Implantation of Aspergillus Section Flavi in French Maize and Consequences on Aflatoxin Contamination of Maize at Harvest: Three-Year Survey
Abstract
1. Introduction
2. Results
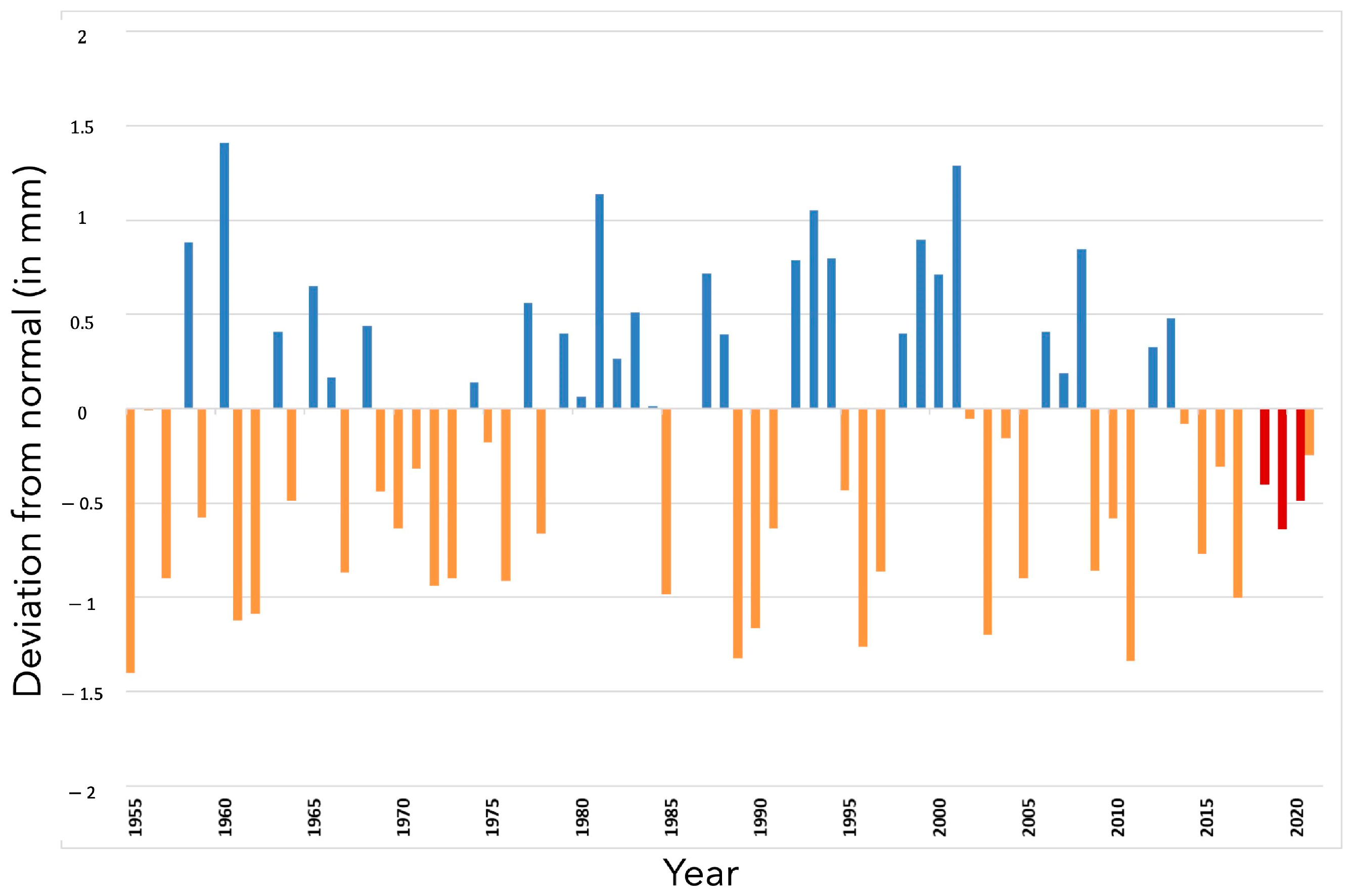
2.1. Climatic Data
2.2. Global Mycoflora of Maize Samples
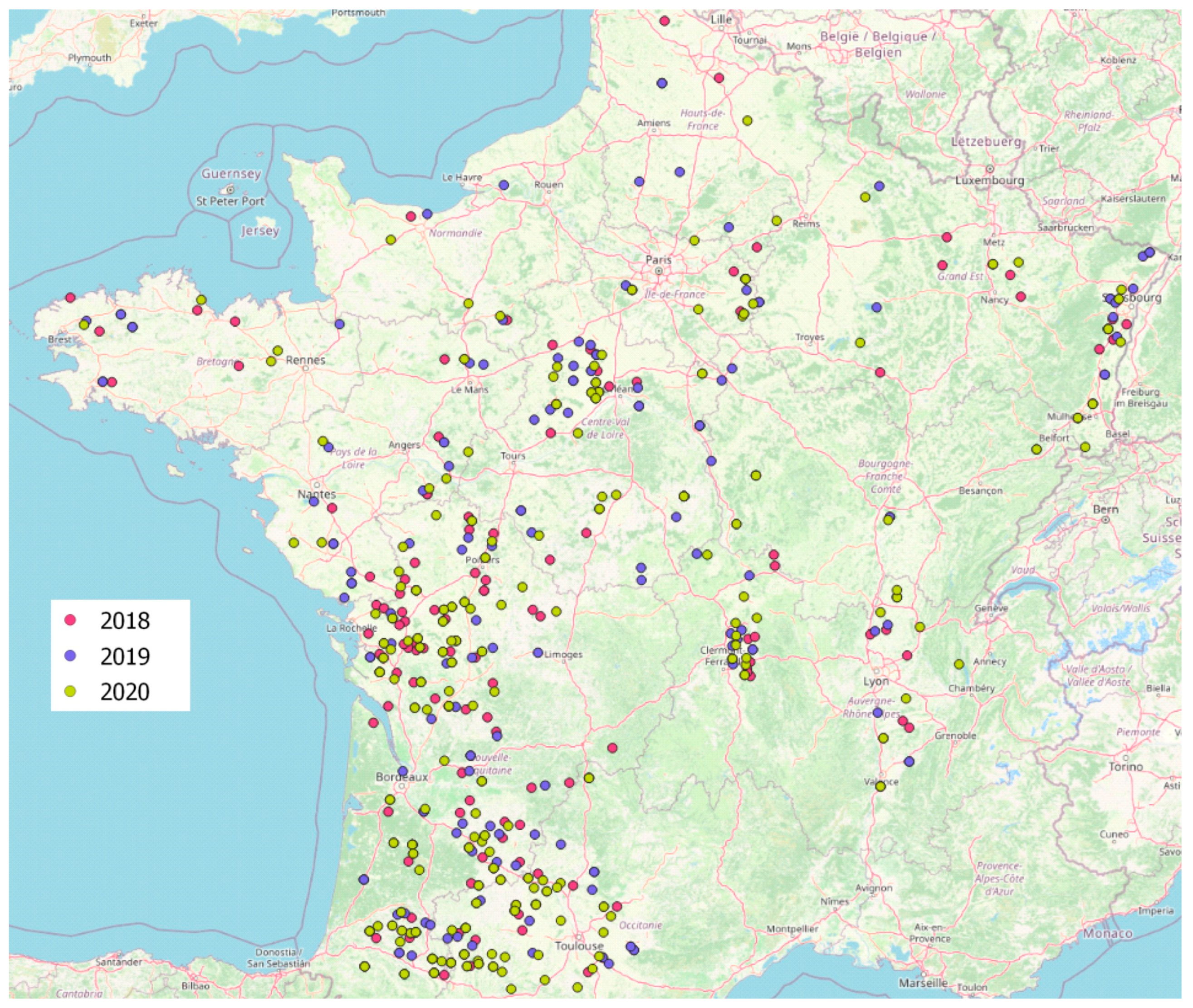
2.3. Presence of Aspergillus Section Flavi in French Maize Samples
2.4. Nature of Aspergillus Section Flavi Species Present in French Fields
2.5. Toxigenic Potential of Isolated Strains
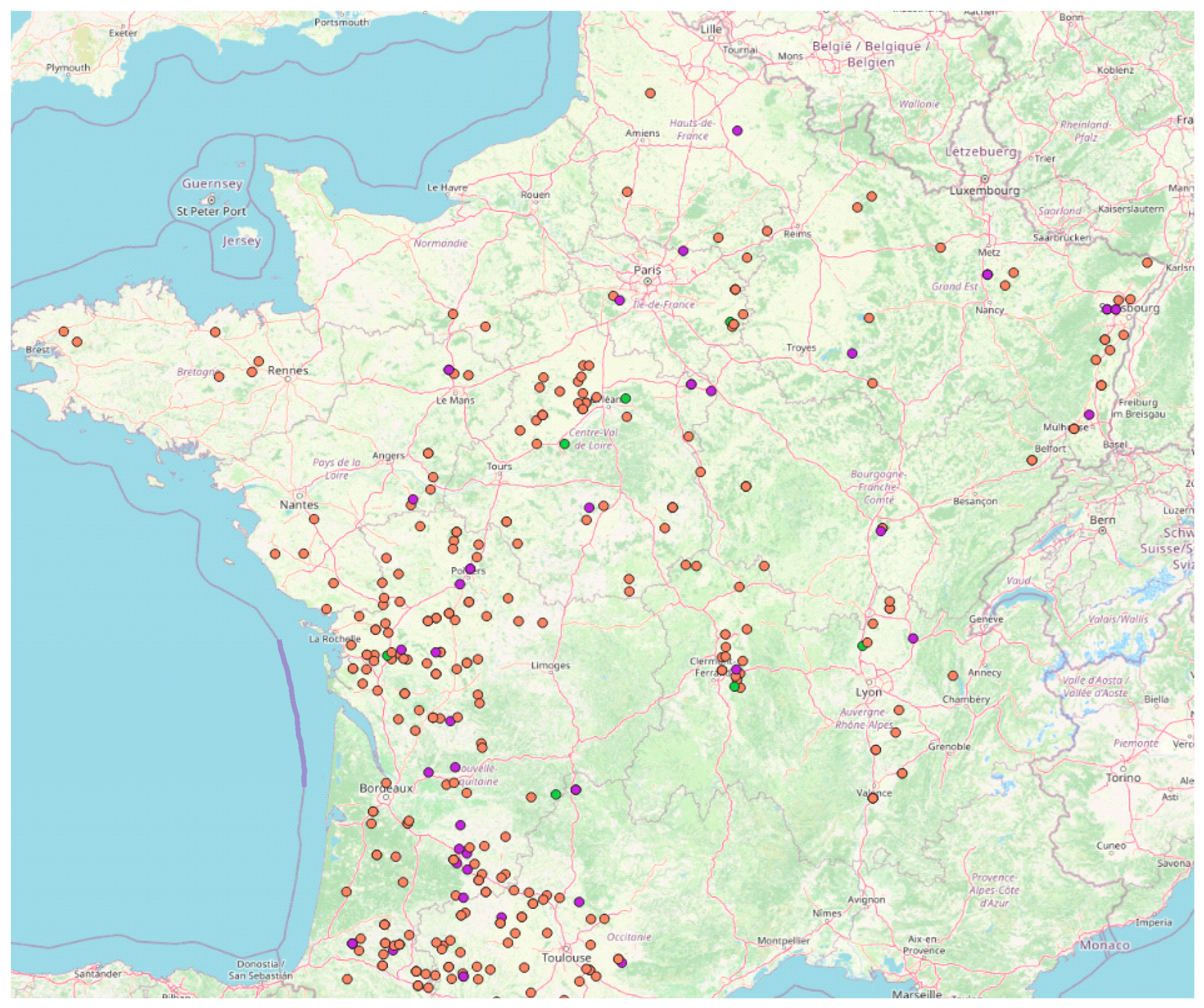
2.6. Impact on AF Contamination of Maize Grains at Harvest
3. Discussion
4. Conclusions
5. Materials and Methods
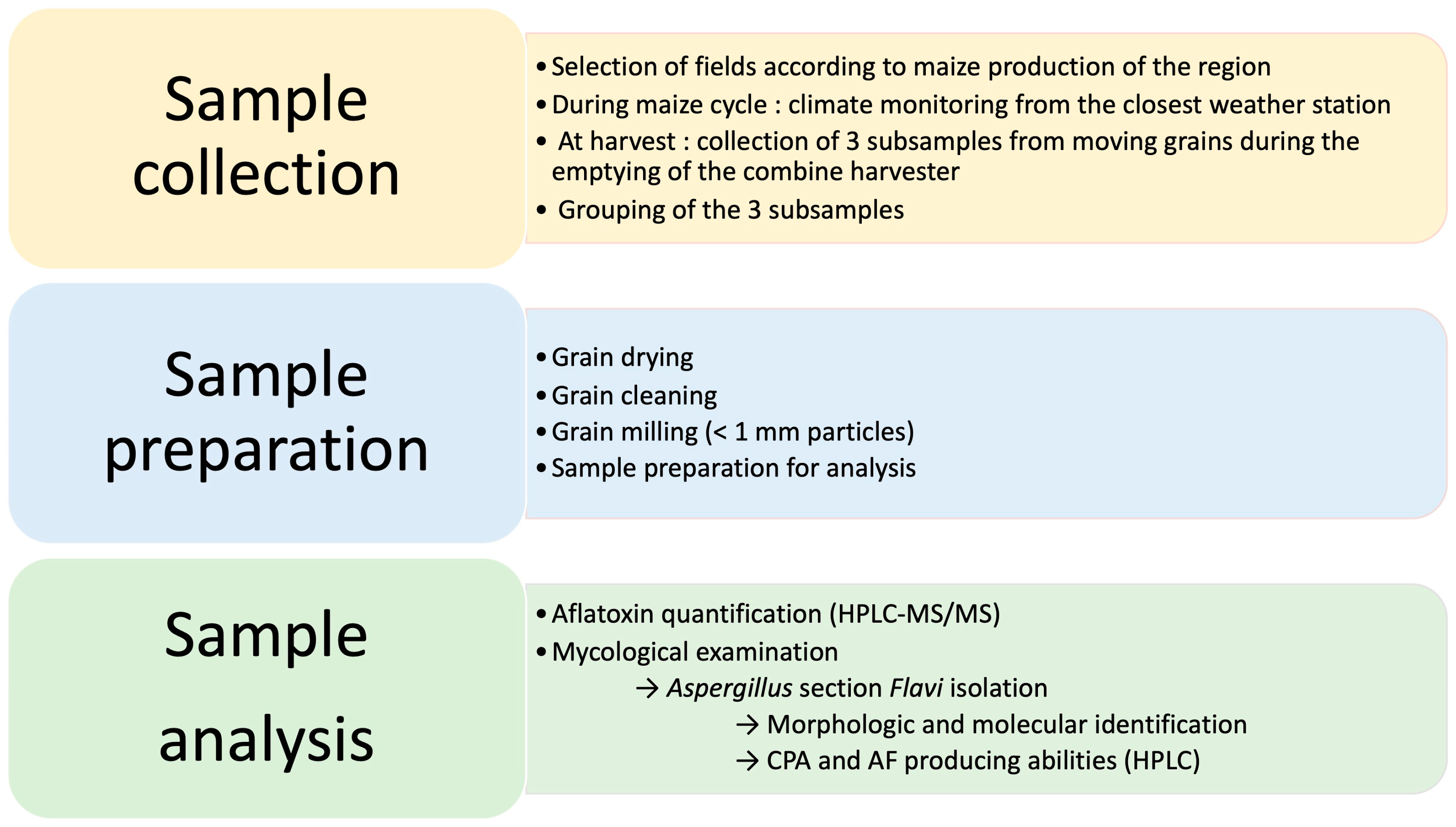
5.1. Sampling
5.2. Climate Monitoring
- -
- The mean of the average daily temperature (in °C) from 1 March to 30 October for each year.
- -
- The mean of the total daily rainfall sum (in mm) from 1 March to 30 October of each year.
- -
- The number of days with a maximum temperature exceeding 30 °C from 1 January to 31 December of each year.
5.3. Aflatoxin Quantification
5.4. Mycological Analysis
5.5. Identification of Aspergillus Section Flavi
5.6. Determination of AFs and CPA Production by Aspergillus Section Flavi Strains
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- IARC. Monographs on the evaluation of carcinogenic risks to humans. Aflatoxins 2012, 100F, 225–244. [Google Scholar]
- Rushing, B.R.; Selim, M.I. Aflatoxin B1: A review on metabolism, toxicity, occurrence in food, occupational exposure and detoxification methods. Food Chem. Toxicol. 2019, 124, 81–100. [Google Scholar] [CrossRef] [PubMed]
- Gibb, H.; Devleesschauwer, B.; Bolger, B.M.; Wu, F.; Ezenman, J.; Cliff, J.; Zeilmaker, M.; Verger, P.; Pitt, J.; Baines, J. World Health Organization estimates of the global and regional disease burden of four foodborne chemical toxins, 2010: A data synthesis. F1000Research 2015, 4, 1393. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Regulation 2023/2019 regarding maximal levels for certain food contaminants. Off. J. Eur. Union 2023, L119, 103–157. [Google Scholar]
- Van Egmond, H.P.; Schothorst, R.C.; Jonker, M.A. Regulation relating to mycotoxins in food: Perspectives in a global and European context. Anal. Bioanal. Chem. 2007, 389, 147–157. [Google Scholar] [CrossRef]
- Singh, P.; Orbach, J.M.; Cotty, J.P. Aspergillus texensis: A novel aflatoxin producer with S morphology from the United States. Toxins 2018, 10, 513. [Google Scholar] [CrossRef]
- Frisvad, J.C.; Hubka, V.; Ezekiel, C.N.; Hong, S.B.; Novakova, A.; Chen, A.J.; Arzanlou, M.; Larsen, T.O.; Sklenar, F.; Mahakarnchanakul, W.; et al. Taxonomy of Aspergillus section Flavi and their production of aflatoxins, ochratoxins and other mycotoxins. Stu. Mycol. 2019, 93, 1–63. [Google Scholar] [CrossRef]
- Uka, V.; Moore, G.G.; Aroyo-Manzanares, N.; Nebija, D.; De Saeger, S.; Di Mavungu, J.D. Unravelling the diversity of the cyclopiazonic acid family of mycotoxins in Aspergillus flavus by UHPLC triple TOF HRMS. Toxins 2017, 9, 35. [Google Scholar] [CrossRef]
- Pitt, J.I.; Hocking, A.D. Fungi and Food Spoilage, 3rd ed.; Springer: London, UK, 2009; 540p, ISBN 978-0-387-92206-5. [Google Scholar]
- Klich, M.A. Aspergillus flavus: The major producer of aflatoxin. Mol. Plant. Pathol. 2007, 8, 713–722. [Google Scholar] [CrossRef]
- Battilani, P.; Toscano, P.; Van der Fels-Klerx, H.J.; Moretti, A.; Carmado Leggieri, M.; Brera, C.; Rortais, A.; Goumperis, T.; Robinson, T. Aflatoxin B1 contamination in maize in Europe increases due to climate change. Sci. Rep. 2016, 6, 24328. [Google Scholar] [CrossRef]
- Tabuc, C.; Marin, D.; Guerre, P.; Sesan, T.; Bailly, J.D. Molds and mycotoxin content of cereals in southeastern Romania. J. Food Prot. 2009, 72, 662–665. [Google Scholar] [CrossRef] [PubMed]
- Amorini, S.; Altafini, A.; Zaghini, A.; Roncada, P. Occurrence of aflatoxin B1 in conventional and organic flour in Italy and the role of sampling. Food Control. 2015, 50, 858–863. [Google Scholar] [CrossRef]
- Pleadin, J.; Vulic, A.; Persi, N.; Skrivanko, M.; Capek, B.; Cvetnic, Z. Annual and regional variations of aflatoxin B1 levels seen in grains and feed coming from Croatian dairy farms over a 5-year period. Food Control. 2015, 47, 221–225. [Google Scholar] [CrossRef]
- Kos, J.; Radic, B.; Radovic, R.; Saric, B.; Jovanov, P.; Saric, L. Aflatoxins in maize, milk and dairy products from Serbia. Food Addit. Contam. Part B Surveill. 2024, 17, 296–307. [Google Scholar] [CrossRef]
- Kollia, E.; Tsourouflis, K.; Markaki, P. Aflatoxin B1 in sesame seeds and sesame products from the Greek market. Food Addit. Contam. Part B Surveill. 2016, 9, 217–222. [Google Scholar] [CrossRef]
- Ketney, O.; Santini, A.; Oancea, S. recent aflatoxin survey data in milk products: A review. Int. J. Dairy Technol. 2017, 70, 320–331. [Google Scholar] [CrossRef]
- Rodriguez-Blanco, M.; Ramos, A.J.; Prim, M.; Sanchis, V.; Marin, S. Usefulness of the analytical control of aflatoxins in feedstuffs for dairy cows for the prevention of aflatoxin M1 in milk. Mycotoxin Res. 2020, 36, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Zentai, A.; Jozwiak, A.; Süth, M.; Farkas, Z. Carry-over of aflatoxin B1 from feed to cow milk—A review. Toxins 2023, 15, 195. [Google Scholar] [CrossRef]
- Bailly, S.; El Mahgubi, A.; Carvajal-Campos, A.; Lorber, S.; Puel, O.; Oswald, I.P.; Bailly, J.-D.; Orlando, B. Occurrence and identification of Aspergillus section Flavi in the context of the emergence of Aflatoxins in French maize. Toxins 2018, 10, 525. [Google Scholar] [CrossRef]
- Zingales, V.; Taroncher, M.; Martino, P.A.; Ruiz, M.-j.; Caloni, F. Climate change and effects on mold and mycotoxins. Toxins 2022, 14, 445. [Google Scholar] [CrossRef]
- Perrone, G.; Susca, A. Penicillium species and their associated mycotoxins. Methods Mol. Biol. 2017, 1542, 107–119. [Google Scholar] [CrossRef]
- Calvo, A.M.; Cary, J.W. Association of fungal secondary metabolism and sclerotial biology. Front. Microbiol. 2015, 6, 62. [Google Scholar] [CrossRef] [PubMed]
- Nsabiyumva, G.; Mutegi, C.K.; Wagacha, J.M.; Mohamed, A.B.; Njeru, N.K.; Ndayihanzamaso, P.; Niyuhire, M.-C.; Atehnkeng, J.; Njukwe, E.; Callicott, K.A.; et al. Aflatoxin contamination of maize and groundnut in Burundi: Distribution of contamination, identification of causal agents and potential biocontrol genotypes of Aspergillus flavus. Front. Microbiol. 2023, 14, 1106543. [Google Scholar] [CrossRef]
- Kachapula, P.W.; Akello, J.; Bandyopadhyay, R.; Cotty, P.J. Aspergillus section Flavi community structure in Zambia influences aflatoxin contamination of maize and groundnut. Int. J. Food Microbiol. 2017, 261, 49–56. [Google Scholar] [CrossRef]
- Aristil, J.; Venturini, G.; Spada, A. Occurrence of toxigenic fungi and aflatoxin potential of Aspergillus spp. strains associated with subsistence farmed crops in Haiti. J. Food Prot. 2017, 80, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Del Palacio, A.; Pan, D. Occurrence and toxigenic potential of Aspergillus section Flavi on wheat and sorghum silages in Uruguay. Mycology 2020, 11, 147–157. [Google Scholar] [CrossRef]
- Yogendrarajah, P.; Devlieghere, F.; Njumbe Ediage, E.; Jacxsens, L.; De Meulenaer, B.; De Saeger, S. Toxigenic potentiality of Aspergillus flavus and Aspergillus parasiticus strains isolated from black pepper assessed by an LC-MS/MS based multi-mycotoxin method. Food Microbiol. 2015, 52, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Nikolic, M.; Savic, I.; Nikolic, A.; Jaukovic, M.; Stevanovic, M.; Stankovic, S. Toxigenic species Aspergillus parasiticus originating from maize kernels grown in Serbia. Toxins 2021, 13, 847. [Google Scholar] [CrossRef]
- Makhlouf, J.; Carvajal-Campos, A.; Querin, A.; Tadrist, S.; Puel, O.; Lorber, S.; Oswald, I.P.; Hamze, M.; Bailly, J.-D.; Bailly, S. Morphologic, molecular and metabolic characterization of Aspergillus section Flavi in spices marketed in Lebanon. Sci. Rep. 2019, 9, 5263. [Google Scholar] [CrossRef]
- Giorni, P.; Magan, N.; Pietri, A.; Bertuzzi, T.; Battilani, P. Studies on Aspergillus section Flavi isolated from maize in northern Italy. Int. J. Food Microbiol. 2007, 113, 330–338. [Google Scholar] [CrossRef]
- Chalivendra, S.C.; DeRobertis, C.; Chang, P.-K.; Damann, K.E. Cyclopiazonic acid is a pathogencity factor for Aspergillus flavus and a promising target for screening germplast for ear rot resistance. Mol. Plant Microbe Interact. 2017, 30, 361–373. [Google Scholar] [CrossRef]
- Maragos, C.M.; Sieve, K.K.; Bobell, J. Detection of cyclopiazonic acid (CPA) in maize by immunoassay. Mycotoxin Res. 2017, 33, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Navale, V.; Vamkudoth, K.R.; Ajmera, S.; Dhuri, V. Aspergillus derived mycotoxins in food and the environment: Prevalence, detection and toxicity. Tox. Rep. 2021, 8, 1008–1030. [Google Scholar] [CrossRef]
- Hymery, N.; Masson, F.; Barbier, G.; Coton, E. Cytotoxicity and immunotoxicity of cyclopiazonic acid on human cells. Toxicol. In Vitro 2014, 28, 940–947. [Google Scholar]
- Deepika, C.; Hariprasanna, K.; Das, I.K.; Jacob, J.; Ronanki, S.; Ratnavathi, C.V.; Bellundagi, A.; Sooganna, D.; Tonapi, V.A. Kodo poisoning: Cause, science and management. J. Food Sci. Technol. 2022, 59, 2517–2526. [Google Scholar] [CrossRef]
- Ma, X.; Ye, Y.; Sun, J.; Ji, J.; Wang, J.-S.; Sun, X. Coexposure of cyclopiazonic acid with aflatoxin B1 involved in disrupting amino acid metabolism and redox homeostasis causing synergistic toxic effects in hepatocyte spheroids. J. Agric. Food Chem. 2022, 27, 5166–5176. [Google Scholar] [CrossRef]
- Rapid Alert System on Food and Feed. Annual Report. 2020. Available online: https://food.ec.europa.eu/document/download/4b178b4f-c40c-4405-9c01-db558aa1392a_en?filename=rasff_pub_annual-report_2020.pdf (accessed on 20 March 2025).
- Ministère de l’Agriculture et de la Souveraineté Alimentaire. Bilan 2021, Plans de Surveillance et Plans de Contrôle. 2023. Available online: https://agriculture.gouv.fr/telecharger/133178 (accessed on 20 March 2025).
- European Food Safety Authority. Chemical Contaminants Occurrence Data (2011–2015). Available online: https://www.efsa.europa.eu/fr/microstrategy/contaminants-occurrence-data (accessed on 20 March 2025).
- Radonic, J.R.; Kocic Tanackov, S.D.; Mihajlovic, I.J.; Grujic, Z.S.; Vojinovic Miloradov, M.B.; Skrinjar, M.M.; Turk Sekulic, M.M. Occurrence of aflatoxin M1 in human milk smaples in Vojvodina, Serbia: Estimation of average daily intake by babies. J. Environ. Sci. Health B 2017, 52, 59–63. [Google Scholar] [CrossRef]
- Milicevic, D.R.; Spiric, D.; Radicevic, T.; Velebit, B.; Stefanovic, S.; Milojevic, L.; Jankovic, S. A review of the current situation of aflatoxin M1 in cow’s milk in Serbia: Risk assessment and regulatory aspects. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2017, 34, 1617–1631. [Google Scholar] [CrossRef]
- Miocinovic, J.; Keskic, T.; Miloradovic, Z.; Kos, A.; Tomasevic, I.; Pudja, P. The aflatoxin M1 crisis in the Serbian dairy sector: The year after. Food Addit. Contam. Part B Surveill. 2017, 10, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Van der Fels-Klerx, H.J. Quantitative modeling of climate change impacts on mycotoxins in cereals: A review. Toxins 2021, 13, 276. [Google Scholar] [CrossRef]
- Agbetiameh, D.; Ortega-Beltran, A.; Awuah, R.T.; Atehnkeng, J.; Elzein, A.; Cotty, P.J.; Bandyopadhyay, R. Field efficacy of two atoxigenic biocontrol products for mitigation of aflatoxin contamination in maize and groundnut in Ghana. Biol. Control 2020, 150, 104351. [Google Scholar] [CrossRef] [PubMed]
- Tafesse Mamo, F.; Shang, B.; Selvaraj, J.N.; Zheng, Y.; Liu, Y. Biocontrol efficacy of atoxigenic Aspergillus flavus strains against aflatoxin contamination in peanut field in Guangdong province, South China. Mycology 2021, 13, 143–152. [Google Scholar] [CrossRef]
- Sweany, R.R.; DeRobertis, C.D.; Kaller, M.D.; Damann, K.E., Jr. Intraspecific growth and aflatoxin inhibition responses to atoxigenic Aspergillus flavus: Evidence of secreted, inhibitory substances in biocontrol. Phytopathology 2022, 112, 2084–2098. [Google Scholar] [CrossRef] [PubMed]
- Météo France: L’API Données Climatologiques de Météo France. Available online: https://portail-api.meteofrance.fr/web/fr/api/DonneesPubliquesClimatologie (accessed on 20 March 2025).
- Norme NF V08-059; Microbiologie des Aliments; Dénombrement des Levures et Moisissures par Comptage des Colonies à 25 °C. Association Française de Normalisation (AFNOR): Paris, France, 2002.
- Carvajal-Campos, A.; Manizan, A.L.; Tadrist, S.; Koffi-Akaki, D.; Koffi-Nevry, R.; Moore, G.G.; Fapohunda, S.O.; Bailly, S.; Montet, D.; Oswald, I.P.; et al. Aspergillus korhogoensis, a novel aflatoxin producing species from the Côte d’Ivoire. Toxins 2017, 9, 353. [Google Scholar] [CrossRef]


| Fungal Genus | Aspergillus | ||||||||
| Section Flavi | Section Nigri | Section Candidi | Section Cremei | ||||||
| Positive Samples a (%) | Mean CFU/g b | Positive Samples a (%) | Mean CFU/g b | Positive Samples a (%) | Mean CFU/g b | Positive Samples a (%) | Mean CFU/g b | ||
| Year | 2018 | 105 (53.8) | 1000 | 69 (35.4) | 1000 | 7 (3.6) | 8000 | 5 (2.6) | 2000 |
| 2019 | 103 (56.3) | 1000 | 87 (47.5) | 1000 | 9 (4.9) | 300 | 4 (2.2) | 600 | |
| 2020 | 140 (79.5) | 10,000 | 123 (69.9) | 5000 | 12 (6.8) | 60 | 9 (5.1) | 400 | |
| Fungal Genus | Aspergillus | Eurotium | |||||||
| Section Fumigati | Section Nidulantes | Section Terrei | |||||||
| Positive Samples a (%) | Mean CFU/g b | Positive Samples a (%) | Mean CFU/g b | Positive Samples a (%) | Mean CFU/g b | Positive Samples a (%) | Mean CFU/g b | ||
| Year | 2018 | 1 (0.03) | 100 | 8 (4.1) | 100 | 4 (2.0) | 60 | 61 (31.2) | 10,000 |
| 2019 | 4 (2.2) | 50,000 | 3 (1.6) | 40 | 2 (1.1) | 300 | 33 (18.0) | 1000 | |
| 2020 | 3 (1.7) | 70 | 17 (9.6) | 500 | 6 (3.4) | 2000 | 59 (33.5) | 9000 | |
| Fungal Genus | Penicillium | Fusarium (Liseola Section) | Acremonium | Cladosporium | |||||
| Positive Samples a (%) | Mean CFU/g b | Positive Samples a (%) | Mean CFU/g b | Positive Samples a (%) | Mean CFU/g b | Positive Samples a (%) | Mean CFU/g b | ||
| Year | 2018 | 190 (97.4) | 70,000 | 186 (95.4) | 30,000 | 161 (82.6) | 6000 | 176 (90.2) | 1000 |
| 2019 | 179 (96.8) | 50,000 | 177 (96.7) | 40,000 | 163 (89.1) | 20,000 | 158 (86.3) | 2000 | |
| 2020 | 173 (98.3) | 20,000 | 167 (94.9) | 20,000 | 156 (88.6) | 5000 | 161 (91.5) | 2000 | |
| Fungal Genus | Mucor | Rhizopus | Absidia | ||||||
| Positive Samples a (%) | Mean CFU/g b | Positive Samples a (%) | Mean CFU/g b | Positive Samples a (%) | Mean CFU/g b | ||||
| Year | 2018 | 142 (72.8) | 5000 | 97 (49.7) | 1000 | 54 (27.7) | 2000 | ||
| 2019 | 154 (84.1) | 9000 | 165 (90.1) | 20,000 | 116 (63.4) | 9000 | |||
| 2020 | 137 (77.8) | 4000 | 167 (94.9) | 10,000 | 52 (29.5) | 2000 | |||
| 2018 | 2019 | 2020 | |
|---|---|---|---|
| Number of samples | 195 | 183 | 176 |
| Number of samples (%) contaminated with Aspergillus section Flavi strains | 105 (54%) | 103 (56%) | 140 (80%) |
| Total number of Aspergillus section Flavi isolated and characterized | 258 | 204 | 386 |
| Mean number of strains per sample | 1.3 | 1.1 | 2.2 |
| Mean number of strains per sample contaminated with AFs | 4.7 | 4.5 | 4.75 |
| 2018 | 2019 | 2020 | ||||
|---|---|---|---|---|---|---|
| Number of Samples (%) | Mean Numeration | Number of Samples (%) | Mean Numeration | Number of Samples (%) | Mean Numeration | |
| Flavi+/AF− | 98 (50%) | 5.102 | 99 (54%) | 7.102 | 112 (64%) | 7.102 |
| Flavi+/AF+ | 7 (4%) | 104 | 4 (2%) | 8.103 | 28 (16%) | 7.104 |
| Species | 2018 | 2019 | 2020 |
|---|---|---|---|
| Aspergillus flavus | 241 (93%) | 189 (93%) | 349 (90%) |
| Aspergillus parasiticus | 16 (6%) | 14 (7%) | 37 (10%) |
| Aspergillus pseudonomius | 1 | - | - |
| Aspergillus tamarii | - | 1 | - |
| Morphotype a | Year | |||
|---|---|---|---|---|
| 2018 (n b = 241) | 2019 (n = 189) | 2020 (n = 346) | ||
| 1 | 1a | 56 (23.2%) | 48 (25.4%) | 46 (13.3%) |
| 1b | 82 (34%) | 46 (24.3%) | 89 (25.7%) | |
| 1c | 4 (1.7%) | 1 (0.5%) | 11 (3.2%) | |
| 2 | 80 (33.2%) | 69 (36.5%) | 161 (46.5%) | |
| 3 | 9 (3.7%) | 12 (6.3%) | 18 (5.2%) | |
| 4 | 10 (4.1%) | 13 (6.9%) | 21 (6%) | |
| Species | Number of Toxigenic Strains | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2018 | 2019 | 2020 | ||||||||
| Number of Strains | AFB | AFG | CPA | AFB | AFG | CPA | AFB | AFG | CPA | |
| A. flavus | 779 | 67 | - | 182 | 40 | - | 136 | 77 | - | 251 |
| A. parasiticus | 67 | 16 | 16 | - | 14 | 14 | - | 32 | 31 | - |
| A. pseudonomius | 1 | 1 | 1 | - | ||||||
| A. tamarii | 1 | - | - | 1 | ||||||
| Year | Range of Contamination (µg/kg) | |||||
|---|---|---|---|---|---|---|
| <LOQ | >LOQ < 2 | 2–4 | 4–10 | >10 | ||
| AFB1 | 2018 | 96.4 | 2 | 0.5 | 1 | 0.5 |
| 2019 | 97.8 | 2.2 | 0 | 0 | 0 | |
| 2020 | 84.1 | 10.7 | 1.2 | 1.7 | 2.3 | |
| Total AF | 2018 | 96.4 | 2 | 0.5 | 1 | 0.5 |
| 2019 | 97.8 | 2.2 | 0 | 0 | 0 | |
| 2020 | 84.1 | 10.1 | 2.2 | 0.6 | 3.4 | |
| Gene | Gene Name | Length bp | Primers | Sequence (Nucleotides: 5′ → 3′) | |
|---|---|---|---|---|---|
| Forward | Reverse | ||||
| benA | ß-tubulin | 541 | Btub2a | 5′-GGTAACCAAATCGGTGCTGCTTTC | |
| Btub2b | 5′-ACCCTCAGTGTAGTGACCCTTGGC | ||||
| cmdA | Calmodulin | 543 | Cmd5 | 5′CCGAGTACAAGGAGGCCTTC-3′ | |
| Cmd6 | 5′-CCGATAGAGGTCATAACGTGG-3′ | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bailly, S.; El Mahgubi, A.; Puel, O.; Lorber, S.; Bailly, J.-D.; Orlando, B. Implantation of Aspergillus Section Flavi in French Maize and Consequences on Aflatoxin Contamination of Maize at Harvest: Three-Year Survey. Toxins 2025, 17, 155. https://doi.org/10.3390/toxins17040155
Bailly S, El Mahgubi A, Puel O, Lorber S, Bailly J-D, Orlando B. Implantation of Aspergillus Section Flavi in French Maize and Consequences on Aflatoxin Contamination of Maize at Harvest: Three-Year Survey. Toxins. 2025; 17(4):155. https://doi.org/10.3390/toxins17040155
Chicago/Turabian StyleBailly, Sylviane, Anwar El Mahgubi, Olivier Puel, Sophie Lorber, Jean-Denis Bailly, and Béatrice Orlando. 2025. "Implantation of Aspergillus Section Flavi in French Maize and Consequences on Aflatoxin Contamination of Maize at Harvest: Three-Year Survey" Toxins 17, no. 4: 155. https://doi.org/10.3390/toxins17040155
APA StyleBailly, S., El Mahgubi, A., Puel, O., Lorber, S., Bailly, J.-D., & Orlando, B. (2025). Implantation of Aspergillus Section Flavi in French Maize and Consequences on Aflatoxin Contamination of Maize at Harvest: Three-Year Survey. Toxins, 17(4), 155. https://doi.org/10.3390/toxins17040155






