Multi-Mycotoxin Contamination in Serbian Maize During 2021–2023: Climatic Influences and Implications for Food and Feed Safety
Abstract
- Climatic factors strongly influence mycotoxin contamination in Serbian maize.
- Maximum aflatoxin B1 concentration (527 µg/kg at 18% moisture) was 105.4 and 26.4 times greater than the maximum EU allowable levels for food and feed.
- Co-contamination of aflatoxins poses food safety and health risks.
- Urges better mitigation and regulations to protect food, feed, and exports.
1. Introduction
2. Results
2.1. Climatic Conditions in Serbia During the Maize Growing and Harvest Seasons of 2021 to 2023
2.2. Occurrence and Concentration of Detected Regulated Mycotoxins
2.3. Influences of Climatic Factors on the Concentration of Mycotoxins
2.4. Implications for Food and Feed Safety
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Sample Collection
5.2. Multi-Mycotoxin Analysis
5.2.1. Sample Preparation
5.2.2. LC-MS/MS Parameters
5.3. Climate Data
5.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Erenstein, O.; Jaleta, M.; Sonder, K.; Mottaleb, K.; Prasanna, B.M. Global maize production, consumption and trade: Trends and R&D implications. Food Secur. 2022, 14, 1295–1319. [Google Scholar]
- Nuss, E.T.; Tanumihardjo, S.A. Maize: A paramount staple crop in the context of global nutrition. Compr. Rev. Food Sci. Food Saf. 2010, 9, 417–436. [Google Scholar] [CrossRef] [PubMed]
- Grčak, M.; Grčak, D.; Penjišević, A.; Simjanović, D.; Orbović, B.; Đukić, N.; Rajičić, V. The trends in maize and wheat production in the Republic of Serbia. Acta Agric. Serbica 2020, 25, 121–127. [Google Scholar] [CrossRef]
- Radić, B.; Kos, J.; Janić Hajnal, E.; Malachová, A.; Krska, R.; Sulyok, M. Fusarium metabolites in maize from regions of Northern Serbia in 2016–2017. Food Addit. Contam. Part B 2021, 14, 295–305. [Google Scholar] [CrossRef]
- Kos, J.; Hajnal, E.J.; Malachová, A.; Steiner, D.; Stranska, M.; Krska, R.; Poschmaier, B.; Sulyok, M. Mycotoxins in maize harvested in the Republic of Serbia in the period 2012–2015. Part 1: Regulated mycotoxins and its derivatives. Food Chem. 2020, 312, 126034. [Google Scholar] [CrossRef] [PubMed]
- Observatory of Economic Complexity. Corn, Serbia. Available online: https://oec.world/en/profile/bilateral-product/corn/reporter/srb (accessed on 19 January 2025).
- Maslac, T.G.; U.S. Department of Agriculture (USDA); Foreign Agricultural Service (FAS). Serbia Grain and Feed Annual; Washington, DC, USA, 2024; pp. 1–17. Available online: https://fas.usda.gov/data/serbia-grain-and-feed-annual-9 (accessed on 19 January 2025).
- Kos, J.; Radić, B.; Radović, R.; Šarić, B.; Jovanov, P.; Šarić, L. Aflatoxins in maize, milk and dairy products from Serbia. Food Addit. Contam. Part B 2024, 17, 296–307. [Google Scholar] [CrossRef]
- Awuchi, C.G.; Ondari, E.N.; Nwozo, S.; Odongo, G.A.; Eseoghene, I.J.; Twinomuhwezi, H.; Ogbonna, C.U.; Upadhyay, A.K.; Adeleye, A.O.; Okpala, C.O.R. Mycotoxins’ toxicological mechanisms involving humans, livestock and their associated health concerns: A review. Toxins 2022, 14, 167. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Song, G.; Lim, W. Effects of mycotoxin-contaminated feed on farm animals. J. Hazard. Mater. 2020, 389, 122087. [Google Scholar] [CrossRef]
- Creppy, E.E. Update of survey, regulation and toxic effects of mycotoxins in Europe. Toxicol. Lett. 2002, 127, 19–28. [Google Scholar] [CrossRef]
- Palumbo, R.; Goncalves, A.; Gkrillas, A.; Logrieco, A.; Dorne, J.-L.; Dall’Asta, C.; Venancio, A.; Battilani, P. Mycotoxins in maize. Phytopathol. Mediterr. 2020, 59, 5–28. [Google Scholar] [CrossRef]
- Raj, J.; Farkaš, H.; Jakovčević, Z.; Medina, A.; Magan, N.; Čepela, R.; Vasiljević, M. Comparison of multiple mycotoxins in harvested maize samples in three years (2018–2020) in four continents. Food Addit. Contam. Part A 2022, 39, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.-C.; Madec, S.; Coton, E.; Hymery, N. Natural co-occurrence of mycotoxins in foods and feeds and their in vitro combined toxicological effects. Toxins 2016, 8, 94. [Google Scholar] [CrossRef] [PubMed]
- Penagos-Tabares, F.; Khiaosa-Ard, R.; Schmidt, M.; Pacífico, C.; Faas, J.; Jenkins, T.; Nagl, V.; Sulyok, M.; Labuda, R.; Zebeli, Q. Fungal species and mycotoxins in mouldy spots of grass and maize silages in Austria. Mycotoxin Res. 2022, 38, 1–20. [Google Scholar] [CrossRef]
- Santos Pereira, C.; Cunha, S.C.; Fernandes, J.O. Prevalent mycotoxins in animal feed: Occurrence and analytical methods. Toxins 2019, 11, 290. [Google Scholar] [CrossRef] [PubMed]
- Gruber-Dorninger, C.; Jenkins, T.; Schatzmayr, G. Global mycotoxin occurrence in feed: A ten-year survey. Toxins 2019, 11, 375. [Google Scholar] [CrossRef]
- Singh, K.; Kumari, A. Mycotoxins co-occurrence poisoning. In Mycotoxins and Mycotoxicoses; Springer: Berlin/Heidelberg, Germany, 2022; pp. 129–136. [Google Scholar]
- Grenier, B.; Oswald, I. Mycotoxin co-contamination of food and feed: Meta-analysis of publications describing toxicological interactions. World Mycotoxin J. 2011, 4, 285–313. [Google Scholar] [CrossRef]
- Alassane-Kpembi, I.; Schatzmayr, G.; Taranu, I.; Marin, D.; Puel, O.; Oswald, I.P. Mycotoxins co-contamination: Methodological aspects and biological relevance of combined toxicity studies. Crit. Rev. Food Sci. Nutr. 2017, 57, 3489–3507. [Google Scholar] [CrossRef]
- Magnoli, A.P.; Poloni, V.L.; Cavaglieri, L. Impact of mycotoxin contamination in the animal feed industry. Curr. Opin. Food Sci. 2019, 29, 99–108. [Google Scholar] [CrossRef]
- Kos, J.; Anć, M.; Radić, B.; Zadravec, M.; Janić Hajnal, E.; Pleadin, J. Climate change—A global threat resulting in increasing mycotoxin occurrence. Foods 2023, 12, 2704. [Google Scholar] [CrossRef]
- Gomez, K.S.; Castañeda Roldán, E.; Ávila Sosa, R.; Munguía-Pérez, R. Mycotoxins and climate change. In The Impact of Climate Change on Fungal Diseases; Springer: Berlin/Heidelberg, Germany, 2022; pp. 239–256. [Google Scholar]
- Duchenne-Moutien, R.A.; Neetoo, H. Climate change and emerging food safety issues: A review. J. Food Prot. 2021, 84, 1884–1897. [Google Scholar] [CrossRef]
- Zingales, V.; Taroncher, M.; Martino, P.A.; Ruiz, M.-J.; Caloni, F. Climate change and effects on molds and mycotoxins. Toxins 2022, 14, 445. [Google Scholar] [CrossRef] [PubMed]
- Pleadin, J.; Kos, J.; Radić, B.; Vulić, A.; Kudumija, N.; Radović, R.; Janić Hajnal, E.; Mandić, A.; Anć, M. Aflatoxins in Maize from Serbia and Croatia: Implications of climate change. Foods 2023, 12, 548. [Google Scholar] [CrossRef] [PubMed]
- Kos, J.; Mastilović, J.; Hajnal, E.J.; Šarić, B. Natural occurrence of aflatoxins in maize harvested in Serbia during 2009–2012. Food Control 2013, 34, 31–34. [Google Scholar] [CrossRef]
- Kos, J.; Hajnal, E.J.; Šarić, B.; Jovanov, P.; Nedeljković, N.; Milovanović, I.; Krulj, J. The influence of climate conditions on the occurrence of deoxynivalenol in maize harvested in Serbia during 2013–2015. Food Control 2017, 73, 734–740. [Google Scholar] [CrossRef]
- Kos, J.; Radić, B.; Lešić, T.; Anć, M.; Jovanov, P.; Šarić, B.; Pleadin, J. Climate Change and Mycotoxins Trends in Serbia and Croatia: A 15-Year Review. Foods 2024, 13, 1391. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EU) 2023/915 of 25 April 2023 on maximum levels for certain contaminants in food and repealing Regulation (EC) No 1881/2006. Off. J. Eur. Union 2023, 119, 103–157. [Google Scholar]
- European Commission. Commission Regulation (EU) 2024/1038 of 9 April 2024 amending Regulation (EU) 2023/915 as regards maximum levels of T-2 and HT-2 toxins in food. Off. J. Eur. Union 2024, 1038, 1–5. [Google Scholar]
- European Commission. Directive 2002/32/EC of the European Parliament and of the Council of 7 May 2002 on undesirable substances in animal feed. Off. J. Eur. Communities 2002. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A02002L0032-20191128 (accessed on 15 March 2025).
- Serbian Regulation. Maximum allowed contents of contaminants in food and feed. Off. Bull. Repub. Serb. 2019, 81, 28. [Google Scholar]
- European Commission. Commission Recommendation of 17 August 2006 on the presence of deoxynivalenol, zearalenone, ochratoxin A, T-2 and HT-2 and fumonisins in products intended for animal feeding (2006/576/EC). Off. J. Eur. Union 2006, 229, 7–9. [Google Scholar]
- European Commission. Commission Recommendation of 27 March 2013 on the presence of T-2 and HT-2 toxin in cereals and cereal products (2013/165/EU). Off J Eur Union L 2013, 91, 12–15. [Google Scholar]
- European Commission. Commission Regulation (EU) 2024/1022 of 8 April 2024 Amending Regulation (EU) 2023/915 as Regards Maximum Levels of Deoxynivalenol in Food. Available online: https://eur-lex.europa.eu/eli/reg/2024/1022/oj (accessed on 15 March 2025).
- Commission Regulation (EU) 2024/1756 of 25 June 2024 Amending and Correcting Regulation (EU) 2023/915 on Maximum Levels for Certain Contaminants in Food. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=OJ:L_202401756 (accessed on 15 March 2025).
- Ostry, V.; Malir, F.; Toman, J.; Grosse, Y. Mycotoxins as human carcinogens—The IARC Monographs classification. Mycotoxin Res. 2017, 33, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chang, C.-C.H.; Marsh, G.M.; Wu, F. Population attributable risk of aflatoxin-related liver cancer: Systematic review and meta-analysis. Eur. J. Cancer 2012, 48, 2125–2136. [Google Scholar] [CrossRef]
- Watson, S.; Gong, Y.Y.; Routledge, M. Interventions targeting child undernutrition in developing countries may be undermined by dietary exposure to aflatoxin. Crit. Rev. Food Sci. Nutr. 2017, 57, 1963–1975. [Google Scholar] [CrossRef]
- Lizárraga-Paulín, E.G.; Moreno-Martínez, E.; Miranda-Castro, S.P. Aflatoxins and their impact on human and animal health: An emerging problem. Aflatoxins-Biochem. Mol. Biol. 2011, 13, 255–262. [Google Scholar]
- Nejad, B.G.; Mostafaei, Z.; Rezaabad, A.B.; Mehravar, F.; Zarei, M.; Dehghani, A.; Estabragh, M.A.R.; Karami-Mohajeri, S.; Alizadeh, H. A systematic review with meta-analysis of the relation of aflatoxin B1 to growth impairment in infants/children. BMC Pediatr. 2023, 23, 614. [Google Scholar] [CrossRef]
- Williams, J.H.; Phillips, T.D.; Jolly, P.E.; Stiles, J.K.; Jolly, C.M.; Aggarwal, D. Human aflatoxicosis in developing countries: A review of toxicology, exposure, potential health consequences, and interventions. Am. J. Clin. Nutr. 2004, 80, 1106–1122. [Google Scholar] [CrossRef]
- Dhanasekaran, D.; Shanmugapriya, S.; Thajuddin, N.; Panneerselvam, A. Aflatoxins and aflatoxicosis in human and animals. Aflatoxins-Biochem. Mol. Biol. 2011, 10, 221–254. [Google Scholar]
- Zentai, A.; Jóźwiak, Á.; Süth, M.; Farkas, Z. Carry-over of aflatoxin B1 from feed to cow milk—A review. Toxins 2023, 15, 195. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Ogunade, I.M.; Vyas, D.; Adesogan, A.T. Aflatoxin in dairy cows: Toxicity, occurrence in feedstuffs and milk and dietary mitigation strategies. Toxins 2021, 13, 283. [Google Scholar] [CrossRef]
- Jauković, M.M.; Rokvić, N.I.; Vuksan, A.D. Recent aflatoxin levels in maize, feed mixtures, milk and cheese in Serbia. Srpska J. Nat. Sci. 2024, 146, 81–89. [Google Scholar] [CrossRef]
- Hoffman, J.M.; Valencak, T.G. A short life on the farm: Aging and longevity in agricultural, large-bodied mammals. Geroscience 2020, 42, 909–922. [Google Scholar] [CrossRef]
- Shruthi, P.; Sujatha, K.; Srilatha, C.; Rayulu, V. Incidence of different tumours in bovines. Open Access J. Sci. 2018, 2, 220–227. [Google Scholar]
- Penagos-Tabares, F.; Mahmood, M.; Sulyok, M.; Rafique, K.; Khan, M.R.; Zebeli, Q.; Krska, R.; Metzler-Zebeli, B. Outbreak of aflatoxicosis in a dairy herd induced depletion in milk yield and high rates of abortion in Pakistan. Toxicon 2024, 246, 107799. [Google Scholar] [CrossRef]
- Marasas, W.F.; Riley, R.T.; Hendricks, K.A.; Stevens, V.L.; Sadler, T.W.; Gelineau-van Waes, J.; Missmer, S.A.; Cabrera, J.; Torres, O.; Gelderblom, W.C. Fumonisins disrupt sphingolipid metabolism, folate transport, and neural tube development in embryo culture and in vivo: A potential risk factor for human neural tube defects among populations consuming fumonisin-contaminated maize. J. Nutr. 2004, 134, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Lumsangkul, C.; Tso, K.-H.; Fan, Y.-K.; Chiang, H.-I.; Ju, J.-C. Mycotoxin fumonisin B1 interferes sphingolipid metabolisms and neural tube closure during early embryogenesis in brown tsaiya ducks. Toxins 2021, 13, 743. [Google Scholar] [CrossRef]
- Wangia-Dixon, R.N.; Nishimwe, K. Molecular toxicology and carcinogenesis of fumonisins: A review. J. Environ. Sci. Health Part C 2020, 39, 44–67. [Google Scholar] [CrossRef] [PubMed]
- Giannitti, F.; Diab, S.S.; Pacin, A.M.; Barrandeguy, M.; Larrere, C.; Ortega, J.; Uzal, F.A. Equine leukoencephalomalacia (ELEM) due to fumonisins B1 and B2 in Argentina. Pesqui. Veterinária Bras. 2011, 31, 407–412. [Google Scholar] [CrossRef]
- Ross, P.; Ledet, A.; Owens, D.; Rice, L.; Nelson, H.; Osweiler, G.; Wilson, T. Experimental equine leukoencephalomalacia, toxic hepatosis, and encephalopathy caused by corn naturally contaminated with fumonisins. J. Vet. Diagn. Investig. 1993, 5, 69–73. [Google Scholar] [CrossRef]
- Haschek, W.M.; Gumprecht, L.A.; Smith, G.; Tumbleson, M.E.; Constable, P.D. Fumonisin toxicosis in swine: An overview of porcine pulmonary edema and current perspectives. Environ. Health Perspect. 2001, 109, 251–257. [Google Scholar]
- Tardieu, D.; Travel, A.; Le Bourhis, C.; Metayer, J.-P.; Mika, A.; Cleva, D.; Boissieu, C.; Guerre, P. Fumonisins and zearalenone fed at low levels can persist several days in the liver of turkeys and broiler chickens after exposure to the contaminated diet was stopped. Food Chem. Toxicol. 2021, 148, 111968. [Google Scholar] [CrossRef]
- Chain, E.P.o.C.i.t.F.; Schrenk, D.; Bignami, M.; Bodin, L.; Chipman, J.K.; del Mazo, J.; Grasl-Kraupp, B.; Hogstrand, C.; Leblanc, J.C.; Nielsen, E. Assessment of information as regards the toxicity of fumonisins for pigs, poultry and horses. EFSA J. 2022, 20, e07534. [Google Scholar]
- Haliburton, J.C.; Buck, W.B. Equine leucoencephalomalacia: An historical review. In Diagnosis of Mycotoxicoses; Springer: Berlin/Heidelberg, Germany, 1986; pp. 75–79. [Google Scholar]
- Mallmann, C.A.; Santurio, J.M.; Dilkin, P. Equine leukoencephalomalacia associated with ingestion of corn contaminated with fumonisin B1. Rev. De Microbiol. 1999, 30, 249–252. [Google Scholar] [CrossRef]
- Wang, S.; Wu, K.; Xue, D.; Zhang, C.; Rajput, S.A.; Qi, D. Mechanism of deoxynivalenol mediated gastrointestinal toxicity: Insights from mitochondrial dysfunction. Food Chem. Toxicol. 2021, 153, 112214. [Google Scholar] [CrossRef]
- Robbana-Barnat, S.; Lafarge-Frayssinet, C.; Cohen, H.; Neish, G.; Frayssinet, C. Immunosuppressive properties of deoxynivalenol. Toxicology 1988, 48, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, K.; Habrowska-Górczyńska, D.E.; Piastowska-Ciesielska, A.W. Zearalenone as an endocrine disruptor in humans. Environ. Toxicol. Pharmacol. 2016, 48, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, T.; Wache, Y.; Laffitte, J.; Taranu, I.; Saeedikouzehkonani, N.; Mori, Y.; Oswald, I.P. Deoxynivalenol impairs the immune functions of neutrophils. Mol. Nutr. Food Res. 2013, 57, 1026–1036. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, A.; Braber, S.; Akbari, P.; Garssen, J.; Fink-Gremmels, J. Deoxynivalenol impairs weight gain and affects markers of gut health after low-dose, short-term exposure of growing pigs. Toxins 2015, 7, 2071–2095. [Google Scholar] [CrossRef]
- Metzler, M.; Pfeiffer, E.; Hildebrand, A. Zearalenone and its metabolites as endocrine disrupting chemicals. World Mycotoxin J. 2010, 3, 385–401. [Google Scholar] [CrossRef]
- Minervini, F.; Dell’Aquila, M.E. Zearalenone and reproductive function in farm animals. Int. J. Mol. Sci. 2008, 9, 2570–2585. [Google Scholar] [CrossRef]
- Wild, C.P.; Stewart, B.W.; Wild, C. World Cancer Report 2014; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Kőszegi, T.; Poór, M. Ochratoxin A: Molecular interactions, mechanisms of toxicity and prevention at the molecular level. Toxins 2016, 8, 111. [Google Scholar] [CrossRef]
- Duarte, S.C.; Lino, C.M.; Pena, A. Ochratoxin A in feed of food-producing animals: An undesirable mycotoxin with health and performance effects. Vet. Microbiol. 2011, 154, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Polak-Śliwińska, M.; Paszczyk, B. Trichothecenes in food and feed, relevance to human and animal health and methods of detection: A systematic review. Molecules 2021, 26, 454. [Google Scholar] [CrossRef] [PubMed]
- Hayes, M.; Wobeser, G. Subacute toxic effects of dietary T-2 toxin in young mallard ducks. Can. J. Comp. Med. 1983, 47, 180–187. [Google Scholar] [PubMed]
- Canady, R.A.; Coker, R.D.; Egan, S.K.; Krska, R.; Olsen, M.; Resnik, S.; Schlatter, J. T-2 and HT-2 toxins. Safety evaluation of certain mycotoxins in food. WHO Food Addit. Ser. 2001, 47, 557–597. [Google Scholar]
- Battilani, P.; Palumbo, R.; Giorni, P.; Dall’Asta, C.; Dellafiora, L.; Gkrillas, A.; Toscano, P.; Crisci, A.; Brera, C.; De Santis, B. Mycotoxin mixtures in food and feed: Holistic, innovative, flexible risk assessment modelling approach: MYCHIF. EFSA Support. Publ. 2020, 17, 1757. [Google Scholar] [CrossRef]
- Speijers, G.J.A.; Speijers, M.H.M. Combined toxic effects of mycotoxins. Toxicol. Lett. 2004, 153, 91–98. [Google Scholar] [CrossRef]
- van den Brand, A.D.; Bokkers, B.G.; Te Biesebeek, J.D.; Mengelers, M.J. Combined exposure to multiple mycotoxins: An example of using a tiered approach in a mixture risk assessment. Toxins 2022, 14, 303. [Google Scholar] [CrossRef]
- Karsauliya, K.; Yahavi, C.; Pandey, A.; Bhateria, M.; Sonker, A.K.; Pandey, H.; Sharma, M.; Singh, S.P. Co-occurrence of mycotoxins: A review on bioanalytical methods for simultaneous analysis in human biological samples, mixture toxicity and risk assessment strategies. Toxicon 2022, 218, 25–39. [Google Scholar] [CrossRef]
- Hove, M.; Van Poucke, C.; Njumbe-Ediage, E.; Nyanga, L.; De Saeger, S. Review on the natural co-occurrence of AFB1 and FB1 in maize and the combined toxicity of AFB1 and FB1. Food Control 2016, 59, 675–682. [Google Scholar] [CrossRef]
- Kolawole, O.; Graham, A.; Donaldson, C.; Owens, B.; Abia, W.A.; Meneely, J.; Alcorn, M.J.; Connolly, L.; Elliott, C.T. Low doses of mycotoxin mixtures below EU regulatory limits can negatively affect the performance of broiler chickens: A longitudinal study. Toxins 2020, 12, 433. [Google Scholar] [CrossRef]
- Di Paola, D.; Iaria, C.; Capparucci, F.; Arangia, A.; Crupi, R.; Cuzzocrea, S.; Spanò, N.; Gugliandolo, E.; Peritore, A.F. Impact of mycotoxin contaminations on aquatic organisms: Toxic effect of aflatoxin B1 and fumonisin B1 mixture. Toxins 2022, 14, 518. [Google Scholar] [CrossRef] [PubMed]
- Jajić, I.; Dudaš, T.; Krstović, S.; Krska, R.; Sulyok, M.; Bagi, F.; Savić, Z.; Guljaš, D.; Stankov, A. Emerging Fusarium mycotoxins fusaproliferin, beauvericin, enniatins, and moniliformin in Serbian maize. Toxins 2019, 11, 357. [Google Scholar] [CrossRef] [PubMed]
- Hajnal, E.J.; Kos, J.; Malachová, A.; Steiner, D.; Stranska, M.; Krska, R.; Sulyok, M. Mycotoxins in maize harvested in Serbia in the period 2012–2015. Part 2: Non-regulated mycotoxins and other fungal metabolites. Food Chem. 2020, 317, 126409. [Google Scholar] [CrossRef]
- Kos, J.; Janić-Hajnal, E.; Malachová, A.; Steiner, D.; Stranska, M.; Krska, R.; Sulyok, M. Maize: Is it a potential source of mycotoxins mixture? In Proceedings of the IV International Congress Food Technology, Quality and Safety, Novi Sad, Serbia, 28–30 October 2018. [Google Scholar]
- Focker, M.; van Eupen, M.; Verweij, P.; Liu, C.; van Haren, C.; Van der Fels-Klerx, H. Effects of climate change on areas suitable for maize cultivation and aflatoxin contamination in Europe. Toxins 2023, 15, 599. [Google Scholar] [CrossRef]
- Casu, A.; Camardo Leggieri, M.; Toscano, P.; Battilani, P. Changing climate, shifting mycotoxins: A comprehensive review of climate change impact on mycotoxin contamination. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13323. [Google Scholar] [CrossRef]
- Polano, C.; Sanseverino, I.; Ermolli, M.; Porcel, R.E.; Bisselink, B.; Pozzoli, L.; Lettieri, T. Mycotoxin Fungal Plant Diseases and Climate Change: State of the Art and Beyond; Publications Office of the European Union: Luxembourg, 2024. [Google Scholar]
- Medina, A.; Akbar, A.; Baazeem, A.; Rodriguez, A.; Magan, N. Climate change, food security and mycotoxins: Do we know enough? Fungal Biol. Rev. 2017, 31, 143–154. [Google Scholar] [CrossRef]
- Battilani, P.; Toscano, P.; Van der Fels-Klerx, H.; Moretti, A.; Camardo Leggieri, M.; Brera, C.; Rortais, A.; Goumperis, T.; Robinson, T. Aflatoxin B1 contamination in maize in Europe increases due to climate change. Sci. Rep. 2016, 6, 24328. [Google Scholar] [CrossRef]
- Fu, Z.; Huang, X.; Min, S. Rapid determination of aflatoxins in corn and peanuts. J. Chromatogr. A 2008, 1209, 271–274. [Google Scholar] [CrossRef]
- Gomes, A.; Sousa, R.; Das Neves, L.; Da Gloria, E.; Burbarelli, M.; Petrus, R.; Fernandes, A. Occurrence and Co-exposure of Aflatoxins and Fumonisins in Conventional and Organic Corn. Food Control 2024, 165, 110628. [Google Scholar] [CrossRef]
- Molnár, K.; Rácz, C.; Dövényi-Nagy, T.; Bakó, K.; Pusztahelyi, T.; Kovács, S.; Adácsi, C.; Pócsi, I.; Dobos, A. The effect of environmental factors on mould counts and AFB1 toxin production by Aspergillus flavus in maize. Toxins 2023, 15, 227. [Google Scholar] [CrossRef]
- Asghar, M.A.; Ahmed, A.; Asghar, M.A. Influence of temperature and environmental conditions on aflatoxin contamination in maize collected from different regions of Pakistan during 2016–2019. J. Stored Prod. Res. 2020, 88, 101637. [Google Scholar] [CrossRef]
- Abel Palacios, H.; Stefanello, A.; García Gavilánez, M.S.; Castro Demera, D.A.; García, M.V.; Vásquez Castillo, W.A.; Almeida Marcano, M.A.; Samaniego Maigua, I.R.; Copetti, M.V. Relationship between the fungal incidence, water activity, humidity, and aflatoxin content in maize samples from the highlands and coast of Ecuador. Toxins 2022, 14, 196. [Google Scholar] [CrossRef]
- Magan, N.; Medina, A.; Aldred, D. Possible climate-change effects on mycotoxin contamination of food crops pre- and postharvest. Plant Pathol. 2011, 60, 150–163. [Google Scholar] [CrossRef]
- Paterson, R.R.M.; Lima, N. How will climate change affect mycotoxins in food? Food Res. Int. 2010, 43, 1902–1914. [Google Scholar] [CrossRef]
- Cao, A.; Santiago, R.; Ramos, A.J.; Souto, X.C.; Aguín, O.; Malvar, R.A.; Butrón, A. Critical environmental and genotypic factors for Fusarium verticillioides infection, fungal growth and fumonisin contamination in maize grown in northwestern Spain. Int. J. Food Microbiol. 2014, 177, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Cao, A.; Santiago, R.; Ramos, A.J.; Marín, S.; Reid, L.M.; Butrón, A. Environmental factors related to fungal infection and fumonisin accumulation during the development and drying of white maize kernels. Int. J. Food Microbiol. 2013, 164, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Gbashi, S.; Adelusi, O.A.; Njobeh, P.B. Insights from modelling sixteen years of climatic and fumonisin patterns in maize in South Africa. Sci. Rep. 2024, 14, 11643. [Google Scholar] [CrossRef]
- Wu, L.; Qiu, L.; Zhang, H.; Sun, J.; Hu, X.; Wang, B. Optimization for the production of deoxynivalenol and zearalenone by Fusarium graminearum using response surface methodology. Toxins 2017, 9, 57. [Google Scholar] [CrossRef]
- Sanchis, V.; Magan, N. Environmental conditions affecting mycotoxins. In Mycotoxins in Food: Detection and Control; CRC Press: Boca Raton, FL, USA, 2004; pp. 174–198. [Google Scholar]
- Marin, S.; Magan, N.; Serra, J.; Ramos, A.; Canela, R.; Sanchis, V. Fumonisin B1 production and growth of Fusarium moniliforme and Fusarium proliferatum on maize, wheat, and barley grain. J. Food Sci. 1999, 64, 921–924. [Google Scholar] [CrossRef]
- Marín, S.; Magan, N.; Bellí, N.; Ramos, A.; Canela, R.; Sanchis, V. Two-dimensional profiles of fumonisin B1 production by Fusarium moniliforme and Fusarium proliferatum in relation to environmental factors and potential for modelling toxin formation in maize grain. Int. J. Food Microbiol. 1999, 51, 159–167. [Google Scholar] [CrossRef]
- Van der Fels-Klerx, H.; van Asselt, E.D.; Madsen, M.S.; Olesen, J.E. Impact of climate change effects on contamination of cereal grains with deoxynivalenol. PLoS ONE 2013, 8, e73602. [Google Scholar] [CrossRef] [PubMed]
- Munkvold, G.P. Cultural and genetic approaches to managing mycotoxins in maize. Annu. Rev. Phytopathol. 2003, 41, 99–126. [Google Scholar] [CrossRef]
- Chain, E.P.o.C.i.t.F. Scientific Opinion on the risks for animal and public health related to the presence of T-2 and HT-2 toxin in food and feed. EFSA J. 2011, 9, 2481. [Google Scholar]
- Wild, C.P.; Gong, Y.Y. Mycotoxins and human disease: A largely ignored global health issue. Carcinogenesis 2010, 31, 71–82. [Google Scholar] [CrossRef]
- Antonissen, G.; Martel, A.; Pasmans, F.; Ducatelle, R.; Verbrugghe, E.; Vandenbroucke, V.; Li, S.; Haesebrouck, F.; Van Immerseel, F.; Croubels, S. The impact of Fusarium mycotoxins on human and animal host susceptibility to infectious diseases. Toxins 2014, 6, 430–452. [Google Scholar] [CrossRef] [PubMed]
- Milićević, D.; Udovički, B.; Petrović, Z.; Janković, S.; Radulović, S.; Gurinović, M.; Rajković, A. Current status of mycotoxin contamination of food and feeds and associated public health risk in Serbia. Meat Technol. 2020, 61, 1–14. [Google Scholar] [CrossRef]
- Fraeyman, S.; Croubels, S.; Devreese, M.; Antonissen, G. Emerging Fusarium and Alternaria mycotoxins: Occurrence, toxicity and toxicokinetics. Toxins 2017, 9, 228. [Google Scholar] [CrossRef]
- Penagos-Tabares, F.; Sulyok, M.; Artavia, J.-I.; Flores-Quiroz, S.-I.; Garzón-Pérez, C.; Castillo-Lopez, E.; Zavala, L.; Orozco, J.-D.; Faas, J.; Krska, R. Mixtures of mycotoxins, phytoestrogens, and other secondary metabolites in whole-plant corn silages and total mixed rations of dairy farms in Central and Northern Mexico. Toxins 2023, 15, 153. [Google Scholar] [CrossRef] [PubMed]
- Groten, J.P.; Feron, V.J.; Sühnel, J. Toxicology of simple and complex mixtures. Trends Pharmacol. Sci. 2001, 22, 316–322. [Google Scholar] [CrossRef]
- Mattsson, J.L. Mixtures in the real world: The importance of plant self-defense toxicants, mycotoxins, and the human diet. Toxicol. Appl. Pharmacol. 2007, 223, 125–132. [Google Scholar] [CrossRef]
- Warne, M.S.J.; Hawker, D.W. The number of components in a mixture determines whether synergistic and antagonistic or additive toxicity predominate: The funnel hypothesis. Ecotoxicol. Environ. Saf. 1995, 31, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Steiner, D.; Malachová, A.; Sulyok, M.; Krska, R. Challenges and future directions in LC-MS-based multiclass method development for the quantification of food contaminants. Anal. Bioanal. Chem. 2021, 413, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Sulyok, M.; Stadler, D.; Steiner, D.; Krska, R. Validation of an LC-MS/MS-based dilute-and-shoot approach for the quantification of >500 mycotoxins and other secondary metabolites in food crops: Challenges and solutions. Anal. Bioanal. Chem. 2020, 412, 2607–2622. [Google Scholar] [CrossRef] [PubMed]
- Steiner, D.; Sulyok, M.; Malachová, A.; Mueller, A.; Krska, R. Realizing the simultaneous liquid chromatography-tandem mass spectrometry-based quantification of >1200 biotoxins, pesticides and veterinary drugs in complex feed. J. Chromatogr. A 2020, 1629, 461502. [Google Scholar] [CrossRef]
- Magan, N.; Aldred, D. Post-harvest control strategies: Minimizing mycotoxins in the food chain. Int. J. Food Microbiol. 2007, 119, 131–139. [Google Scholar] [CrossRef]
- Choudhary, A.K.; Kumari, P. Management of mycotoxin contamination in preharvest and postharvest crops: Present status and future prospects. J. Phytol. 2010, 2, 37–47. [Google Scholar]
- Quesada-Vázquez, S.; Codina Moreno, R.; Della Badia, A.; Castro, O.; Riahi, I. Promising phytogenic feed additives used as anti-mycotoxin solutions in animal nutrition. Toxins 2024, 16, 434. [Google Scholar] [CrossRef]
- Vieira, D.J.; Fonseca, L.M.; Poletti, G.; Martins, N.P.; Grigoletto, N.T.; Chesini, R.G.; Tonin, F.G.; Cortinhas, C.S.; Acedo, T.S.; Artavia, I. Anti-mycotoxin feed additives: Effects on metabolism, mycotoxin excretion, performance, and total tract digestibility of dairy cows fed artificially multi-mycotoxin-contaminated diets. J. Dairy Sci. 2024, 107, 1123–1134. [Google Scholar] [CrossRef]
- Catellani, A.; Ghilardelli, F.; Trevisi, E.; Cecchinato, A.; Bisutti, V.; Fumagalli, F.; Swamy, H.; Han, Y.; van Kuijk, S.; Gallo, A. Effects of supplementation of a mycotoxin mitigation feed additive in lactating dairy cows fed Fusarium mycotoxin-contaminated diet for an extended period. Toxins 2023, 15, 546. [Google Scholar] [CrossRef]
- Di Gregorio, M.C.; Neeff, D.V.d.; Jager, A.V.; Corassin, C.H.; Carão, Á.C.d.P.; Albuquerque, R.d.; Azevedo, A.C.d.; Oliveira, C.A.F. Mineral adsorbents for prevention of mycotoxins in animal feeds. Toxin Rev. 2014, 33, 125–135. [Google Scholar] [CrossRef]
- Adamović, M.; Stojanović, M.; Grubišić, M.; Ileš, D.; Milojković, J. Importance of aluminosilicate minerals in safe food production. Appl. Clay Sci. 2011, 53, 145–154. [Google Scholar] [CrossRef]
- Hojnik, N.; Cvelbar, U.; Tavčar-Kalcher, G.; Walsh, J.L.; Križaj, I. Mycotoxin decontamination of food: Cold atmospheric pressure plasma versus “classic” decontamination. Toxins 2017, 9, 151. [Google Scholar] [CrossRef]
- Čolović, R.; Puvača, N.; Cheli, F.; Avantaggiato, G.; Greco, D.; Đuragić, O.; Kos, J.; Pinotti, L. Decontamination of mycotoxin-contaminated feedstuffs and compound feed. Toxins 2019, 11, 617. [Google Scholar] [CrossRef] [PubMed]
- Pleadin, J.; Frece, J.; Markov, K. Mycotoxins in food and feed. Adv. Food Nutr. Res. 2019, 89, 297–345. [Google Scholar] [PubMed]
- Patriarca, A.; Pinto, V.F. Prevalence of mycotoxins in foods and decontamination. Curr. Opin. Food Sci. 2017, 14, 50–60. [Google Scholar] [CrossRef]
- Farkas, H.; Raj, J.; Cepela, R.; Vukovic, G.; Vasiljevic, M. Development of a multi-mycotoxins analysis method for animal feed samples using Agilent 6460 LC-MS/MS. In De Saeger, S.; Logrieco, A. Report from the 1st MYCOKEY International Conference Global Mycotoxin Reduction in the Food and Feed Chain Held in Ghent, Belgium, 11–14 September 2017. Toxins 2017, 9, 276. [CrossRef]
- European Commission. Guidance Document on Identification of Mycotoxins in Food and Feed, SANTE/12089/2016; European Commission: Brussels, Belgium, 2016. [Google Scholar]
- European Commission. Guidance Document on Analytical Quality Control and Method Validation for Pesticide Residues Analysis in Food and Feed, SANTE/12682/2019; European Commission: Brussels, Belgium, 2019. [Google Scholar]
- Schober, P.; Boer, C.; Schwarte, L.A. Correlation coefficients: Appropriate use and interpretation. Anesth. Analg. 2018, 126, 1763–1770. [Google Scholar] [CrossRef]



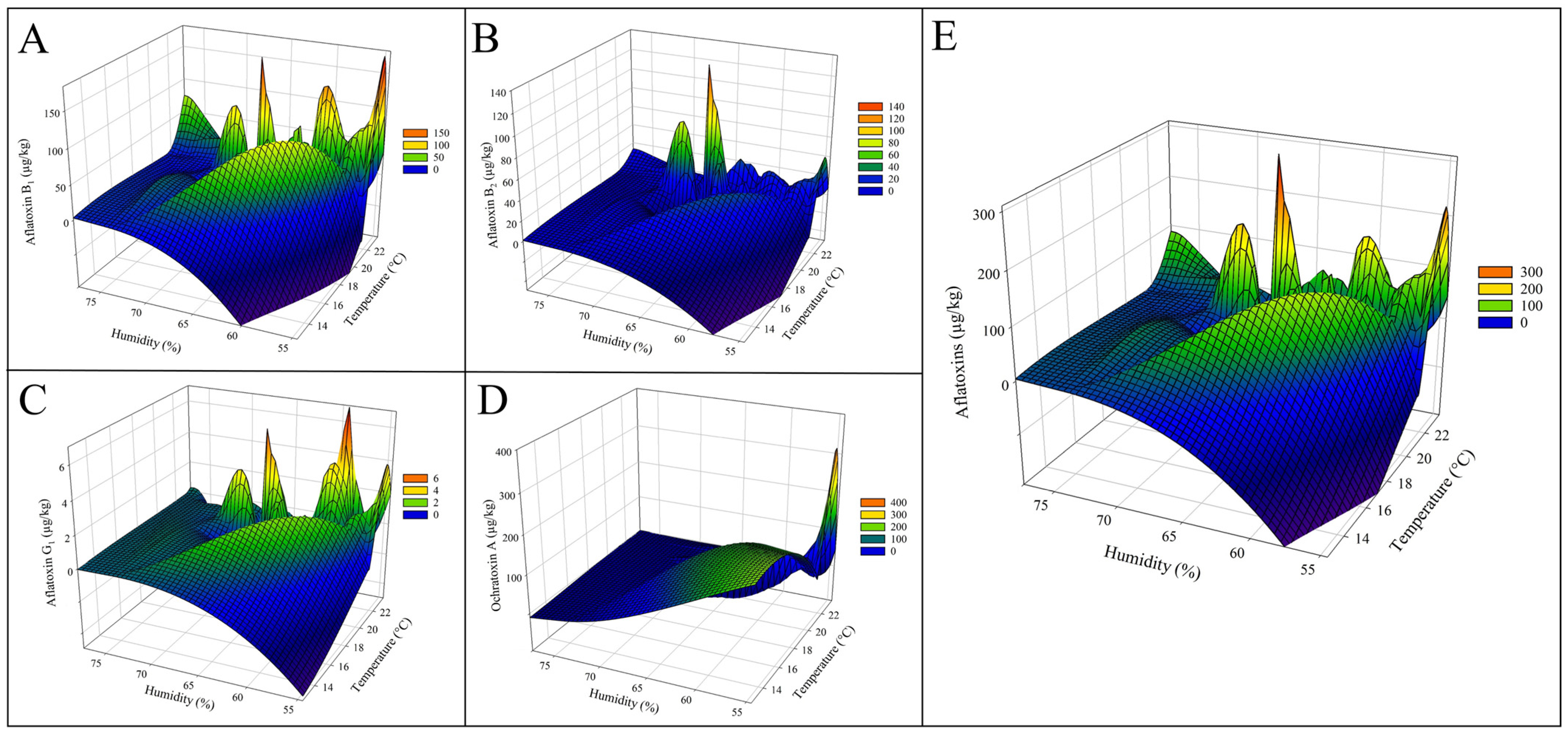
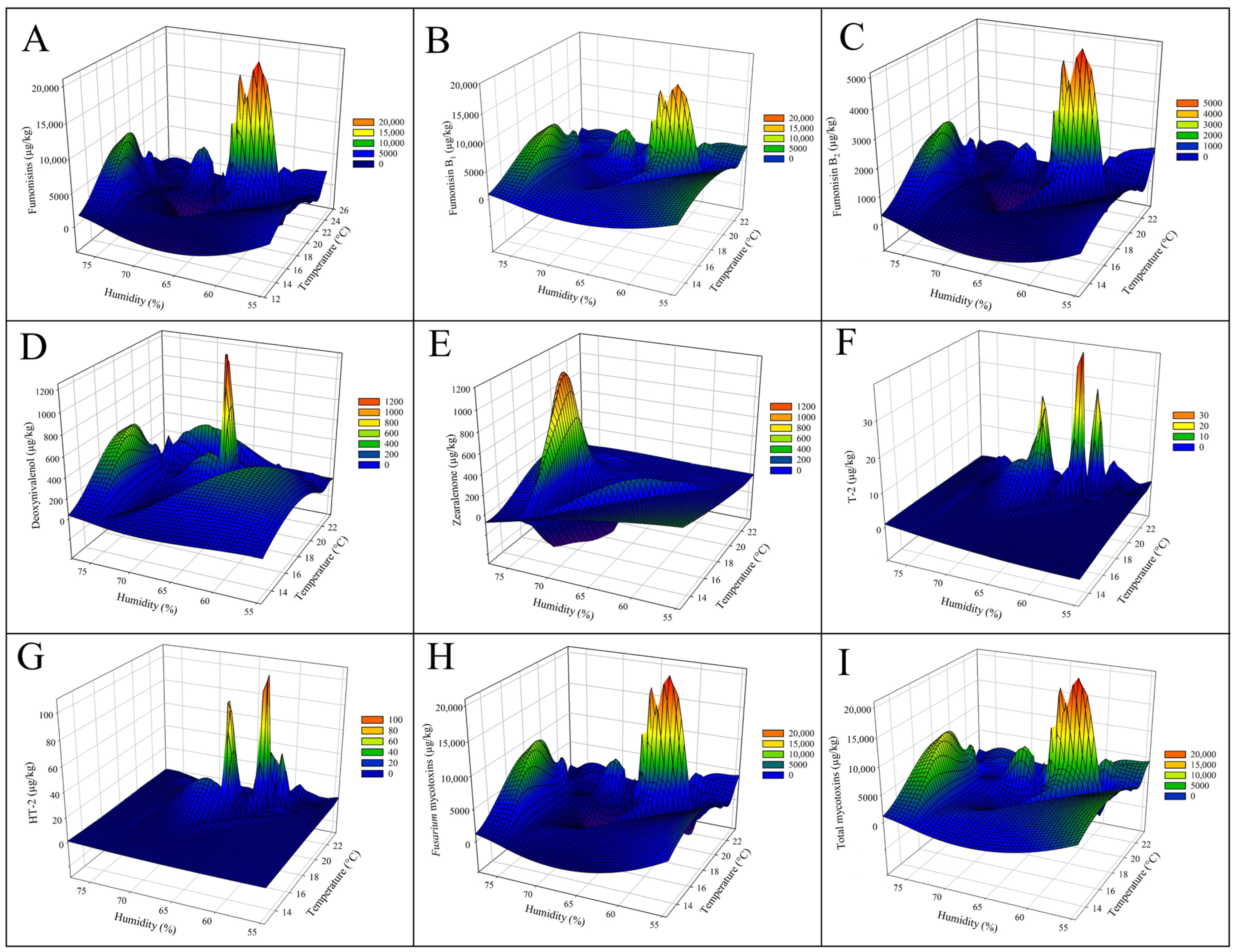
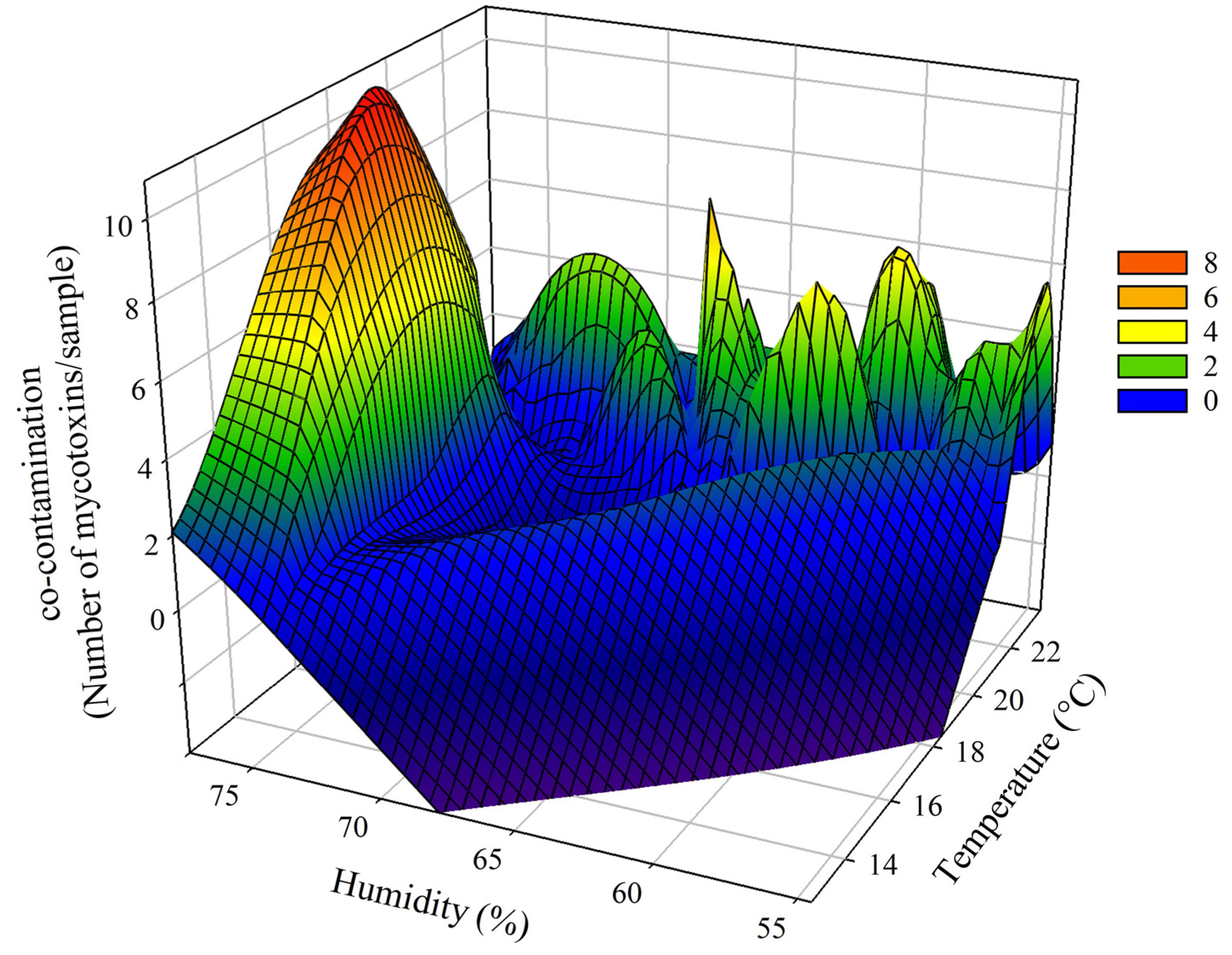

| Parameter | Year | Average ± SD | Range | ||||
|---|---|---|---|---|---|---|---|
| Altitude (m.a.s.l.) | 266.3 | ± | 423.2 | 42 | – | 2017 | |
| Temperature (harvest’s month 1) (°C) | 2021 | 16.9 | ± | 2.1 | 9.2 | – | 19.4 |
| 2022 | 16.2 | ± | 2.0 | 8.5 | – | 18.1 | |
| 2023 | 19.8 | ± | 2.2 | 11.9 | – | 22.2 | |
| Temperature (growing season 2) (°C) | 2021 | 21.2 | ± | 2.2 | 13.0 | – | 23.6 |
| 2022 | 21.2 | ± | 2.3 | 12.6 | – | 23.4 | |
| 2023 | 21.5 | ± | 2.2 | 13.4 | – | 23.7 | |
| Temperature (annual) (°C) | 2021 | 11.7 | ± | 1.9 | 4.3 | – | 13.8 |
| 2022 | 12.4 | ± | 2.0 | 5.0 | – | 14.5 | |
| 2023 | 12.9 | ± | 2.0 | 5.4 | – | 14.8 | |
| Humidity (harvest’s month 1) (%) | 2021 | 76.3 | ± | 4.4 | 70.2 | – | 86.5 |
| 2022 | 75.0 | ± | 4.4 | 63.0 | – | 80.1 | |
| 2023 | 70.8 | ± | 4.9 | 62.0 | – | 85.4 | |
| Humidity (growing season 2) (%) | 2021 | 62.2 | ± | 4.8 | 54.1 | – | 73.0 |
| 2022 | 64.4 | ± | 5.4 | 54.5 | – | 73.9 | |
| 2023 | 70.5 | ± | 6.0 | 55.5 | – | 79.5 | |
| Rainfall (harvest month 1) (mm) | 2021 | 70.2 | ± | 22.3 | 38.6 | – | 107.5 |
| 2022 | 11.6 | ± | 8.6 | 1.5 | – | 31.0 | |
| 2023 | 13.5 | ± | 7.5 | 1.1 | – | 30.6 | |
| Rainfall (growing season 2) (mm) | 2021 | 194.9 | ± | 56.2 | 89.8 | – | 306.6 |
| 2022 | 305.5 | ± | 87.3 | 189.7 | – | 530.1 | |
| 2023 | 298.7 | ± | 73.4 | 158.8 | – | 448.8 | |
| Mycotoxin | Year | Occurrence (%) 1 | Concentration (µg/kg) 2 | Kruskal–Wallis Test p-Value | |||||
|---|---|---|---|---|---|---|---|---|---|
| Average (±SD) | Range | ||||||||
| 2021 | 18.5 | 10.0 B | ± | 16.5 | 0.4 | – | 101 | <0.0001 * | |
| Aflatoxin B1 | 2022 | 73.2 | 80.6 A | ± | 115.3 | 0.5 | – | 527 | |
| 2023 | 34.1 | 29.2 AB | ± | 43.3 | 0.6 | – | 218 | ||
| 2021 | 9.3 | 2.5 B | ± | 4.0 | 0.4 | – | 17 | <0.0001 * | |
| Aflatoxin B2 | 2022 | 54.8 | 26.3 A | ± | 57.7 | 0.5 | – | 390 | |
| 2023 | 23.2 | 6.5 B | ± | 11.7 | 0.4 | – | 61.6 | ||
| 2021 | 1.4 | 5.3 | ± | 2.9 | 1.4 | – | 8.4 | 0.6260 | |
| Aflatoxin G1 | 2022 | 23.8 | 6.9 | ± | 9.7 | 0.5 | – | 51.8 | |
| 2023 | 0 | nd | |||||||
| 2021 | 0 | nd | – | ||||||
| Aflatoxin G2 | 2022 | 4.7 | 37.3 | ± | 73.7 | 0.6 | – | 231 | |
| 2023 | 0 | nd | |||||||
| Total of aflatoxins | 2021 | 18.5 | 12.0 B | ± | 20.0 | 0.4 | – | 118 | <0.0001 * |
| 2022 | 73.2 | 105 A | ± | 162.2 | 0.5 | – | 897 | ||
| 2023 | 34.1 | 33.6 B | ± | 52.1 | 0.6 | – | 245 | ||
| 2021 | 2.3 | 37.6 | ± | 34.6 | 4.8 | – | 102 | 0.8173 | |
| Ochratoxin A | 2022 | 29.8 | 65.2 | ± | 89.5 | 1.6 | – | 325 | |
| 2023 | 3.7 | 76.6 | ± | 66.1 | 2.6 | – | 190 | ||
| 2021 | 3.2 | 40.0 | ± | 13.9 | 20.7 | – | 65.5 | 0.3596 | |
| Zearalenone | 2022 | 2.4 | 357.6 | ± | 347 | 21.8 | – | 851 | |
| 2023 | 1.2 | 221.6 | ± | 164 | 57.2 | – | 386 | ||
| 2021 | 87.5 | 291 | ± | 188 | 95.3 | – | 606 | 0.6973 | |
| Deoxynivalenol | 2022 | 87.5 | 304 | ± | 248 | 64.4 | – | 904 | |
| 2023 | 70.7 | 222 | ± | 123 | 71.0 | – | 474 | ||
| 2021 | 87.1 | 4532 A | ± | 4617 | 45.0 | – | 28,781 | <0.0001 * | |
| Fumonisin B1 | 2022 | 87.6 | 2202 B | ± | 2158 | 42.3 | – | 11,775 | |
| 2023 | 70.7 | 885 C | ± | 981 | 43.4 | – | 5731 | ||
| 2021 | 81.9 | 1653 A | ± | 2115 | 45.1 | – | 15,479 | <0.0001 * | |
| Fumonisin B2 | 2022 | 79.2 | 637 B | ± | 689 | 40.4 | – | 3868 | |
| 2023 | 59.8 | 280 C | ± | 291 | 42.2 | – | 1848 | ||
| Total of fumonisins | 2021 | 87.5 | 6080 A | ± | 6659 | 45.0 | – | 44,260 | <0.0001 * |
| 2022 | 87.5 | 2778 B | ± | 2816 | 42.3 | – | 15,643 | ||
| 2023 | 72.0 | 1102 C | ± | 1249 | 43.4 | – | 7168 | ||
| 2021 | 5.1 | 48.7 | ± | 28.5 | 18.9 | – | 114 | 0.0690 | |
| HT-2 | 2022 | 6.5 | 54.7 | ± | 98.2 | 9.8 | – | 362 | |
| 2023 | 1.2 | 84.3 | ± | 31.7 | 52.6 | – | 116 | ||
| 2021 | 2.8 | 49.0 | ± | 23.8 | 15.1 | – | 86.4 | 0.4655 | |
| T-2 | 2022 | 4.1 | 36.4 | ± | 31.1 | 9.3 | – | 107 | |
| 2023 | 1.2 | 27.9 | ± | 10.3 | 17.6 | – | 38.1 | ||
| Total of HT-2 and T-2 | 2021 | 5.1 | 75.4 | ± | 55.2 | 18.9 | – | 200 | 0.1801 |
| 2022 | 7.1 | 71.4 | ± | 123 | 9.8 | – | 469 | ||
| 2023 | 1.8 | 74.8 | ± | 41.7 | 17.6 | – | 116 | ||
| Total of Trichothecenes 3 | 2021 | 10.1 | 196 | ± | 179 | 18.9 | – | 606 | 0.6772 |
| 2022 | 14.8 | 241 | ± | 247 | 9.8 | – | 904 | ||
| 2023 | 13.4 | 202 | ± | 126 | 17.6 | – | 474 | ||
| Total of Fusarium toxins 4 | 2021 | 89.4 | 5978 A | ± | 6639 | 20.7 | – | 44,289 | <0.0001 * |
| 2022 | 88.7 | 2791 B | ± | 2799 | 42.3 | – | 15,643 | ||
| 2023 | 72.0 | 1144 C | ± | 1262 | 43.4 | – | 7214 | ||
| Total of mycotoxins | 2021 | 89.8 | 5951 A | ± | 6635 | 7.7 | – | 44,289 | <0.0001 * |
| 2022 | 89.9 | 2861 B | ± | 2809 | 0.8 | – | 15,669 | ||
| 2023 | 75.6 | 1107 C | ± | 1253 | 0.6 | – | 7225 | ||
| Mycotoxins | Proportion of Contaminated Maize Samples (%) | ||
|---|---|---|---|
| 2021 | 2022 | 2023 | |
| AFB1 + AFB2 | 9.3 | 54.8 | 23.2 |
| AFB1 + AFG1 | 1.4 | 23.8 | 0.0 |
| AFB1 + AFG2 | 0.0 | 4.8 | 0.0 |
| AFB1 + AFB2 + AFG1 | 1.4 | 23.2 | 0.0 |
| AFB1 + AFB2 + AFG2 | 9.3 | 4.8 | 0.0 |
| AFB1 + AFB2 + AFG1 + AFG2 | 0.0 | 4.8 | 0.0 |
| FB1 + FB2 | 81.9 | 79.2 | 58.5 |
| Afs * + FB1 + FB2 | 18.1 | 69.6 | 29.3 |
| Afs * + FB1 | 18.1 | 72.0 | 30.5 |
| Afs * + FB2 | 18.1 | 69.6 | 29.3 |
| DON + ZEN + FB1 + FB2 | 0.0 | 1.2 | 1.2 |
| DON + ZEN + FB1 | 0.0 | 1.2 | 1.2 |
| DON + ZEN + FB2 | 0.0 | 1.2 | 1.2 |
| Mycotoxin | Samples Above Compliance Levels for Human Consumption (%) 1 | Samples Above Compliance Levels for Animal Consumption (%) 1 | ||||||
|---|---|---|---|---|---|---|---|---|
| ML 2,3,4 (µg/kg) | Year | ML 6/GL 7,8,9 (µg/kg) | Year | |||||
| 2021 | 2022 | 2023 | 2021 | 2022 | 2023 | |||
| Aflatoxin B1 | 5 2 | 8.3 | 55.4 | 23.2 | 20 6/30 7 | 2.3/0.9 | 40.5/33.9 | 12.2/9.8 |
| Sum of aflatoxins | 10 2 | 6.5 | 50.3 | 19.5 | - | - | - | - |
| Ochratoxin A | 5 2 | 1.9 | 20.8 | 2.4 | 250 8 | 0.0 | 1.8 | 0.0 |
| Zearalenone | 350 2 | 0.0 | 1.2 | 0.6 | 2000 8/4000 6 | 0.0 | 0.0 | 0.0 |
| Deoxynivalenol | 1500 3 | 0.0 | 0.0 | 0.0 | 8000 8 | 0.0 | 0.0 | 0.0 |
| Sum of fumonisins | 4000 2,4 | 40.1 | 22.5 | 1.8 | 60.000 8 | 0.0 | 0.0 | 0.0 |
| Sum of HT-2 and T-2 | 100 5 | 1.4 | 0.6 | 0.6 | 250 9 | 0.0 | 0.6 | 0.0 |
| Mycotoxin | LOQ (μg/kg) | Apparent Recovery (%) |
|---|---|---|
| AFB1 | 0.4 | 92 |
| AFB2 | 0.4 | 90 |
| AFG1 | 0.4 | 86 |
| AFG2 | 0.4 | 81 |
| OTA | 1.6 | 96 |
| ZEN | 16.0 | 92 |
| DON | 64.0 | 87 |
| FB1 | 40.0 | 97 |
| FB2 | 40.0 | 95 |
| HT-2 | 9.6 | 93 |
| T-2 | 9.6 | 97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Penagos-Tabares, F.; Todorov, A.; Raj, J.; Farkaš, H.; Grubješić, G.; Jakovčević, Z.; Ćujić, S.; Nedeljković-Trailović, J.; Vasiljević, M. Multi-Mycotoxin Contamination in Serbian Maize During 2021–2023: Climatic Influences and Implications for Food and Feed Safety. Toxins 2025, 17, 227. https://doi.org/10.3390/toxins17050227
Penagos-Tabares F, Todorov A, Raj J, Farkaš H, Grubješić G, Jakovčević Z, Ćujić S, Nedeljković-Trailović J, Vasiljević M. Multi-Mycotoxin Contamination in Serbian Maize During 2021–2023: Climatic Influences and Implications for Food and Feed Safety. Toxins. 2025; 17(5):227. https://doi.org/10.3390/toxins17050227
Chicago/Turabian StylePenagos-Tabares, Felipe, Anastasija Todorov, Jog Raj, Hunor Farkaš, Goran Grubješić, Zdenka Jakovčević, Svetlana Ćujić, Jelena Nedeljković-Trailović, and Marko Vasiljević. 2025. "Multi-Mycotoxin Contamination in Serbian Maize During 2021–2023: Climatic Influences and Implications for Food and Feed Safety" Toxins 17, no. 5: 227. https://doi.org/10.3390/toxins17050227
APA StylePenagos-Tabares, F., Todorov, A., Raj, J., Farkaš, H., Grubješić, G., Jakovčević, Z., Ćujić, S., Nedeljković-Trailović, J., & Vasiljević, M. (2025). Multi-Mycotoxin Contamination in Serbian Maize During 2021–2023: Climatic Influences and Implications for Food and Feed Safety. Toxins, 17(5), 227. https://doi.org/10.3390/toxins17050227






