Toxin Production by Alternaria alternata in Black Spot Disease of Chrysanthemum morifolium ‘Fubai’: Accumulation of Altenuene and Tenuazonic Acid in Flowers
Abstract
1. Introduction
2. Results
2.1. UHPLC-MS/MS Qualitative Detection
2.2. UPLC Quantitative Detection for ATs
2.3. Concentration of ALT and TeA in Culture Filtrates of Strains
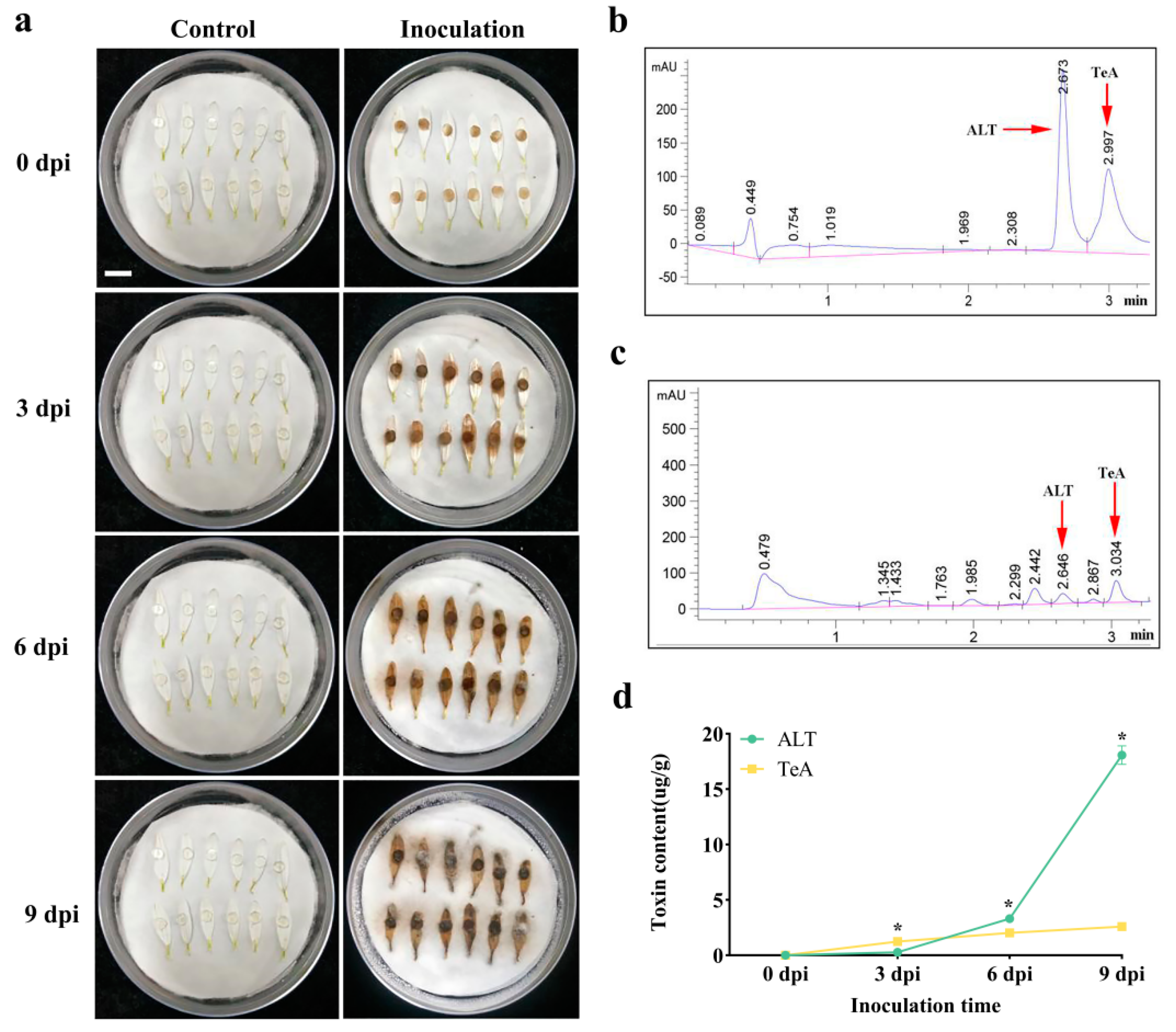
2.4. Concentration of ALT and TeA in Inoculated Chrysanthemum Petals
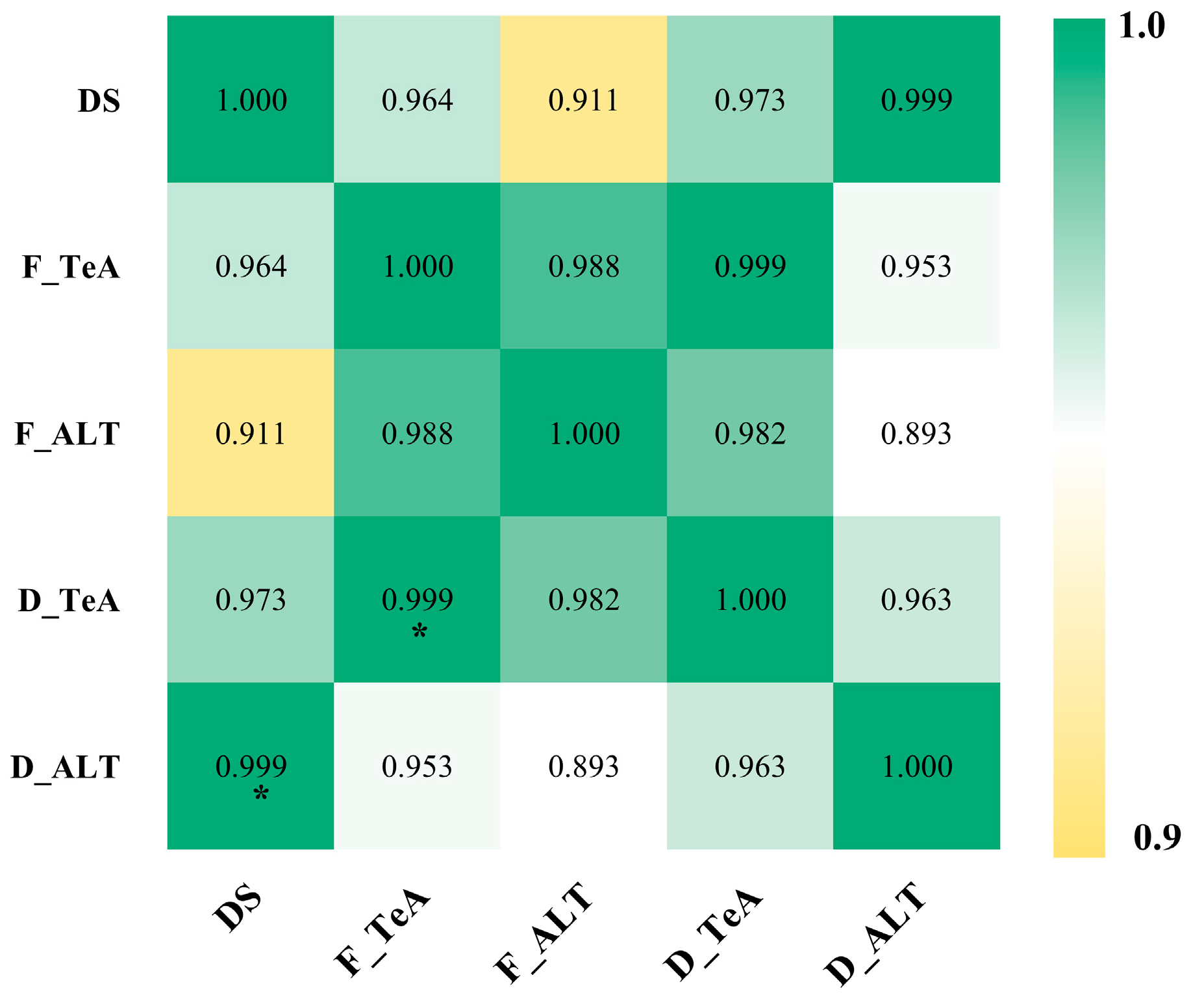
2.5. Analysis of Toxin Contamination in Field-Collected Flower Samples
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Materials and Reagents
5.1.1. Fungal Material
5.1.2. Culture Media and Chemical Reagents
5.2. Instruments
5.2.1. Chromatography Systems
5.2.2. Auxiliary Equipment
5.3. Sample Collection
5.3.1. Fungal Culture Filtrate
5.3.2. Artificially Inoculated Petal Samples
5.3.3. Naturally Infected Field Samples
5.4. Sample Pretreatment
5.4.1. Fungal Culture Filtrate Extraction
5.4.2. Petal Tissue Extraction
5.4.3. Fresh and Dried Flower Extraction
5.5. UPLC-MS/MS Detection Conditions
5.6. UPLC Detection Conditions
5.7. Method Validation
5.8. Data Processing and Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luo, X.J.; Wang, H.Z.; Wang, M.S. Genomic Sequence Data of Alternaria alternata hznu325 Causing Black Leaf Spot on Chrysanthemum morifolium. Plant Dis. 2022, 106, 2506–2510. [Google Scholar] [CrossRef] [PubMed]
- Aparecido, L.; Torsoni, G.B.; de Lima, R.F.; Baratti, A.; Rossi, M.; Dos Santos, A.F.; Peche, P.M. Climate zoning: Identifying suitable regions for the occurrence of Alternaria Brown spot in tangerine trees in Brazil. Pest Manag. Sci. 2024, 80, 1615–1631. [Google Scholar] [CrossRef]
- Liao, K.; He, M.; Fang, W.J.; Liu, H.; Jin, C.; Chen, Y.; Guo, K. First Report of Leaf Spot Caused by Alternaria alternata on Chard (Beta vulgaris var. cicla L.) in China. Plant Dis. 2024, 108, 1. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.J.; Zhang, B.; Gai, Y.P.; Sun, X.P.; Chung, K.R.; Li, H.Y. Cell-wall-degrading enzymes required for virulence in the host selective toxin-producing necrotroph Alternaria alternata of Citrus. Front. Microbiol. 2019, 10, 2514. [Google Scholar] [CrossRef]
- Escrivá, L.; Oueslati, S.; Font, G.; Manyes, L. Alternaria mycotoxins in food and feed: An overview. J. Food Qual. 2017, 2017, 1–20. [Google Scholar] [CrossRef]
- Zwickel, T.; Kahlm, S.M.; Rychlik, M.; Müller, M. Chemotaxonomy of mycotoxigenic small-spored Alternaria fungi—Do Multitoxin Mixtures act as an indicator for species differentiation? Front. Microbiol. 2018, 9, 1368. [Google Scholar] [CrossRef]
- Woudenberg, J.; Seidl, M.F.; Groenewald, J.Z.; de Vries, M.; Stielow, J.B.; Thomma, B.; Crous, P.W. Alternaria section Alternaria: Species, formae speciales or pathotypes? Stud. Mycol. 2015, 82, 1–21. [Google Scholar] [CrossRef]
- Bacha, S.; Li, Y.P.; Nie, J.Y.; Xu, G.F.; Han, L.X.; Farooq, S. Comprehensive review on patulin and Alternaria toxins in fruit and derived products. Front. Plant Sci. 2023, 14, 1139757. [Google Scholar] [CrossRef]
- Moreno, M.; Alonso, I.G.; de Santos, R.M.; Lacarra, T.G. The importance of genus Alternaria in mycotoxins production and human diseases. Nutr. Hosp. 2012, 27, 1772–1781. [Google Scholar]
- Richards, K.; Klaffke, H.; Rychlik, M.; Zwickel, T. Development of a high performance liquid chromatography tandem mass spectrometry based analysis for the simultaneous quantification of various Alternaria toxins in wine, vegetable juices and fruit juices. J. Chromatogr. A 2016, 1455, 74–85. [Google Scholar]
- Nguyen, T.; Kim, J.; Jeon, S.J.; Lee, C.W.; Magan, N.; Lee, H.B. Mycotoxin production of Alternaria strains isolated from Korean barley grains determined by LC-MS/MS. Int. J. Food Microbiol. 2018, 268, 44–52. [Google Scholar]
- Hickert, S.; Bergmann, M.; Ersen, S.; Cramer, B.; Humpf, H.U. Survey of Alternaria toxin contamination in food from the German market, using a rapid HPLC-MS/MS approach. Mycotoxin Res. 2016, 32, 7–18. [Google Scholar] [CrossRef]
- Fraeyman, S.; Devreese, M.; Broekaert, N.; De Mil, T.; Antonissen, G.; De Baere, S.; De Backer, P.; Rychlik, M.; Croubels, S. Quantitative determination of tenuazonic acid in pig and broiler chicken plasma by LC-MS/MS and Its Comparative Toxicokinetics. J. Agric. Food Chem. 2015, 63, 8560–8567. [Google Scholar]
- Rychlik, M.; Lepper, H.; Weidner, C.; Asam, S. Risk evaluation of the Alternaria mycotoxin tenuazonic acid in foods for adults and infants and subsequent risk management. Food Control 2016, 68, 181–185. [Google Scholar]
- Logrieco, A.; Moretti, A.; Solfrizzo, M. Alternaria toxins and plant diseases: An overview of origin, occurrence and risks. World Mycotoxin J. 2009, 2, 129–140. [Google Scholar]
- Saleh, I.; Zeidan, R.; Abu-Dieyeh, M. The characteristics, occurrence, and toxicological effects of alternariol: A mycotoxin. Arch. Toxicol. 2024, 98, 1659–1683. [Google Scholar]
- Chen, S.G.; Qiang, S. Recent advances in tenuazonic acid as a potential herbicide. Pestic. Biochem. Physiol. 2017, 143, 252–257. [Google Scholar]
- Hou, S.L.; Zhang, Y.M.; Wang, X.; Ma, J.J.; Cheng, Y.Q.; Wang, M.; Wang, Z.F.; Wang, H.G.; Sun, J.H.; Zhang, W.Y.; et al. Establishment of an immunolateral chromatographic quantitative method for the detection of alternariol based on quantum dot fluorescent nanospheres in fruit. LWT-Food Sci. Technol. 2024, 203, 116353. [Google Scholar] [CrossRef]
- Jassim, N.S.; Ahmed, A.N. The isolation and molecular identification of the main fungus caused leaf spots on date palms (Phoenix dactylifera L.). Arch. Phytopathol. Plant Prot. 2024, 57, 542–554. [Google Scholar]
- Schmey, T.; Tominello-Ramirez, C.S.; Brune, C.; Stam, R. Alternaria diseases on potato and tomato. Mol. Plant Pathol. 2024, 25, e13435. [Google Scholar]
- Zhang, Q.; Branca, F.; Li, N.; Liu, N.; Ding, Y.H. Identification of Black Spot Resistance in Broccoli (Brassica oleracea L. var. italica) Germplasm Resources. Appl. Sci. 2024, 14, 2883. [Google Scholar] [CrossRef]
- Haro, M.; Cabrera, G.; Pinto, V.F.; Patriarca, A. Alternaria toxins in tomato products from the Argentinean market. Food Control 2023, 147, 109607. [Google Scholar]
- Ji, X.F.; He, Y.Y.; Xiao, Y.P.; Liang, Y.; Lyu, W.; Yang, H. The fate of Alternaria toxin tenuazonic acid (TeA) during the processing chain of wheat flour products and risk control strategies for mycotoxins. Food Res. Int. 2024, 194, 114941. [Google Scholar] [CrossRef] [PubMed]
- Mihalache, O.A.; Torrijos, R.; Dall’Asta, C. Occurrence of mycotoxins in meat alternatives: Dietary exposure, potential health risks, and burden of disease. Environ. Int. 2024, 185, 108537. [Google Scholar]
- Lin, H.L.; Ni, L.; Chen, H.F.; Xu, W.S. A simple and versatile strategy for sensitive SIDA-UHPLC-MS/MS analysis of Alternaria toxins in olive oil. Anal. Chim. Acta 2022, 1232, 340451. [Google Scholar]
- Arcella, D.; Eskola, M.; Ruiz, J.A.G. Dietary exposure assessment to Alternaria toxins in the European population, EFSA report. EFSA J. 2016, 14, e04654. [Google Scholar]
- Zhou, J.; Xu, J.J.; Cai, Z.X.; Huang, B.F.; Jin, M.C.; Ren, Y.P. Simultaneous determination of five Alternaria toxins in cereals using QuEChERS-based methodology. J. Chromatogr. B 2017, 1068–1069, 15–23. [Google Scholar]
- Fernandes, C.; Casadevall, A.; Gonçalves, T. Mechanisms of Alternaria pathogenesis in animals and plants. Fems Microbiol. Rev. 2023, 47, fuad061. [Google Scholar]
- Mao, X.; Chen, A.Q.; Qu, J.Y.; Luo, P.J.; You, Y.L.; Gao, Y.L.; Dong, F.G.; Wu, Y.N.; Li, Y.S. New insights into in mycotoxins production in Alternaria infected apple during postharvest storage. Postharvest Biol. Technol. 2023, 198, 112238. [Google Scholar] [CrossRef]
- Ali, S.; Tyagi, A.; Rajarammohan, S.; Mir, Z.A.; Bae, H. Revisiting Alternaria-host interactions: New insights on its pathogenesis, defense mechanisms and control strategies. Sci. Hortic. 2023, 322, 112424. [Google Scholar] [CrossRef]
- Chen, H.; Liu, J.; Hu, L.; Yang, J.; Wang, Y.; Sun, W.; Wang, R.; Ding, G.; Li, Y. Mycotoxins from Alternaria Panax, the specific plant pathogen of Panax ginseng. Mycology-An Int. J. 0n Fungal Biol. 2023, 14, 381–392. [Google Scholar]
- Zhang, K.; Zhao, S.; Chen, H.; Ni, J.; Jiang, X.; Chen, F.; Fang, W. Effects of crude toxin from Alternaria alternata on the growth and physiological metabolism of chrysanthemum ’Jinba’ seedlings. Yingyong Shengtai Xuebao 2018, 29, 1190–1196. [Google Scholar]
- Liu, L.N.; Liu, Y.; Li, Y.Y.; Chen, F.D.; Chen, S.M.; Guan, Z.Y. Resistance identification to black spot disease of 38 cultivars of chrysanthemum for tea at seedling stage. J. Nanjing Agric. Univ. 2021, 44, 68–77. [Google Scholar]
- Shi, J.L.; Wang, H.; Li, M.; Mi, L.R.; Gao, Y.Z.; Qiang, S.; Zhang, Y.; Chen, D.; Dai, X.B.; Ma, H.Y.; et al. Alternaria TeA toxin activates a chloroplast retrograde signaling pathway to facilitate JA-dependent pathogenicity. Plant Commun. 2024, 5, 100775. [Google Scholar] [CrossRef]
- Shi, J.L.; Zhang, M.; Gao, L.W.; Yang, Q.; Kalaji, H.M.; Qiang, S.; Strasser, R.J.; Chen, S.G. Tenuazonic acid-triggered cell death Is the essential prerequisite for Alternaria alternata (Fr.) Keissler to infect successfully host Ageratina adenophora. Cells 2021, 10, 1010. [Google Scholar] [CrossRef]
- Zhou, B.; Wang, H.; Meng, B.; Wei, R.; Wang, L.; An, C.F.; Chen, S.G.; Yang, C.L.; Qiang, S. An evaluation of tenuazonic acid, a potential biobased herbicide in cotton. Pest Manag. Sci. 2019, 75, 2482–2489. [Google Scholar]
- Mi, L.R.; Liu, H.; Zhang, J.; Guo, Y.J.; Shi, J.L.; Lu, Y.P.; Cheng, J.; Wang, H.; Cheng, D.; Valverde, B.E.; et al. Low-temperature-induced singlet oxygen adaptation decreases susceptibility to the mycotoxin TeA in invasive plant Ageratina adenophora. Plant Physiol. Biochem. 2025, 220, 109508. [Google Scholar]
- Peach, J.T.; Puntscher, H.; Höger, H.; Marko, D.; Warth, B. Rats exposed to Alternaria toxins in vivo exhibit altered liver activity highlighted by disruptions in riboflavin and acylcarnitine metabolism. Arch. Toxicol. 2024, 98, 3477–3489. [Google Scholar] [CrossRef]
- Visintin, L.; Lu, E.H.; Lin, H.C.; Bader, Y.; Nguyen, T.N.; Michailidis, T.M.; De Saeger, S.; Chiu, W.A.; De Boevre, M. Derivation of human toxicokinetic parameters and internal threshold of toxicological concern for tenuazonic acid through a human intervention trial and hierarchical Bayesian population modeling. J. Expo. Sci. Environ. Epidemiol. 2025; Online ahead of print. [Google Scholar]
- Wang, C.; Fan, Y.Y.; He, W.Z.; Hu, D.Q.; Wu, A.B.; Wu, W.L. Development and application of a QuEChERS-Based liquid chromatography tandem mass spectrometry method to quantitate multi-component Alternaria toxins in Jujube. Toxins 2018, 10, 382. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Liu, C.; van der Fels-klerx, H.J. Occurrence, toxicity, dietary exposure, and management of Alternaria mycotoxins in food and feed: A systematic literature review. Compr. Rev. Food Sci. Food Saf. 2025, 24, e70085. [Google Scholar] [CrossRef]
- Pavicich, M.A.; De Boevre, M.; Vidal, A.; Mikula, H.; Warth, B.; Marko, D.; De Saeger, S.; Patriarca, A. Natural Occurrence, Exposure Assessment & Risk Characterization of Alternaria Mycotoxins in Apple By-Products in Argentina. Expo. Health 2024, 16, 149–158. [Google Scholar]
- Ji, X.F.; Deng, T.; Xiao, Y.P.; Jin, C.H.; Lyu, W.; Wu, Z.; Wang, W.; Wang, X.L.; He, Q.F.; Yang, H. Evaluation of Alternaria toxins in fruits, vegetables and their derivatives marketed in China using a QuEChERS method coupled with ultra-high performance liquid chromatography-tandem mass spectrometry: Analytical methods and occurrence. Food Control 2023, 147, 109563. [Google Scholar]
- Zhao, X.S.; Liu, D.; Yang, X.Q.; Zhang, L.; Yang, M.H. Detection of seven Alternaria toxins in edible and medicinal herbs using ultra-high performance liquid chromatography-tandem mass spectrometry. Food Chem. X 2022, 13, 100186. [Google Scholar] [CrossRef] [PubMed]
- Noser, J.; Schneider, P.; Rother, M.; Schmutz, H. Determination of six Alternaria toxins with UPLC-MS/MS and their occurrence in tomatoes and tomato products from the Swiss market. Mycotoxin Res. 2011, 27, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Estiarte, N.; Crespo-Sempere, A.; Marín, S.; Sanchis, V.; Ramos, A.J. Effect of 1-methylcyclopropene on the development of black mold disease and its potential effect on alternariol and alternariol monomethyl ether biosynthesis on tomatoes infected with Alternaria alternata. Int. J. Food Microbiol. 2016, 236, 74–82. [Google Scholar] [CrossRef] [PubMed]
- SANTE/11945/2015; Guidance Document on Analytical Quality Control and Method Validation Procedures for Pesticides Residues Analysis in Food and Feed. European Commission: Brussels, Belgium, 2015.



| Component | Linear Equation | R2 | Linear Range (μg/mL) | LODs (μg/mL) | LOQs (μg/mL) |
|---|---|---|---|---|---|
| ALT | y = 47.722x − 8.022 | 0.997 | 0.1–500 | 0.023 | 0.077 |
| TeA | y = 58.055x − 826.750 | 0.996 | 0.1–500 | 0.026 | 0.086 |
| Component | Spiked (μg/mL) | Average Recovery (%) | RSD (%) |
|---|---|---|---|
| ALT | 0.1 | 85.65 | 4.3 |
| 1.0 | 91.37 | 5.7 | |
| 10.0 | 95.83 | 3.9 | |
| TeA | 0.1 | 88.25 | 5.0 |
| 1.0 | 93.41 | 4.5 | |
| 10.0 | 96.78 | 3.2 |
| Component | Linear Equation | Correlation Coefficient (R2) | Linear Range (μg·g−1) | LODs (μg·g−1) | LOQs (μg·g−1) |
|---|---|---|---|---|---|
| ALT | y = 112.07x + 94.335 | 0.999 | 0.05–100 | 0.021 | 0.172 |
| TeA | y = 197.610x − 149.550 | 0.999 | 0.05–100 | 0.816 | 0.915 |
| Type | Infection Severity | TeA | ALT | ||||
|---|---|---|---|---|---|---|---|
| Detection Rate (%) | Content Range | Average Value (μg.kg−1) | Detection Rate (%) | Content Range | Average Value (μg.kg−1) | ||
| Fresh samples | Healthy | ND | 0 | 0 | ND | 0 | |
| Mildly infected | 100 | 0.19~0.62 | 0.32 ± 0.18 | 80 | 0~4.91 | 1.86 ± 2.23 | |
| Severely infected | 100 | 0.9~1.88 | 1.22 ± 0.38 | 100 | 7.89~24.89 | 17.15 ± 7.18 | |
| Dried samples | Healthy | ND | 0 | 0 | ND | 0 | |
| Mildly infected | 80 | 0.5~3.57 | 1.95 ± 1.17 | 100 | 22.1~77.62 | 51.31 ± 22.60 | |
| Severely infected | 100 | 4.58~9.04 | 6.16 ± 1.85 | 100 | 80.07~105.5 | 95.79 ± 10.30 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhan, Q.; Liu, L.; Li, W.; Lu, J.; Jiang, J.; Chen, F.; Liu, Y.; Guan, Z. Toxin Production by Alternaria alternata in Black Spot Disease of Chrysanthemum morifolium ‘Fubai’: Accumulation of Altenuene and Tenuazonic Acid in Flowers. Toxins 2025, 17, 181. https://doi.org/10.3390/toxins17040181
Zhan Q, Liu L, Li W, Lu J, Jiang J, Chen F, Liu Y, Guan Z. Toxin Production by Alternaria alternata in Black Spot Disease of Chrysanthemum morifolium ‘Fubai’: Accumulation of Altenuene and Tenuazonic Acid in Flowers. Toxins. 2025; 17(4):181. https://doi.org/10.3390/toxins17040181
Chicago/Turabian StyleZhan, Qingling, Lina Liu, Wenjie Li, Jingshan Lu, Jiafu Jiang, Fadi Chen, Ye Liu, and Zhiyong Guan. 2025. "Toxin Production by Alternaria alternata in Black Spot Disease of Chrysanthemum morifolium ‘Fubai’: Accumulation of Altenuene and Tenuazonic Acid in Flowers" Toxins 17, no. 4: 181. https://doi.org/10.3390/toxins17040181
APA StyleZhan, Q., Liu, L., Li, W., Lu, J., Jiang, J., Chen, F., Liu, Y., & Guan, Z. (2025). Toxin Production by Alternaria alternata in Black Spot Disease of Chrysanthemum morifolium ‘Fubai’: Accumulation of Altenuene and Tenuazonic Acid in Flowers. Toxins, 17(4), 181. https://doi.org/10.3390/toxins17040181









