A Sting Operation: Risk Assessment and Venom Expenditure by Arizona Bark Scorpions (Centruroides sculpturatus) in a Defensive Context
Abstract
1. Introduction
2. Results
2.1. Number of Presentations
2.2. Sting Use and Number of Stings
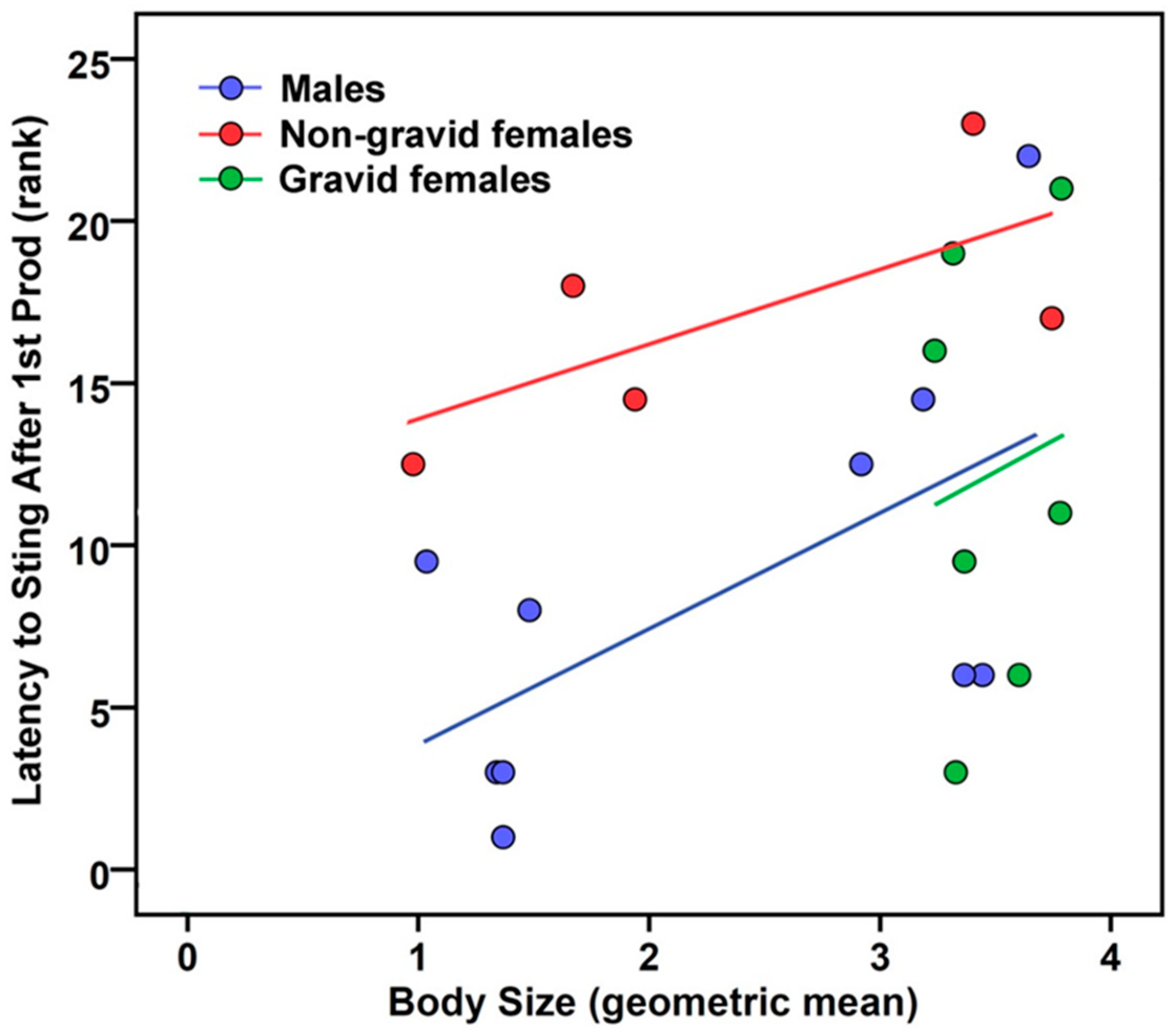
2.3. Latency to Sting After First Prod
2.4. Contact Duration of Stings
2.5. Percentage of Wet Stings
2.6. Mouse Versus Beaker Targets
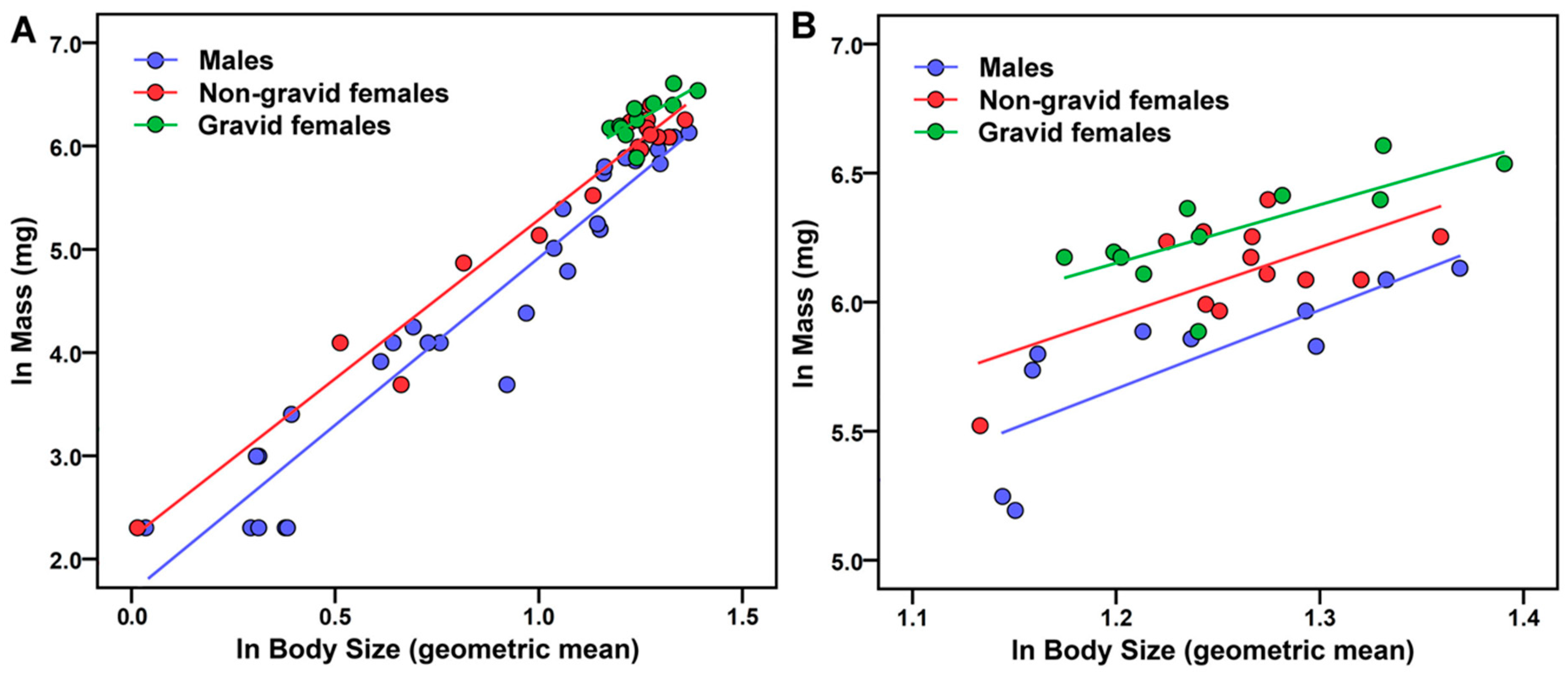
2.7. Relative Mass of Male and Female Scorpions
3. Discussion
3.1. Sex Differences (Hypothesis 1)
3.2. Vertical Versus Horizontal Orientation (Hypothesis 2)
3.3. Body Size Variation (Hypothesis 3)
3.4. Target Differences and Threat Assessment (Hypothesis 4)
3.5. Wet Versus Dry Stings and Venom Metering
3.6. Unaccounted Variation in Environmental Conditions
4. Conclusions
5. Materials and Methods
5.1. Ethics Statement
5.2. Defensive Behavior Trials
5.3. Morphological Measurements
5.4. Behavioral Measurements
5.5. Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviation
| ANCOVA | Analysis of covariance |
References
- Hayes, W.K. Venom metering by juvenile prairie rattlesnakes, Crotalus v. viridis: Effects of prey size and experience. Anim. Behav. 1995, 50, 33–40. [Google Scholar] [CrossRef]
- Hayes, W.K.; Lavin-Murcio, P.A.; Kardong, K.V. Northern Pacific Rattlesnakes meter venom when feeding on prey of different sizes. Copeia 1995, 1995, 337–343. [Google Scholar] [CrossRef]
- Hayes, W.K.; Herbert, S.S.; Rehling, G.C.; Gennaro, J.F. Factors that influence venom expenditure in viperids and other snake species during predatory and defensive contexts. In Biology of the Vipers; Schuett, G.W., Höggren, M., Douglas, M.E., Greene, H.W., Eds.; Eagle Mountain Publ.: Eagle Mountain, UT, USA, 2002; pp. 207–233. [Google Scholar]
- Wigger, E.; Kuhn-Nentwig, L.; Nentwig, W. The venom optimisation hypothesis: A spider injects large venom quantities only into difficult prey types. Toxicon 2002, 40, 749–752. [Google Scholar] [CrossRef] [PubMed]
- Hayes, W.K. The snake venom-metering controversy: Levels of analysis, assumptions, and evidence. In The Biology of Rattlesnakes; Hayes, W.K., Beaman, K.R., Cardwell, M.D., Bush, S.P., Eds.; Loma Linda University Press: Loma Linda, CA, USA, 2008; pp. 191–220. [Google Scholar]
- Morgenstern, D.; King, G.F. The venom optimization hypothesis revisited. Toxicon 2013, 63, 120–128. [Google Scholar] [CrossRef]
- Cooper, A.M.; Nelsen, D.R.; Hayes, W.K. The strategic use of venom by spiders. In Evolution of Venomous Animals and Their Toxins; Gopalakrishnakone, P., Malhotra, A., Eds.; Toxinology series; Springer Science+Business Media: Dordrecht, The Netherlands, 2017; pp. 1–18. [Google Scholar] [CrossRef]
- Evans, E.R.; Northfield, T.D.; Daly, N.L.; Wilson, D.T. Venom costs and optimization in scorpions. Front. Ecol. Evol. 2019, 7, 196. [Google Scholar] [CrossRef]
- Nisani, Z.; Dunbar, S.G.; Hayes, W.K. Cost of venom regeneration in Parabuthus transvaalicus (Arachnida: Buthidae). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2007, 147, 509–513. [Google Scholar] [CrossRef]
- Nisani, Z.; Boskovic, D.S.; Dunbar, S.G.; Kelln, W.; Hayes, W.K. Investigating the chemical profile of regenerated scorpion (Parabuthus transvaalicus) venom in relation to metabolic cost and toxicity. Toxicon 2012, 60, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Hayes, W.K.; Person, C.E.; Fox, G.A.; King, J.L.; Briggs, E.; Gren, E.C. Paradoxical exception to island tameness: Increased defensiveness in an insular population of rattlesnakes. Toxins 2024, 16, 157. [Google Scholar] [CrossRef]
- Nelsen, D.R.; Kelln, W.; Hayes, W.K. Poke but don’t pinch: Risk assessment and venom metering in the western black widow spider, Latrodectus hesperus. Anim. Behav. 2014, 89, 107–114. [Google Scholar] [CrossRef]
- Nisani, Z.; Hayes, W.K. Defensive stinging by Parabuthus transvaalicus scorpions: Risk assessment and venom metering. Anim. Behav. 2011, 81, 627–633. [Google Scholar] [CrossRef]
- Nisani, Z.; Hayes, W.K. Venom-spraying behavior of the scorpion Parabuthus transvaalicus (Arachnida: Buthidae). Behav. Process. 2015, 115, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Van Der Meijden, A.; Coelho, P.; Rasko, M. Variability in venom volume, flow rate and duration in defensive stings of five scorpion species. Toxicon 2015, 100, 60–66. [Google Scholar] [CrossRef]
- Lira, A.F.A.; Santos, A.B.; Silva, N.A.; Martins, R.D. Threat level influences the use of venom in a scorpion species, Tityus stigmurus (Scorpiones, Buthidae). Acta Ethol. 2017, 20, 291–295. [Google Scholar] [CrossRef]
- Marston, L.A. A Comparison of Defensive Stinging Behavior Between Two Sonoran Scorpion Species. Master’s Thesis, Loma Linda University, Loma Linda, CA, USA, 2020. [Google Scholar]
- Laborieux, L. Biomechanics of venom delivery in South America’s first toxungen-spraying scorpion. Zool. J. Linn. Soc. 2024, zlae161. [Google Scholar] [CrossRef]
- El Joud, Y.; El Bouazzaoui, A.; El-Ghali, S.; Laghzaoui, E.M.; Toulon, O.; Larradia, M.A.; Elmourid, A.; Hamdan, Y.A.; Rhazi, M.; Kahime, K.; et al. Assessing the effects of temperature, diet and threat conditions on defensive behaviour and venom regeneration in scorpion (Buthus atlantis). J. Therm. Biol. 2024, 124, 103966. [Google Scholar] [CrossRef]
- Rasko, M.; Coelho, P.; Simone, Y.; van der Meijden, A. How to attack a scorpion: Venom metering during a repeated attack. Anim. Behav. 2018, 145, 125–129. [Google Scholar] [CrossRef]
- Nisani, Z.; Curiel, R. Antipredator responses of Hadrurus arizonensis (Scorpiones: Caraboctonidae) to chemosensory cue from a mammalian predator. J. Arachnol. 2019, 47, 389–391. [Google Scholar] [CrossRef]
- Evans, E.R.J. Venom Chemistry and Ecology of Australian Scorpions. Ph.D. Thesis, James Cook University, Townsville, QLD, Australia, 2022; 164p. [Google Scholar]
- Carlson, B.E.; McGinley, S.; Rowe, M.P. Meek males and fighting females: Sexually-dimorphic antipredator behavior and locomotor performance is explained by morphology in bark scorpions (Centruroides vittatus). PLoS ONE 2014, 9, e97648. [Google Scholar] [CrossRef]
- Carlson, B.E.; Rowe, M.P. Temperature and desiccation effects on the antipredator behavior of Centruroides vittatus (Scorpiones: Buthidae). J. Arachnol. 2009, 37, 321–330. [Google Scholar] [CrossRef]
- Miller, D.W.; Jones, A.D.; Goldston, J.S.; Rowe, M.P.; Rowe, A.H. Sex differences in defensive behavior and venom of the striped bark scorpion Centruroides vittatus (Scorpiones: Buthidae). Integr. Comp. Biol. 2016, 56, 1022–1031. [Google Scholar] [CrossRef]
- Lira, A.F.A.; Almeida, F.M.F.; de Albuquerque, C.M.R. Reaction under the risk of predation: Effects of age and sexual plasticity on defensive behavior in scorpion Tityus pusillus (Scorpiones: Buthidae). J. Ethol. 2020, 38, 13–19. [Google Scholar] [CrossRef]
- de Albuquerque, K.B.C.; de Araujo Lira, A.F. Sex-based defensive behavior influenced by threat level in the scorpion Tityus pusillus (Scorpiones: Buthidae). J. Arachnol. 2021, 49, 402–406. [Google Scholar] [CrossRef]
- Cooper, W.E.; Blumstein, D.T. (Eds.) Escaping from Predators: An Integrative View of Escape Decisions; Cambridge University Press: Cambridge, UK.
- Domenici, P.; Blagburn, J.M.; Bacon, J.P. Animal escapology I: Theoretical issues and emerging trends in escape trajectories. J. Exp. Biol. 2011, 214, 2463–2473. [Google Scholar] [CrossRef] [PubMed]
- Domenici, P.; Blagburn, J.M.; Bacon, J.P. Animal escapology II: Escape trajectory case studies. J. Exp. Biol. 2011, 214, 2474–2494. [Google Scholar] [CrossRef] [PubMed]
- Nelsen, D.R.; David, E.M.; Harty, C.N.; Hector, J.B.; Corbit, A.G. Risk assessment and the effects of refuge availability on the defensive behaviors of the southern unstriped scorpion (Vaejovis carolinianus). Toxins 2020, 12, 534. [Google Scholar] [CrossRef]
- Brown, C.A.; O’ Connell, D.J. Plant climbing behavior in the scorpion Centruroides vittatus. Am. Midl. Nat. 2000, 144, 406–418. [Google Scholar] [CrossRef]
- McReynolds, C.N. The effect of microhabitat use on the foraging and diet of the striped bark scorpion, Centruroides vittatus (Buthidae: Scorpiones) in blackbrush habitat of south Texas. J. Arachnol. 2022, 50, 90–100. [Google Scholar] [CrossRef]
- Humphreys, R.K.; Ruxton, G.D. Dropping to escape: A review of an under-appreciated antipredator defence. Biol. Rev. 2019, 94, 575–589. [Google Scholar] [CrossRef]
- Higham, T.E.; Korchari, P.; McBrayer, L.D. How to climb a tree: Lizards accelerate faster, but pause more, when escaping on vertical surfaces. Biol. J. Linn. Soc. 2011, 102, 83–90. [Google Scholar] [CrossRef]
- Van Der Meijden, A.; Coelho, P.L.; Sousa, P.; Herrel, A. Choose your weapon: Defensive behavior is associated with morphology and performance in scorpions. PLoS ONE 2013, 8, e78955. [Google Scholar] [CrossRef]
- Forde, A.; Jacobsen, A.; Dugon, M.M.; Healy, K. Species with smaller body sizes and narrower chelae have the highest venom potency. Toxins 2022, 14, 219. [Google Scholar] [CrossRef] [PubMed]
- Coelho, P.; Kaliontzopoulou, A.; Rasko, M.; van der Meijden, A. A “striking” relationship: Scorpion defensive behaviour and its relation to morphology and performance. Funct. Ecol. 2017, 31, 1390–1404. [Google Scholar] [CrossRef]
- McReynolds, C.N. Microhabitat preferences for the errant scorpion, Centruroides vittatus (Scorpiones, Buthidae). J. Arachnol. 2008, 36, 557–564. [Google Scholar] [CrossRef]
- Stahnke, H.L. The genus Centruroides (Buthidae) and its venom. In Arthropod Venoms: Handbook of Experimental Pharmacology; Bettini, S., Ed.; Springer: Berlin, Germany, 1978; Volume 48, pp. 277–307. [Google Scholar]
- McReynolds, C.N. Temporal patterns in microhabitat use for the scorpion Centruroides vittatus (Scorpiones: Buthidae). Euscorpius 2004, 17, 35–45. [Google Scholar]
- Fox, G.A. The Design of Complex Weapons Systems in Scorpions: Sexual, Ontogenetic, and Interspecific Variation. Ph.D. Dissertation, Loma Linda University, Loma Linda, CA, USA, 2018. [Google Scholar]
- Yamashita, T.; Rhoads, D.D. Species delimitation and morphological divergence in the scorpion Centruroides vittatus (Say, 1821): Insights from phylogeography. PLoS ONE 2013, 8, e68282. [Google Scholar] [CrossRef]
- McLean, C.J.; Garwood, R.J.; Brassey, C.A. Sexual dimorphism in the arachnid orders. PeerJ 2018, 6, e5751. [Google Scholar] [CrossRef]
- González-Gómez, J.C.; Valenzuela-Rojas, J.C.; García, L.F.; Franco Pérez, L.M.; Guevara, G.; Buitrago, S.; Cubillos, A.; Van Der Meijden, A. Sexual dimorphism in the biomechanical and toxicological performance in prey incapacitation of two morphologically distinct scorpion species (Chactas sp. and Centruroides sp.). Biol. J. Linn. Soc. 2020, 129, 190–198. [Google Scholar] [CrossRef]
- Nelsen, D. Ontogeny of Venom Use and Venom Composition in the Western Widow Spider Latrodectus hesperus. Ph.D. Dissertation, Loma Linda University, Loma Linda, CA, USA, 2013. [Google Scholar]
- Eiben, B.; Persons, M. The effect of prior exposure to predator cues on chemically-mediated defensive behavior and survival in the wolf spider Rabidosa rabida (Araneae: Lycosidae). Behaviour 2007, 144, 889–906. [Google Scholar]
- Greggor, A.L.; Thornton, A.; Clayton, N.S. Neophobia is not only avoidance: Improving neophobia tests by combining cognition and ecology. Curr. Opin. Behav. Sci. 2015, 6, 82–89. [Google Scholar] [CrossRef]
- Crane, A.L.; Ferrari, M.C. Patterns of predator neophobia: A meta-analytic review. Proc. R. Soc. B 2017, 284, 20170583. [Google Scholar] [CrossRef]
- Crane, A.L.; Feyten, L.E.; Preagola, A.A.; Ferrari, M.C.; Brown, G.E. Uncertainty about predation risk: A conceptual review. Biol. Rev. 2024, 99, 238–252. [Google Scholar] [CrossRef] [PubMed]
- Antiqueira, P.A.P.; Vieira, C.; Viana, J.V.D.A.; Romero, G.Q. Floral and insect traits predict predation risk and neophobia impacts on flower-visiting insects’ behavior. Behav. Ecol. Sociobiol. 2025, 79, 1–13. [Google Scholar] [CrossRef]
- Harrison, N.D.; Phillips, B.L.; Hemmi, J.M.; Wayne, A.F.; Steven, R.; Mitchell, N.J. Identifying the most effective behavioural assays and predator cues for quantifying anti-predator responses in mammals: A systematic review protocol. Environ. Evid. 2021, 10, 38. [Google Scholar] [CrossRef]
- Hossie, T.; Landolt, K.; Murray, D.L. Determinants and co-expression of anti-predator responses in amphibian tadpoles: A meta-analysis. Oikos 2017, 126, 173–184. [Google Scholar] [CrossRef]
- Rowe, A.H.; Rowe, M.P. Physiological resistance of grasshopper mice (Onychomys spp.) to Arizona bark scorpion (Centruroides exilicauda) venom. Toxicon 2008, 52, 597–605. [Google Scholar] [CrossRef]
- Nelsen, D.R.; Nisani, Z.; Cooper, A.M.; Fox, G.A.; Gren, E.C.; Corbit, A.G.; Hayes, W.K. Poisons, toxungens, and venoms: Redefining and classifying toxic biological secretions and the organisms that employ them. Biol. Rev. 2014, 89, 450–465. [Google Scholar] [CrossRef]
- Cardoso, F.J.T. Escorpionismo na Amazônia: A Epidemiologia, a Clínica e a Vulnerabilidade aos Acidentes Escorpiônicos em Rurópolis, Pará, Brasil. Ph.D. Dissertation, Universidade de São Paulo, São Paulo, Brazil, 2019. [Google Scholar]
- Alahyane, H.; El-Mansoury, B.; Hakem, A.; Elmourid, A.; Ali, D.A.; El Koutbi, M.; Kaoutar, K.; El Houate, B.; El Hidan, M.A.; Gamrani, H.; et al. Assessment of knowledge about first aid methods, diagnosis, and treatment of scorpion stings among health workers in Ouarzazate region, Morocco: A cross-sectional study. Toxicon 2024, 250, 108085. [Google Scholar] [CrossRef]
- Inceoglu, B.; Lango, J.; Jing, J.; Chen, L.; Doymaz, F.; Pessah, I.N.; Hammock, B.D. One scorpion, two venoms: Prevenom of Parabuthus transvaalicus acts as an alternative type of venom with distinct mechanism of action. Proc. Natl. Acad. Sci. USA 2003, 100, 922–927. [Google Scholar] [CrossRef] [PubMed]
- Gangur, A.N.; Smout, M.; Liddell, M.J.; Seymour, J.E.; Wilson, D.; Northfield, T.D. Changes in predator exposure, but not in diet, induce phenotypic plasticity in scorpion venom. Proc. R. Soc. B 2017, 284, 20171364. [Google Scholar] [CrossRef]
- Polis, G.A. Seasonal patterns and age-specific variation in the surface activity of a population of desert scorpions in relation to environmental factors. J. Anim. Ecol. 1980, 49, 1–18. [Google Scholar] [CrossRef]
- Camp, E.A.; Gaffin, D.D. Escape behavior mediated by negative phototaxis in the scorpion Paruroctonus utahensis (Scorpiones, Vaejovidae). J. Arachnol. 1999, 27, 679–684. [Google Scholar]
- Machan, L. Spectral sensitivity of scorpion eyes and the possible role of shielding pigment effect. J. Exp. Biol. 1968, 49, 95–105. [Google Scholar] [CrossRef]
- Blass, G.R.; Gaffin, D.D. Light wavelength biases of scorpions. Anim. Behav. 2008, 76, 365–373. [Google Scholar] [CrossRef]
- Bradley, R.A. The influence of weather and biotic factors on the behaviour of the scorpion Paruroctonus utahensis. J. Anim. Ecol. 1988, 57, 533–551. [Google Scholar] [CrossRef]
- Nime, M.F.; Casanoves, F.; Vrech, D.E.; Mattoni, C.I. Relationship between environmental variables and surface activity of scorpions in the Arid Chaco ecoregion of Argentina. Invertebr. Biol. 2013, 132, 145–155. [Google Scholar] [CrossRef]
- Nisani, Z.; Cardenas, V.; Cox, J. Thermal ecology and the relationship between temperature and sprint speed in adult females Paruroctonus marksi (Scorpiones: Vaejovidae). J. Arid Environ. 2022, 197, 104675. [Google Scholar] [CrossRef]
- Martin, C.E.; Fox, G.A.; Putman, B.J.; Hayes, W.K. Social security: Can rattlesnakes reduce acute stress through social buffering? Front. Ethol. 2023, 2, 1181774. [Google Scholar] [CrossRef]
- Suarez, A.V.; Tsutsui, N.D.; Holway, D.A.; Case, T.J. Behavioral and genetic differentiation between native and introduced populations of the Argentine ant. Biol. Invas. 1999, 1, 43–53. [Google Scholar] [CrossRef]
- Kralj-Fišer, S.; Gregorič, M. Spider welfare. In The Welfare of Invertebrate Animals. Animal Welfare; Carere, C., Mather, J., Eds.; Springer: Cham, Switzerland, 2019; Volume 18, pp. 105–122. [Google Scholar]
- Underwood, W.; Anthony, R. AVMA Guidelines for the Euthanasia of Animals: 2020 Edition; American Veterinary Medical Association: Schaumburg, IL, USA, 2020. [Google Scholar]
- Stahnke, H.L. UV light, a useful field tool. BioScience 1972, 22, 604–607. [Google Scholar] [CrossRef]
- Gaffin, D.D.; Bumm, L.A.; Taylor, M.S.; Popokina, N.V.; Mann, S. Scorpion fluorescence and reaction to light. Anim. Behav. 2012, 83, 429–436. [Google Scholar] [CrossRef]
- Rowe, A.H.; Rowe, M.P. Risk assessment by grasshopper mice (Onychomys spp.) feeding on neurotoxic prey (Centruroides spp.). Anim. Behav. 2006, 71, 725–734. [Google Scholar] [CrossRef]
- Mosimann, J.E. Size-allometry: Size and shape variables with characterizations of the lognormal and generalized gamma distributions. J. Am. Stat. Assoc. 1970, 65, 930–945. [Google Scholar] [CrossRef]
- Mosimann, J.E.; James, F.C. New statistical methods for allometry with application to Florida red-winged blackbirds. Evolution 1979, 33, 444–459. [Google Scholar] [CrossRef] [PubMed]
- Fox, G.A.; Cooper, A.M.; Hayes, W.K. The dilemma of choosing a reference character for measuring sexual size dimorphism, sexual body component dimorphism, and character scaling: Cryptic dimorphism and allometry in the scorpion Hadrurus arizonensis. PLoS ONE 2015, 10, e0120392. [Google Scholar] [CrossRef]
- Pike, N. Using false discovery rates for multiple comparisons in ecology and evolution. Methods Ecol. Evol. 2011, 2, 278–282. [Google Scholar] [CrossRef]
- Nakagawa, S.A. Farewell to Bonferroni: The problems of low statistical power and publication bias. Behav. Ecol. 2004, 15, 1044–1045. [Google Scholar] [CrossRef]
- Moran, M.D. Arguments for rejecting the sequential Bonferroni in ecological studies. Oikos 2003, 100, 403–405. [Google Scholar] [CrossRef]
- Tabachnick, B.G.; Fidell, L.S. Using Multivariate Statistics, 6th ed.; Pearson/Allyn & Bacon: Boston, MA, USA, 2013. [Google Scholar]
- Field, A. Discovering Statistics Using IBM SPSS Statistics, 4th ed.; Sage: Los Angeles, CA, USA, 2015. [Google Scholar]
- Cohen, J. Statistical Power Analysis for The Behavioral Sciences, 2nd ed.; Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1988. [Google Scholar]


| Dependent Measures and Main Effects a | Experiment 1 Mouse Trials (June–July) | Experiment 2 Beaker Trials (July, September) |
|---|---|---|
| Sting use (%) | ||
| Males | 71.4 (14) | 52.9 (17) |
| Non-gravid females | 83.3 (6) | 75.0 (12) |
| Gravid females | 72.7 (11) | — |
| Horizontal orientation | 71.4 (14) | 38.5 (13) |
| Vertical orientation | 68.4 (19) | 81.3 (16) |
| Main effect of sex | p = 0.709 b | p = 0.090, OR = 0.67–252.90 b |
| Main effect of orientation | p = 0.668, OR = 0.09–4.76 b | p = 0.021, OR = 1.62–314.40 b |
| Main effect of size | p = 0.321, OR = 0.57–5.48 b | p = 0.701, OR = 0.16–3.37 b |
| Number of stings before fleeing | ||
| Males | 1.1 ± 0.2 (14) | 0.9 ± 0.3 (17) |
| Non-gravid females | 1.0 ± 0.3 (6) | 1.8 ± 0.5 (12) |
| Gravid females | 0.9 ± 0.2 (11) | — |
| Horizontal orientation | 0.9 ± 0.2 (14) | 1.0 ± 0.5 (13) |
| Vertical orientation | 1.0 ± 0.2 (19) | 1.6 ± 0.3 (16) |
| Main effect of sex | — | p = 0.136, η2 = 0.09 c |
| Main effect of orientation | — | p = 0.221, η2 = 0.06 c |
| Main effect of size | — | p = 0.975, η2 < 0.01 c |
| Latency to sting after first prod (s) | ||
| Males | 0.21 ± 0.11 (10) | 0.31 ± 0.08 (9) |
| Non-gravid females | 1.77 ± 1.47 (5) | 0.28 ± 0.08 (8) |
| Gravid females | 0.36 ± 0.13 (8) | — |
| Horizontal orientation | 0.98 ± 0.75 (10) | 0.32 ± 0.06 (5) |
| Vertical orientation | 0.31 ± 0.09 (13) | 0.28 ± 0.07 (12) |
| Main effect of sex | p = 0.039, η2 = 0.35 c | p = 0.827, η2 < 0.01 c |
| Main effect of orientation | p = 0.817, η2 < 0.01 c | p = 0.473, η2 = 0.04 c |
| Main effect of size | p = 0.047, η2 = 0.24 b (+) | p = 0.638, η2 = 0.02 c |
| Contact duration of first sting (s) | ||
| Males | 0.17 ± 0.07 (10) | 0.38 ± 0.09 (9) |
| Non-gravid females | 0.20 ± 0.05 (5) | 0.36 ± 0.12 (8) |
| Gravid females | 0.09 ± 0.01 (8) | — |
| Horizontal orientation | 0.14 ± 0.05 (10) | 0.42 ± 0.15 (5) |
| Vertical orientation | 0.15 ± 0.04 (13) | 0.35 ± 0.08 (12) |
| Main effect of sex | p = 0.071, η2 = 0.30 c | p = 0.749, η2 = 0.01 c |
| Main effect of orientation | p = 0.253, η2 = 0.09 c | p = 0.736, η2 = 0.01 c |
| Main effect of size | p = 0.639, η2 = 0.02 c | p = 0.649, η2 = 0.02 c |
| Percentage of wet stings (%) | ||
| Males | — | 87.5 (8) |
| Females | — | 88.9 (9) |
| Horizontal orientation | — | 100.0 (4) |
| Vertical orientation | — | 84.6 (13) |
| Main effect of sex | — | p = 1.000, ϕ = 0.02 d |
| Main effect of orientation | — | p = 1.000, ϕ = 0.20 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marston, L.A.; Fox, G.A.; Hung, K.Y.; Delo, S.J.; Hayes, W.K. A Sting Operation: Risk Assessment and Venom Expenditure by Arizona Bark Scorpions (Centruroides sculpturatus) in a Defensive Context. Toxins 2025, 17, 198. https://doi.org/10.3390/toxins17040198
Marston LA, Fox GA, Hung KY, Delo SJ, Hayes WK. A Sting Operation: Risk Assessment and Venom Expenditure by Arizona Bark Scorpions (Centruroides sculpturatus) in a Defensive Context. Toxins. 2025; 17(4):198. https://doi.org/10.3390/toxins17040198
Chicago/Turabian StyleMarston, Lindsay A., Gerad A. Fox, Kim Y. Hung, Shannon J. Delo, and William K. Hayes. 2025. "A Sting Operation: Risk Assessment and Venom Expenditure by Arizona Bark Scorpions (Centruroides sculpturatus) in a Defensive Context" Toxins 17, no. 4: 198. https://doi.org/10.3390/toxins17040198
APA StyleMarston, L. A., Fox, G. A., Hung, K. Y., Delo, S. J., & Hayes, W. K. (2025). A Sting Operation: Risk Assessment and Venom Expenditure by Arizona Bark Scorpions (Centruroides sculpturatus) in a Defensive Context. Toxins, 17(4), 198. https://doi.org/10.3390/toxins17040198






