis and additive-based approaches. Sci. Rep. 2019, 9, 16038. [Google Scholar] [CrossRef]
- Ma, Y.; Yao, M.; Li, B.; Ding, M.; He, B.; Chen, S.; Zhou, X.; Yuan, Y. Enhanced poly (ethylene terephthalate) hydrolase activity by protein engineering. Engineering 2018, 4, 888–893. [Google Scholar] [CrossRef]
- Huang, Q.; Zhang, X.; Chen, Q.; Tian, S.; Tong, W.; Zhang, W.; Chen, Y.; Ma, M.; Chen, B.; Wang, J.B. Discovery of a P450-catalyzed oxidative defluorination mechanism toward chiral organofluorines: Uncovering a hidden pathway. ACS Catal. 2021, 12, 265–272. [Google Scholar] [CrossRef]
- Beyazit, N.; Cakran, H.S.; Cabir, A.; Akiscan, Y.; Demetgul, C. Synthesis, characterization and antioxidant activity of chitosan Schiff base derivatives bearing (−)-gossypol. Carbohydr. Polym. 2020, 240, 116333. [Google Scholar] [CrossRef]
- Romero, A.C.; Calori-Domingues, M.A.; Abdalla, A.L.; Augusto, P.E.D. Evaluation of ozone technology as an alternative for degradation of free gossypol in cottonseed meal: A prospective study. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2021, 38, 659–669. [Google Scholar] [CrossRef]
- Zhang, B.; Li, L.; Liu, Y.; Wang, Q. Antiviral mechanism study of gossypol and its Schiff base derivatives based on reactive oxygen species (ROS). RSC Adv. 2016, 6, 87637–87648. [Google Scholar] [CrossRef]
- Zhou, S.F. Screening of High-Efficiency Gossypol-Degrading Microbial Strains, Degradation Mechanism, and Solid-State Fermentation Process. Ph.D. Dissertation, Gansu Agricultural University, Lanzhou, China, 2011. [Google Scholar]
- Abou-Donia, M.B.; Dieckert, J.W. Metabolic fate of gossypol: The metabolism of [14C] gossypol in swine. Toxicol. Appl. Pharmacol. 1975, 31, 32–46. [Google Scholar] [CrossRef]
- Witkop, B.; Beiler, T.W. Studies on Schiff bases in connection with the mechanism of transamination. J. Am. Chem. Soc. 1954, 76, 5589–5597. [Google Scholar] [CrossRef]
- Alley, P.W.; Shirley, D.A. Some New Anil Derivatives of Gossypol1. J. Org. Chem. 1959, 24, 1534–1536. [Google Scholar] [CrossRef]
- Cater, C.M.; Lyman, C.M. Reaction of gossypol with amino acids and other amino compounds. J. Am. Oil Chem. Soc. 1969, 46, 649–653. [Google Scholar] [CrossRef]
- Habeeb, A.; Hiramoto, R. Reaction of proteins with glutaraldehyde. Arch. Biochem. Biophys. 1968, 126, 16–26. [Google Scholar] [CrossRef]
- Sleight, S.C.; Bartley, B.A.; Lieviant, J.A.; Sauro, H.M. In-Fusion BioBrick assembly and re-engineering. Nucleic Acids Res. 2010, 38, 2624–2636. [Google Scholar] [CrossRef]
- Murray, V.; Chen, J.; Huang, Y.; Li, Q.; Wang, J. Preparation of very-high-yield recombinant proteins using novel high-cell-density bacterial expression methods. Cold Spring Harb. Protoc. 2010, 2010, pdb-prot5475. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, Y.; Chen, S.; Devine, G.; Denholm, I.; Jewess, P.; Moores, G. The involvement of microsomal oxidases in pyrethroid resistance in Helicoverpa armigera from Asia. Insect Biochem. Mol. Biol. 2004, 34, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Rahma, E.H.; Rao, M.N. Gossypol removal and functional properties of protein produced by extraction of glanded cottonseed with different solvents. J. Food Sci. 1984, 49, 1057–1060. [Google Scholar] [CrossRef]
- Liu, H.; Naismith, J.H. An efficient one-step site-directed deletion, insertion, single and multiple-site plasmid mutagenesis protocol. BMC Biotechnol. 2008, 8, 91. [Google Scholar] [CrossRef]
- Wen, B.; Mei, Z.; Zeng, C.; Liu, S. metaX: A flexible and comprehensive software for processing metabolomics data. BMC Bioinform. 2017, 18, 183. [Google Scholar] [CrossRef] [PubMed]





| Group | Item | Compound | m/z | RT (min) | Regulate |
|---|---|---|---|---|---|
| P450BM3 | 1 | C28H28O9 | 507.16 | 6.64 | Up |
| 2 | C27H2807 | 465.19 | 6.79 | Down | |
| 3 | C25H24O6 | 419.15 | 6.76 | Down | |
| 4 | C16H14O6 | 301.07 | 6.98 | Up | |
| 5 | C16H18N2 | 239.15 | 5.50 | Up | |
| R162H | 1 | C28H30O7 | 477.19 | 6.96 | Down |
| 2 | C25H24O5 | 405.17 | 6.76 | Down | |
| 3 | C16H14O6 | 301.07 | 6.98 | Up | |
| 4 | C16H20N2O3 | 367.18 | 6.82 | Up | |
| Q129H | 1 | C28H30O8 | 493.19 | 6.45 | Down |
| 2 | C28H28O8 | 493.19 | 6.76 | Down | |
| 3 | C27H28O9 | 497.18 | 6.25 | Down | |
| 4 | C27H28O7 | 465.20 | 6.12 | Down | |
| 5 | C26H26O6 | 435.18 | 6.67 | Down | |
| 6 | C20H26N2O2 | 349.18 | 9.33 | Down | |
| 7 | C29H26N2O4 | 457.20 | 6.54 | Down | |
| 8 | C16H20N2O2 | 273.16 | 5.57 | Up |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, W.; Cui, J.; Xu, T.; Xu, S.; Ainiwaer, Z.; Luo, Q.; Wang, C. Heterologous Overexpression of Cytochrome P450BM3 from Bacillus megaterium and Its Role in Gossypol Reduction. Toxins 2025, 17, 253. https://doi.org/10.3390/toxins17050253
Fan W, Cui J, Xu T, Xu S, Ainiwaer Z, Luo Q, Wang C. Heterologous Overexpression of Cytochrome P450BM3 from Bacillus megaterium and Its Role in Gossypol Reduction. Toxins. 2025; 17(5):253. https://doi.org/10.3390/toxins17050253
Chicago/Turabian StyleFan, Wenpeng, Jingjing Cui, Tongxiang Xu, Shiheng Xu, Zulibina Ainiwaer, Qiyu Luo, and Caidie Wang. 2025. "Heterologous Overexpression of Cytochrome P450BM3 from Bacillus megaterium and Its Role in Gossypol Reduction" Toxins 17, no. 5: 253. https://doi.org/10.3390/toxins17050253
APA StyleFan, W., Cui, J., Xu, T., Xu, S., Ainiwaer, Z., Luo, Q., & Wang, C. (2025). Heterologous Overexpression of Cytochrome P450BM3 from Bacillus megaterium and Its Role in Gossypol Reduction. Toxins, 17(5), 253. https://doi.org/10.3390/toxins17050253





