A Comprehensive Probabilistic Risk Assessment Strategy of Aflatoxin B1 Exposure from Medical Areca Nuts Consumption
Abstract
1. Introduction
2. Results and Discussion
2.1. Results of AFB1 Prevalence in Medical Areca Nuts
2.2. Risk Assessment Results
2.2.1. Estimated Daily Intake of AFB1 for Medical Areca Nuts
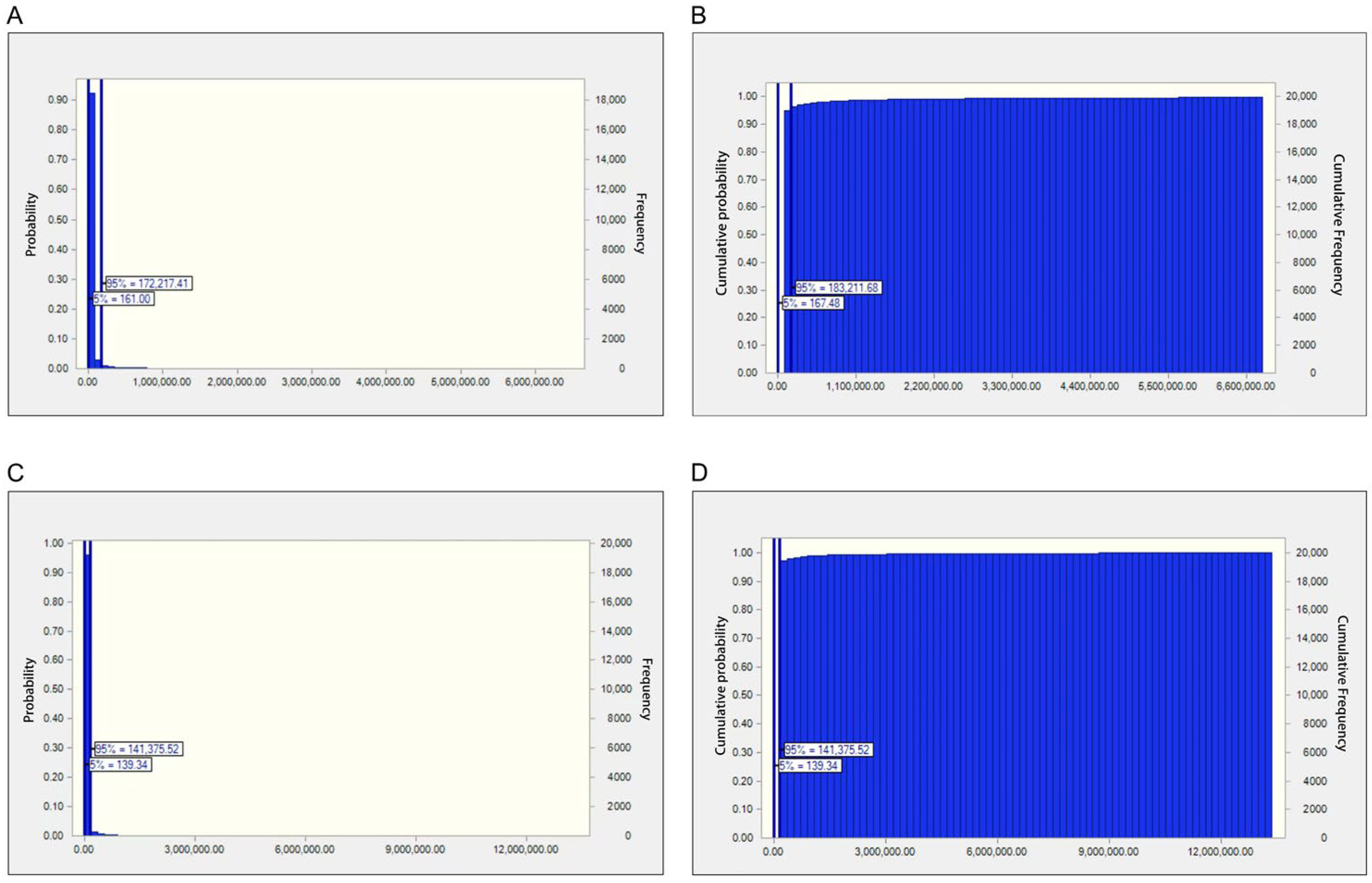
2.2.2. Risk Assessment Based on the MOE Approach
2.2.3. Risk Assessment via the Quantitative HCC Risk Approach
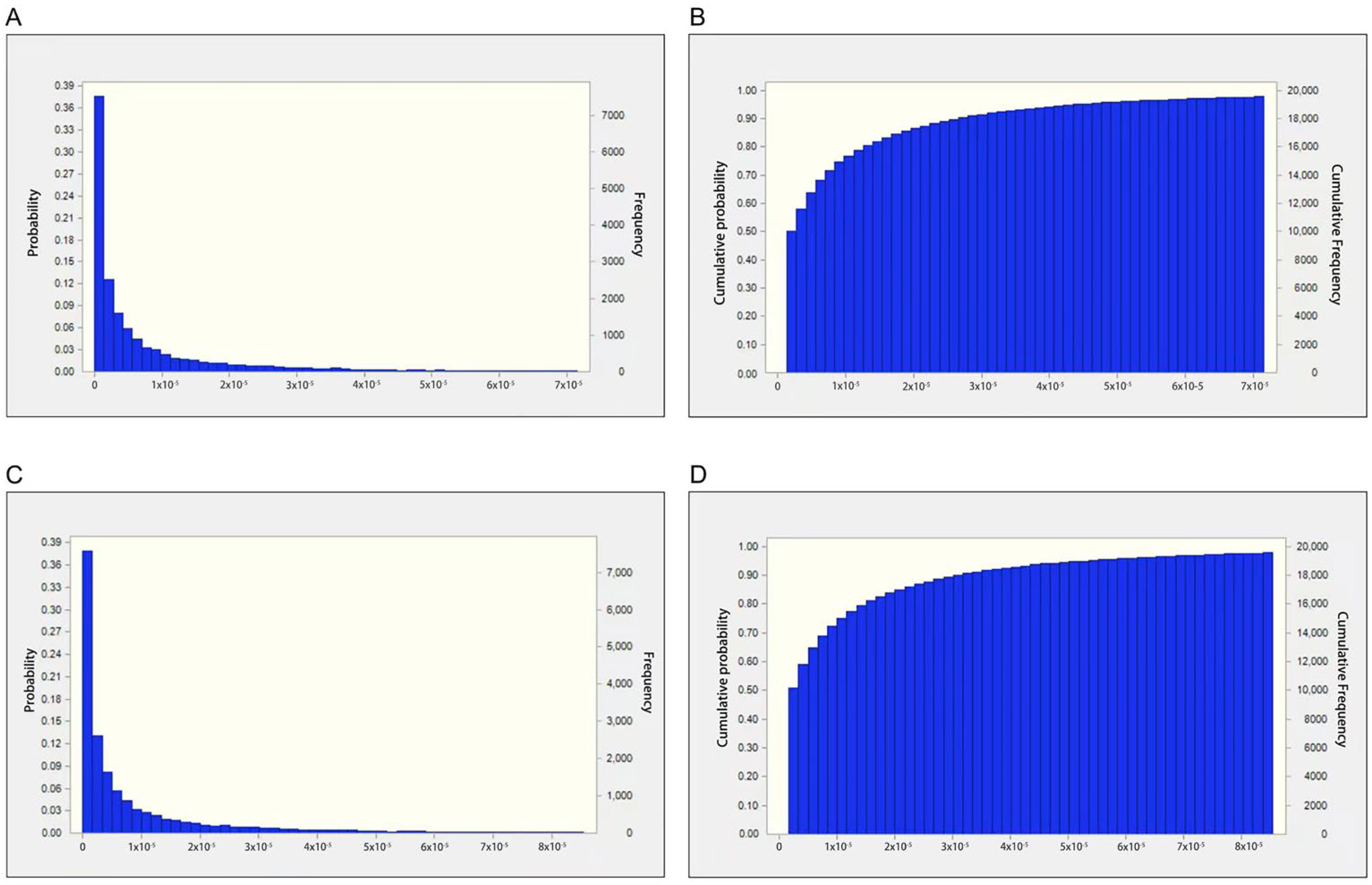
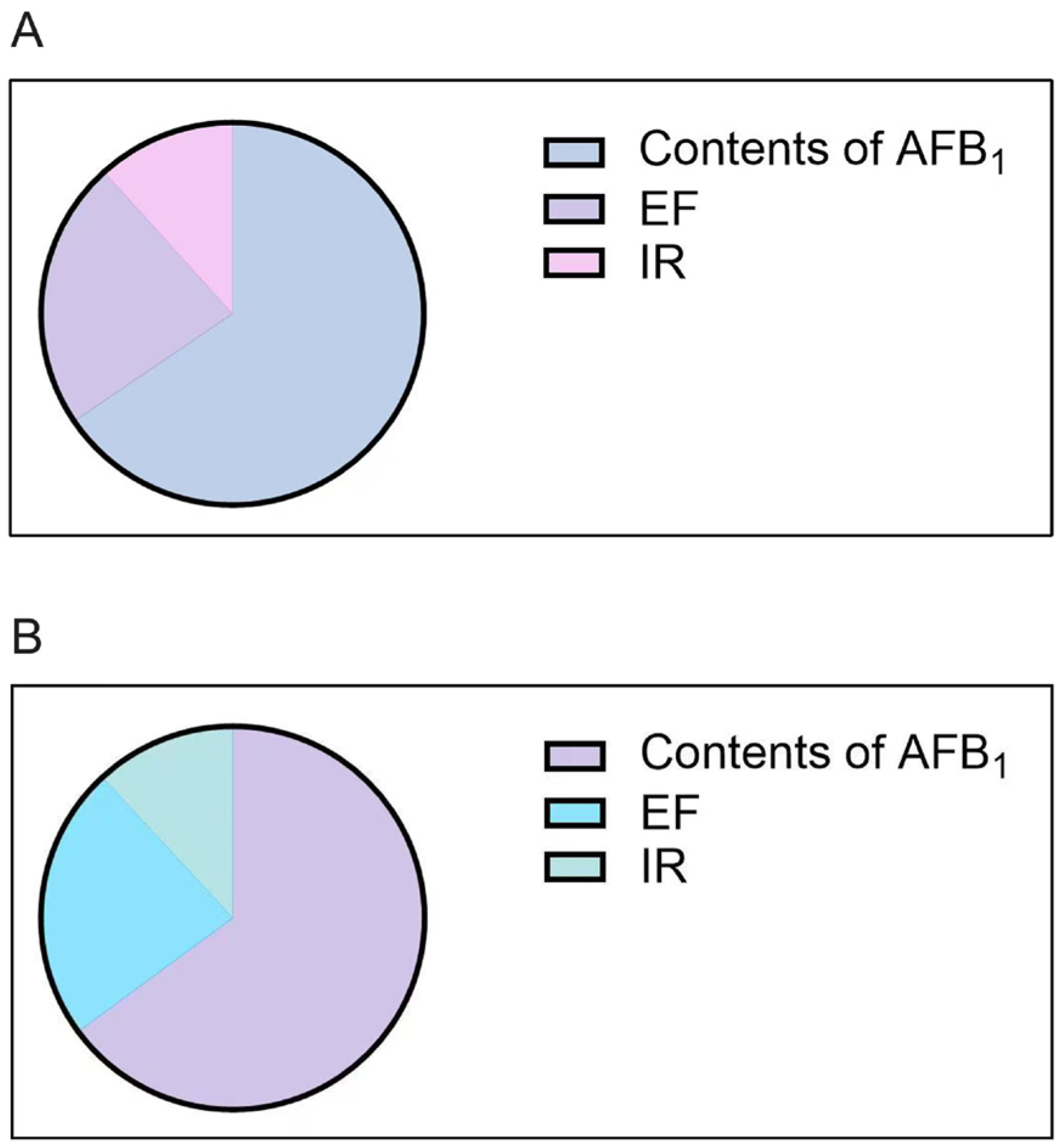
2.3. Sensitivity Analysis Results
2.4. Discussion
2.5. Conclusions
3. Materials and Methods
3.1. Sample Collection
3.2. Sample Preparation and Analysis
3.3. Analytical Procedure
3.4. Exposure Assessment
3.5. Risk Characterization
3.5.1. The MOE Approach
3.5.2. Quantitative HCC Risk Approach
3.5.3. Probabilistic Assessment via Monte Carlo Simulation (MCS)
3.6. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liew, W.P.; Mohd-Redzwan, S. Mycotoxin: Its Impact on Gut Health and Microbiota. Front. Cell. Infect. Microbiol. 2018, 8, 60. [Google Scholar] [CrossRef] [PubMed]
- Pleadin, J.; Frece, J.; Markov, K. Mycotoxins in food and feed. Adv. Food Nutr. Res. 2019, 89, 297–345. [Google Scholar] [PubMed]
- Paterson, R.R.; Lima, N. Toxicology of mycotoxins. Mol. Clin. Environ. Toxicol. 2010, 100, 31–63. [Google Scholar]
- Saleemi, M.K.; Raza, A.; Khatoon, A.; Zubair, M.; Xu, Y.; Murtaza, B.; Li, X.; Jamil, M.; Imran, M.; Muhammad, F.; et al. Toxic Effects of Aflatoxin B1 on Hematobiochemical and Histopathological Parameters of Juvenile White Leghorn Male Birds and their Amelioration with Vitamin E and Moringa oleifera. Pak. Vet. J. 2023, 43, 405–411. [Google Scholar]
- Tarhane, S.; Dursun, İ.; Mor, B.; Kanıcı, T.A.; Kızıltepe, Ş.; Filazi, İ.; Güven, A. Determination of the mycotoxin activity of filamentous fungi isolated from the intestinal region of adult honey bees by the PCR and UHPLCOrbitrap-HRMS methods. Kafkas Univ. Vet. Fak. Derg. 2023, 29, 473–481. [Google Scholar] [CrossRef]
- Javed, S.; Shoaib, A.; Malik, A.; Ijaz, B.; Perveen, S. Rose and eucalyptus essential oil as potent anti-liver canceragents. Asian J. Agric. Biol. 2023, 2023, 2022141. [Google Scholar]
- Alharbi, A.A. In vitro and in vivo anticancer, anti-inflammatory, and antioxidant activity of Dhimran (Ocimum forsskaolii benth) extract and essential oil on carbon tetrachlorideinduced hepatotoxicity in mice. Kafkas Univ. Vet. Fak. Derg. 2024, 30, 729–740. [Google Scholar] [CrossRef]
- Latham, R.L.; Boyle, J.T.; Barbano, A.; Loveman, W.G.; Brown, N.A. Diverse Mycotoxin Threats to Safe Food and Feed Cereals. Essays Biochem. 2023, 67, 797–809. [Google Scholar]
- Azziz-Baumgartner, E.; Lindblade, K.; Gieseker, K.; Rogers, H.S.; Kieszak, S.; Njapau, H.; Schleicher, R.; McCoy, L.F.; Misore, A.; DeCock, K.; et al. Case-control study of an acute aflatoxicosis outbreak, Kenya, 2004. Environ. Health Perspect. 2005, 113, 1779–1783. [Google Scholar] [CrossRef]
- Kachapulula, P.W.; Akello, J.; Bandyopadhyay, R.; Cotty, P.J. Aflatoxin contamination of groundnut and maize in Zambia: Observed and potential concentrations. J. Appl. Microbiol. 2017, 122, 1471–1482. [Google Scholar] [CrossRef]
- Nazareth, T.M.; Torrijos, R.; Bocate, K.P.; Mañes, J.; Luciano, F.B.; Meca, G.; Vila-Donat, P. Development of an Antifungal Device Based on Oriental Mustard Flour to Prevent Fungal Growth and Aflatoxin B1 Production in Almonds. Toxins 2021, 14, 5. [Google Scholar] [CrossRef] [PubMed]
- Marin, S.; Ramos, A.J.; Cano-Sancho, G.; Sanchis, V. Mycotoxins: Occurrence, toxicology, and exposure assessment. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2013, 60, 218–237. [Google Scholar] [CrossRef] [PubMed]
- Marchese, S.; Polo, A.; Ariano, A.; Velotto, S.; Costantini, S.; Severino, L. Aflatoxin B1 and M1: Biological Properties and Their Involvement in Cancer Development. Toxins 2018, 10, 214. [Google Scholar] [CrossRef]
- Abrar, M.; Anjum, F.M.; Butt, M.S.; Pasha, I.; Randhawa, M.A.; Saeed, F.; Waqas, K. Aflatoxins: Biosynthesis, occurrence, toxicity, and remedies. Crit. Rev. Food Sci. Nutr. 2013, 53, 862–874. [Google Scholar] [CrossRef]
- Liu, Y.; Chang, C.C.; Marsh, G.M.; Wu, F. Population attributable risk of aflatoxin-related liver cancer: Systematic review and meta-analysis. Eur. J. Cancer 2012, 48, 2125–2136. [Google Scholar] [CrossRef]
- Iqbal, S.Z.A.; Ariño, M.R.; Affatoxins, A. Reference Module in Life Sciences; Elsevier: Amsterdam, The Netherlands, 2017; ISBN 9780128096338. [Google Scholar]
- CODEX, 2015. General Standard for Contaminants and Toxins in Food and Feed (CODEX STAN 193-1995). Codex Alimentarius Comission, JECFA, Rome. Vol. CODEX STAN 193-1995. Available online: http://www.fao.org/fao-who-codexalimentarius/codex-texts/list-standards/en/ (accessed on 29 December 2017).
- Guo, Y.; Zuo, T.; Gong, S.; Chen, A.; Jin, H.; Liu, J.; Wang, Q.; Liu, J.; Kang, S.; Li, P.; et al. Multi-Element Fingerprinting Combined with Chemometrics for Identification of Seaweeds and Innovative Risk-Benefit Assessment. Foods 2024, 13, 4159–8412. [Google Scholar] [CrossRef]
- European Commission. In Setting Maximum Levels for Certain Contaminants in Foodstuffs. 2006, Vol. No. 1881/2006. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex%3A32006R1881 (accessed on 29 December 2017).
- FDA. The United States Food and Drug Administration Guidilines for Aflatoxin Levels. 2017. Available online: http://agriculture.mo.gov/plants/feed/aflatoxin.php (accessed on 10 July 2017).
- Tassaneeyakul, W.; Razzazi-Fazeli, E.; Porasuphatana, S.; Bohm, J. Contamination of aflatoxins in herbal medicinal products in Thailand. Mycopathologia 2004, 158, 239–244. [Google Scholar] [CrossRef]
- Liu, L.; Jin, H.; Sun, L.; Ma, S.; Lin, R. Determination of aflatoxins in medicinal herbs by high-performance liquid chromatography-tandem mass spectrometry. Phytochem. Anal. PCA 2012, 23, 469–476. [Google Scholar] [CrossRef]
- Huang, G.; Zeng, D.; Liang, T.; Liu, Y.; Cui, F.; Zhao, H.; Lu, W. Recent Advance on Biological Activity and Toxicity of Arecoline in Edible Areca (Betel) Nut: A Review. Foods. 2024, 13, 3825–3838. [Google Scholar] [CrossRef] [PubMed]
- Rarau, P.; Vengiau, G.; Gouda, H.; Phuanukoonon, S.; Kevau, I.H.; Bullen, C.; Scragg, R.; Riley, I.; Marks, G.; Umezaki, M.; et al. Prevalence of non-communicable disease risk factors in three sites across Papua New Guinea: A cross-sectional study. BMJ Glob. Health 2017, 2, e000221. [Google Scholar] [CrossRef]
- Rumgay, H.; Nethan, S.T.; Shah, R.; Vignat, J.; Ayo-Yusuf, O.; Chaturvedi, P.; Guerra, E.N.S.; Gupta, P.C.; Gupta, R.; Liu, S.; et al. Global burden of oral cancer in 2022 attributable to smokeless tobacco and areca nut consumption: A population attributable fraction analysis. Lancet. Oncol. 2024, 25, 1413–1423. [Google Scholar] [CrossRef] [PubMed]
- Teerawichitchainan, B.; Knodel, J. Tradition and Change in Marriage Payments in Vietnam, 1963–2000. Asian Popul. Stud. 2012, 8, 151–172. [Google Scholar] [CrossRef]
- Gu, X.; Hao, D.; Xiao, P. Research progress of Chinese herbal medicine compounds and their bioactivities: Fruitful 2020. Chin. Herb. Med. 2022, 14, 171–186. [Google Scholar] [CrossRef] [PubMed]
- Volgin, A.D.; Bashirzade, A.; Amstislavskaya, T.G.; Yakovlev, O.A.; Demin, K.A.; Ho, Y.J.; Wang, D.; Shevyrin, V.A.; Yan, D.; Tang, Z.; et al. DARK Classics in Chemical Neuroscience: Arecoline. ACS Chem. Neurosci. 2019, 10, 2176–2185. [Google Scholar] [CrossRef]
- Abbas, G.; Naqvi, S.; Erum, S.; Ahmed, S.; Atta Ur, R.; Dar, A. Potential antidepressant activity of Areca catechu nut via elevation of serotonin and noradrenaline in the hippocampus of rats. Phytother. Res. PTR 2013, 27, 39–45. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, Z.; Luo, Y.; Ma, L.; Hu, X.; Chen, F.; Li, D. A review of the traditional uses, pharmacology, and toxicology of areca nut. Phytomed 2024, 134, 156005. [Google Scholar] [CrossRef]
- Lee, H.G.; Song, J.A.; Han, D.S.; Woo, K.W.; Yoon, M.H. Antiallodynic Effects of Intrathecal Areca Nut for Spinal Nerve-Ligated and Chemotherapy-Induced Neuropathic Pain in Rats. Pharmacology 2018, 102, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.B.; Jeon, S.M.; Byun, S.J.; Kim, H.S.; Choi, M.S. Absorption of intestinal free cholesterol is lowered by supplementation of Areca catechu L. extract in rats. Life Sci. 2002, 70, 1849–1859. [Google Scholar] [CrossRef]
- Dhanraj, M.; Veerakumari, L. Effect of ethanol extract of Areca catechu on Fumarate reductase and Succinate dehydrogenase of Cotylophoron cotylophorum. Int. J. Res. Dev. Pharm. Life Sci. 2018, 5, 2117–2123. [Google Scholar]
- Wei, P.L.; Hung, C.S.; Lu, H.H.; Batzorig, U.; Huang, C.Y.; Chang, Y.J. Areca nut extract (ANE) inhibits the progression of hepatocellular carcinoma cells via activation of ROS production and activation of autophagy. Int. J. Med. Sci. 2021, 18, 3452–3462. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, P.; Li, X.; Wang, X.; Song, J.; Peng, W.; Wu, C. Comparative Researches of Semen Arecae and Charred Semen Arecae on Gastrointestinal Motility, Motilin, Substance P, and CCK in Chronically Stressed Rats. Evid. Based Complement. Altern. Med. Ecam. 2017, 2017, 1273561. [Google Scholar] [CrossRef] [PubMed]
- Zuo, T.T.; Jin, H.Y.; Zhang, L.; Liu, Y.L.; Nie, J.; Chen, B.L.; Fang, C.F.; Xue, J.; Bi, X.Y.; Zhou, L.; et al. Innovative health risk assessment of heavy metals in Chinese herbal medicines based on extensive data. Pharmacol. Res. 2021, 163, 105268. [Google Scholar] [CrossRef] [PubMed]
- Zuo, T.T.; Liu, J.; Zan, K.; Liu, L.N.; Wang, Q.; Wang, Z.; Xu, W.Y.; Liu, Y.X.; Guo, Y.S.; Kang, S.; et al. Bioaccessibility and bioavailability of exogenous and endogenous toxic substances in traditional Chinese medicine and their significance in risk assessment. Pharmacol. Res. 2024, 208, 107388. [Google Scholar] [CrossRef] [PubMed]
- Joint FAO/WHO Expert Committee on Food Additive. Evaluation of certain food additives and contaminants. WHO Tech. Rep. Ser. 1987, 759, 359. [Google Scholar]
- Majeed, S.; De Boevre, M.; De Saeger, S.; Rauf, W.; Tawab, A.; Fazale, H.; Rahman, M.; Iqbal, M. Multiple Mycotoxins in Rice:Occurrence and Health Risk Assessment in Children and Adults of Punjab, Pakistan. Toxins 2018, 10, 77. [Google Scholar] [CrossRef]
- EFSA. Opinion of the scientific committee on a request from EFSA related to A Harmonised approach for risk assessment of substances which are both genotoxic and carcinogenic. EFSA J. 2005, 3, 282. [Google Scholar] [CrossRef]
- Farzadkia, M.; Djahed, B.; Shahsavani, S.; Dehghanifard, E. Prediction of gas emission and derived electrical power generation from shiraz landfill. Glob. Nest J. 2015, 17, 487–497. [Google Scholar]
- Fallahzadeh, R.A.; Khosravi, R.; Dehdashti, B.; Ghahramani, E.; Omidi, F.; Adli, A.; Miri, M. Spatial distribution variation and probabilistic risk assessment of exposure to chromium in ground water supplies; a case study in the east of Iran. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2018, 115, 260–266. [Google Scholar] [CrossRef]
- Djahed, B.; Taghavi, M.; Farzadkia, M.; Norzaee, S.; Miri, M. Stochastic exposure and health risk assessment of rice contamination to the heavy metals in the market of Iranshahr, Iran. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2018, 115, 405–412. [Google Scholar] [CrossRef]
- Zuo, T.T.; Liu, J.L.; Jin, H.Y.; Chang, Y.; Wei, F.; Wei, S.; Kang, S.; Ma, S.C. A novel bioaccessibility-based probabilistic risks assessment of potentially toxic elements (PTEs) in earthworm. Front. Pharmacol. 2024, 15, 1398394. [Google Scholar] [CrossRef]
- Li, X.; Chi, W.; Tian, H.; Zhang, Y.; Zhu, Z. Probabilistic ecological risk assessment of heavy metals in western Laizhou Bay, Shandong Province, China. PLoS ONE 2019, 14, e0213011. [Google Scholar] [CrossRef] [PubMed]
- Ginsberg, G.L.; Belleggia, G. Use of Monte Carlo analysis in a risk-based prioritization of toxic constituents in house dust. Environ. Int. 2017, 109, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Emenike, P.C.; Tenebe, I.; Ogarekpe, N.; Omole, D.; Nnaji, C. Probabilistic risk assessment and spatial distribution of potentially toxic elements in groundwater sources in Southwestern Nigeria. Sci. Rep. 2019, 9, 15920. [Google Scholar] [CrossRef]
- Ma, S.C.; Jin, H.Y.; Liu, L.N.; Wang, Y.; Sun, L. Risk Control of Exogenous Harmful Residues in Traditional Chinese Medicines. J. Chin. Pharm. Sci. 2015, 50, 99–103. [Google Scholar]
- Ab Dullah, S.S.; Sabran, M.R.; Hasiah, A.H.; Abdullah, R. Risk assessment of aflatoxin B(1) in herbal medicines and plant food supplements marketed in Malaysia using margin of exposure and RISK21 approaches. Genes Environ. Off. J. Jpn. Environ. Mutagen Soc. 2023, 45, 31. [Google Scholar] [CrossRef]
- Fang, L.; Zhao, B.; Zhang, R.; Wu, P.; Zhao, D.; Chen, J.; Pan, X.; Wang, J.; Wu, X.; Zhang, H.; et al. Occurrence and exposure assessment of aflatoxins in Zhejiang province, China. Environ. Toxicol. Pharmacol. 2022, 92, 103847. [Google Scholar] [CrossRef]
- Samimi, P.; Aslani, R.; Molaee-Aghaee, E.; Sadighara, P.; Shariatifar, N.; Jahed Khaniki, G.; Ozcakmak, S.; Reshadat, Z. Determination and risk assessment of aflatoxin B1 in the kernel of imported raw hazelnuts from Eastern Azerbaijan Province of Iran. Sci. Rep. 2024, 14, 6864. [Google Scholar] [CrossRef]
- Mehri, F.; Heidarinejad, Z.; Pilevar, Z.; Fakhri, Y.; Sarafraz, M.; Mousavi Khaneghah, A. Investigating the Concentration of Potentially Toxic Elements (PTEs) in Bird Eggs: A Comprehensive Systematic Review, Meta-Analysis, and Probabilistic Risk Assessment. Biol. Trace Elem. Res. 2025, 1–11, Online ahead of print. [Google Scholar] [CrossRef]
- Fakhri, Y.; Mahmoudizeh, A.; Hemmati, F.; Adiban, M.; Esfandiari, Z.; Mousavi Khaneghah, A. The concentration of malachite green in fish: A systematic review, meta-analysis, and probabilistic risk assessment. Int. J. Environ. Health Res. 2025, 1–16, Online ahead of print. [Google Scholar] [CrossRef]
- Wu, F.; Khlangwiset, P. Health economic impacts and cost-effectiveness of aflatoxin-reduction strategies in Africa: Case studies in biocontrol and post-harvest interventions. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2010, 27, 496–509. [Google Scholar] [CrossRef]
- Zuo, T.T.; Luo, F.Y.; He, H.Z.; Jin, H.Y.; Sun, L.; Xing, S.X.; Li, B.; Gao, F.; Ma, S.C.; He, L.C. Novel bioavailability-based risk assessment of Cd in earthworms and leeches utilizing in vitro digestion/Caco-2 and MDCK cells. Environ. Sci. Pollut. Res. Int. 2022, 29, 26513–26523. [Google Scholar] [CrossRef] [PubMed]
- Benford, D.; Leblanc, J.C.; Setzer, R.W. Application of the margin of exposure (MoE) approach to substances in food that are genotoxic and carcinogenic: Example: Aflatoxin B1 (AFB1). Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2010, 48 (Suppl. S1), S34–S41. [Google Scholar] [CrossRef] [PubMed]
- Nugraha, A.; Khotimah, K. Risk assessment of aflatoxin B1 exposure from maize and peanut consumption in Indonesia using the margin of exposure and liver cancer risk estimation approaches. Food Chem. Toxicol. 2018, 113, 134–144. [Google Scholar] [CrossRef]
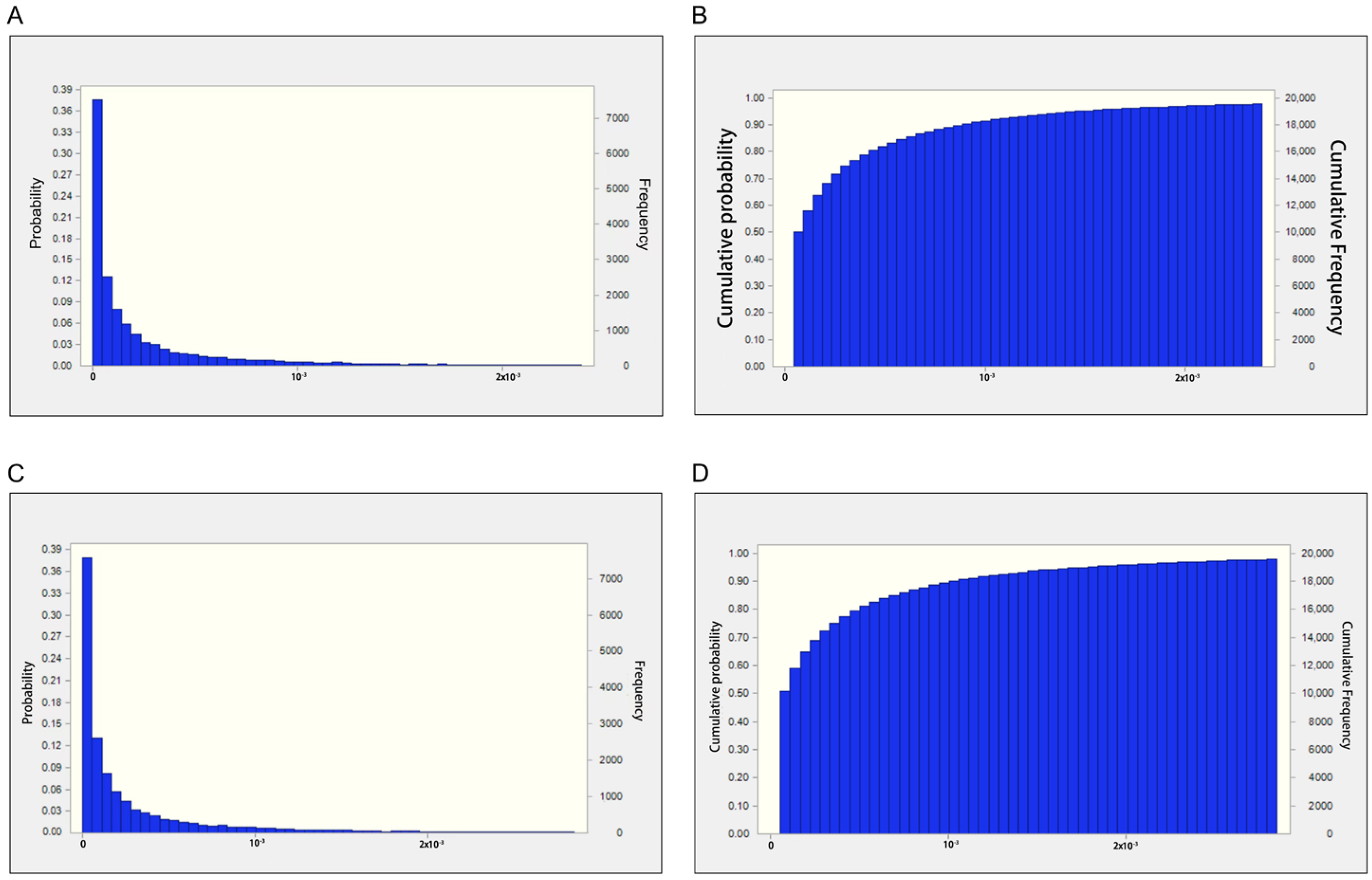
| Min | P25 | Mean | P50 | P75 | Max | |
|---|---|---|---|---|---|---|
| Medical areca nut | Non-detected | 1 | 7.7 | 3 | 18 | 146 |
| Stir-fried medical areca nut | Non-detected | Non-detected | 13.0 | 1.5 | 12.5 | 31 |
| Parameter | EF | IR | Contents of AFB1 |
|---|---|---|---|
| Type of distribution | Triangular distribution | Uniform distribution | Weibull distribution |
| P5 | P10 | P25 | P50 | P75 | P90 | P95 | Max | ||
|---|---|---|---|---|---|---|---|---|---|
| Male | EDI | 1.51 × 10−3 | 4.35 × 10−3 | 2.07 × 10−2 | 9.24 × 10−2 | 0.33 | 0.89 | 1.50 | 13.48 |
| MOE | 166.40 | 280.96 | 756.01 | 2704.11 | 12,077.28 | 57,475.22 | 165,247.18 | 401,863,050.72 | |
| HCC | 4.53 × 10−4 | 0.001 | 0.006 | 0.028 | 0.099 | 0.267 | 0.450 | 4.044 | |
| Female | EDI | 1.78 × 10−3 | 5.13 × 10−3 | 2.44 × 10−2 | 0.11 | 0.39 | 1.05 | 1.77 | 15.90 |
| MOE | 139.96 | 238.25 | 619.16 | 2320.56 | 10,659.60 | 50,906.95 | 148,286.36 | 517,480,061.53 | |
| HCC | 5.35 × 10−4 | 0.002 | 0.007 | 0.033 | 0.117 | 0.315 | 0.531 | 4.770 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuo, T.-T.; Zhang, Y.-Z.; Zhai, H.-Y.; Chen, R.-Y.; Pu, J.-Z.; Jin, H.-Y.; Liu, J.; Cheng, X.-L.; Wei, F. A Comprehensive Probabilistic Risk Assessment Strategy of Aflatoxin B1 Exposure from Medical Areca Nuts Consumption. Toxins 2025, 17, 252. https://doi.org/10.3390/toxins17050252
Zuo T-T, Zhang Y-Z, Zhai H-Y, Chen R-Y, Pu J-Z, Jin H-Y, Liu J, Cheng X-L, Wei F. A Comprehensive Probabilistic Risk Assessment Strategy of Aflatoxin B1 Exposure from Medical Areca Nuts Consumption. Toxins. 2025; 17(5):252. https://doi.org/10.3390/toxins17050252
Chicago/Turabian StyleZuo, Tian-Tian, Ya-Zhong Zhang, Hong-Yan Zhai, Rong-Yao Chen, Jing-Zhe Pu, Hong-Yu Jin, Jing Liu, Xian-Long Cheng, and Feng Wei. 2025. "A Comprehensive Probabilistic Risk Assessment Strategy of Aflatoxin B1 Exposure from Medical Areca Nuts Consumption" Toxins 17, no. 5: 252. https://doi.org/10.3390/toxins17050252
APA StyleZuo, T.-T., Zhang, Y.-Z., Zhai, H.-Y., Chen, R.-Y., Pu, J.-Z., Jin, H.-Y., Liu, J., Cheng, X.-L., & Wei, F. (2025). A Comprehensive Probabilistic Risk Assessment Strategy of Aflatoxin B1 Exposure from Medical Areca Nuts Consumption. Toxins, 17(5), 252. https://doi.org/10.3390/toxins17050252





