Cytotoxic Profiles of Beauvericin, Citrinin, Moniliformin, and Patulin and Their Binary Combinations: A Literature-Based Comparison and Experimental Validation in SH-SY5Y Cells
Abstract
1. Introduction
| Mycotoxin | Cell Line | Dose and Exposure Time | Results | Ref. |
|---|---|---|---|---|
| BEA | CHO-K1 | 0–20 μM 24 h, 48 h, and 72 h | Reduction from 96% to 0% (p ≤ 0.05) in cell proliferation in a time-dependent and concentration-dependent manner. | [11] |
| 0–20 μM 24 h, 48 h, and 72 h | Reduction in cell viability in a time-dependent and concentration-dependent manner. The most significant reduction occurred from 2.5 μM and 0% cell viability is reached (20 μM). IC50 (24 h): 10.7 ± 3.7 μM. IC50 (48 h): 2.5 ± 3.3 μM. IC50 (72 h): 2.2 ± 3.3 μM. | [56] | ||
| 1–100 μM 24 h | IC50 (24 h): 12.08 ± 1.10 μM. | [50] | ||
| Jurkat T-cells | 0–15 μM 24 h, 48 h, and 72 h | Reduction in cell viability in a time-dependent and concentration-dependent manner, reaching values of 50% (after 24 h), 45% (after 48 h), and 25% (after 72 h). IC50 (24 h): 7.5 ± 0.4 μM. IC50 (48 h): 5.0 ± 0.3 μM. IC50 (72 h): 3.0 ± 0.6 μM. | [51] | |
| RGA cells | 0–10 μM 48 h | No cytotoxicity was detected in any of the exposed RGA cell lines, with cell viability consistently maintained between 85% and 100%. However, a significant decrease in cell viability (p ≤ 0.001) was observed at a concentration of 10 μM BEA. | [57] | |
| HepG2 | 0–25 μM 24 h, 48 h, and 72 h | There was a 1.04-fold increase in cell viability after 48 h and 72 h of exposure to 0.9 μM BEA. This increase was mitigated, most notably, from 8.3 μM (p ≤ 0.001). From this concentration, cell viability is less than 50% in all cases. | [58] | |
| IPEC-J2 | 0–10 μM 72 h | Cell viability was not significantly reduced for any of the concentrations used. The percentage of cell viability was maintained, in all cases, between 100% and 80%. | [59] | |
| CTN | SH-SY5Y | 15–500 μM 24 h | There was a 95% decrease in cell viability with 500 μM (23.27 times lower than the control). IC50: 250.90 μM. | [5] |
| 2-50 μM 72 h | An amount of 50 μM results in a 0.82 relative viability (1.2 times lower than the control). Lower concentrations (2–20 μM) were not significant. | [26] | ||
| 0–50 μM 24 h | The higher concentration of CTN used (50 μM) managed to reduce the percentage of cell viability to a value of 70–75%. An amount of 38.75 μM reduced cell viability by 20% compared to control. | [24] | ||
| 10–80 μM 72 h | For the maximum concentrations of CTN used, cell viability was considerably reduced, reaching values of 20%. | [32] | ||
| HEK293 | 20–120 μM 24 h, 48 h, and 72 h | CTN caused a reduction in concentration and was time-dependent. At 80 μM, viability dropped to 54% compared to control (72 h). | [38] | |
| BoMacs | 2.5–320 μM 48 h | IC50: 91.20 μM and IC25: 52.72 μM. | [60] | |
| HCT 116 | 150 μM 24 h | CTN caused a reduction in cell viability (p < 0.05), decreasing it to 40%, which is 2.5 times lower than the control. | [61] | |
| HepG2 | 0–300 μM 24 h | Decrease in viability depending on the dose (24 h) compared to the control. CTN 50 μM: 25% reduction. CTN 200 μM: 56% reduction. CTN 300 μM: 80% reduction. The study established that the changes detected were significant (p < 0.05) in all the concentrations studied, except for CTN 100 μM. | [48] | |
| FHS 74 Int. | 0–20 μg/mL 72 h | CTN did not induce sufficient cytotoxicity and the IC50 value could not be determined. | [52] | |
| TM4 Sertoli | 25–200 μM 6 h, 12 h, 24 h, 48 h, and 72 h | After 24 h of exposure, cell viability decreased depending on concentration. The results were significant from 50 μM. From this concentration, the reduction in cell viability occurs in a percentage ranging from 25% to 65% (200 μM) (p < 0.001). | [62] | |
| MON | Caco-2 | 0.2–100 μM 48 h | The maximum concentration tested (100 μM) showed less than 30% inhibition of cell proliferation compared to the control. | [63] |
| FHS 74 Int. | 0–20 μg/mL 72 h | MON did not induce sufficient cytotoxicity and the IC50 value could not be determined. | [52] | |
| IPEC-J1 | 0–10 μM 72 h | Exposure to MON did not affect cell viability in any case during the 72 h period. | [59] | |
| THP-1 | 0.001–10 μM 48 h | The maximum concentration used (10 μM) of MON showed a 30% reduction in cell viability (p < 0.05). | [64] | |
| HepaRS | 0.001–10 μM 48 h | Only a 10% reduction in cell viability was achieved with MON 10 μM (p < 0.05). | [65] | |
| PAT | AML-12 | 4 μM 36 h | PAT 4 μM reduced viability by 25% compared to the control (1.33 times lower cell viability than the control). | [66] |
| DBTRG-05MG | 10–60 μM 24 h | PAT induced a concentration-dependent decrease, with cell viability being 20 times lower at 60 μM (p < 0.05). | [41] | |
| HEK293 | 2.5–15 μM 8 h | There was a decrease in cell viability in a dose-dependent manner. Maximum of 63% reduction with PAT 15 μM (p < 0.005). | [49] | |
| −2 μM 24 h, 48 h, and 72 h | PAT showed significant effects from 1 μM (2.2 times lower than the control) after 24 h. PAT 1 μM and 2 μM showed 1.72 and 4.16 times less viability than the control, respectively, after 48 h. After 72 h, these were 2.2 times lower and 10 times lower than the control with concentrations of 1 μM and 2 μM, respectively. | [38] | ||
| V79 | 0.35–1.55 μM 24 h | Only from 1 μM was there a significant reduction in viability. Regarding control, 0.35 μM PAT showed 1.17 times less viability; 0.95 μM showed 1.21 times less viability; 1.55 μM showed 5 times less viability. | [67] | |
| CHO-K1 | 0–6.25 μM 24 h, 48 h, and 72 h | Significant reduction in cell viability at higher exposure concentrations (p ≤ 0.05). An amount of 3.125 μM showed 45% cell viability and 6.25 μM showed 16% cell viability. | [11] | |
| 0.2–25 μM 24 h | The IC50 values for PAT were 0.69 ± 0.03 μM, respectively. PAT inhibited the growth of CHO-K1 cells more strongly than BEA. | [50] | ||
| BoMacs | 0.0038–4.8 μM 48 h | Cytotoxicity was significant at concentrations greater than 2.4 μM for PAT. IC50: 0.56 μM and IC25: 0.32 μM. | [60] | |
| HepG2 | 1–100 μM 48 h | Reduction in metabolic activity in a dose-dependent manner. IC50: 8.43 μM. After reaching this value, there was no dose-dependent decrease in metabolic activity and the cells were viable, albeit at low levels. | [37] | |
| 0–30 μM 24 h | Dose-dependent relationship between PAT concentration and cytotoxic effect. | [68] | ||
| NRK52E | 0–5000 nM 24 h | With PAT 50 and 100 nM, viability increased by about 20–30% with respect to the control value, since up to 500 nM there was no significant loss of cell viability. These results suggest that a low concentration of PAT may increase cell proliferation; however, higher concentrations (1–5 μM) of PAT significantly caused cytotoxicity (p < 0.001). | [69] | |
| BEA + PAT | CHO-K1 | BEA: 0.156–1.25 μM PAT: 0.049–0.39 μM BEA + PAT [3.2:1] 24 h, 48 h, and 72 h | The combination is equally cytotoxic when they are tested separately. In addition, at none of the exposure times was cell viability less than 75%. | [11] |
| CTN + PAT | BoMacs | IC25 | It did not cause a significant effect on the percentage of cell viability. | [60] |
2. Results
2.1. State of the Art of Cytotoxicity Assays with BEA, CTN, MON, and PAT
2.2. Cytotoxicity Assay of the Individual Treatment of BEA, CTN, MON, and PAT in the SH-SY5Y Cell Line
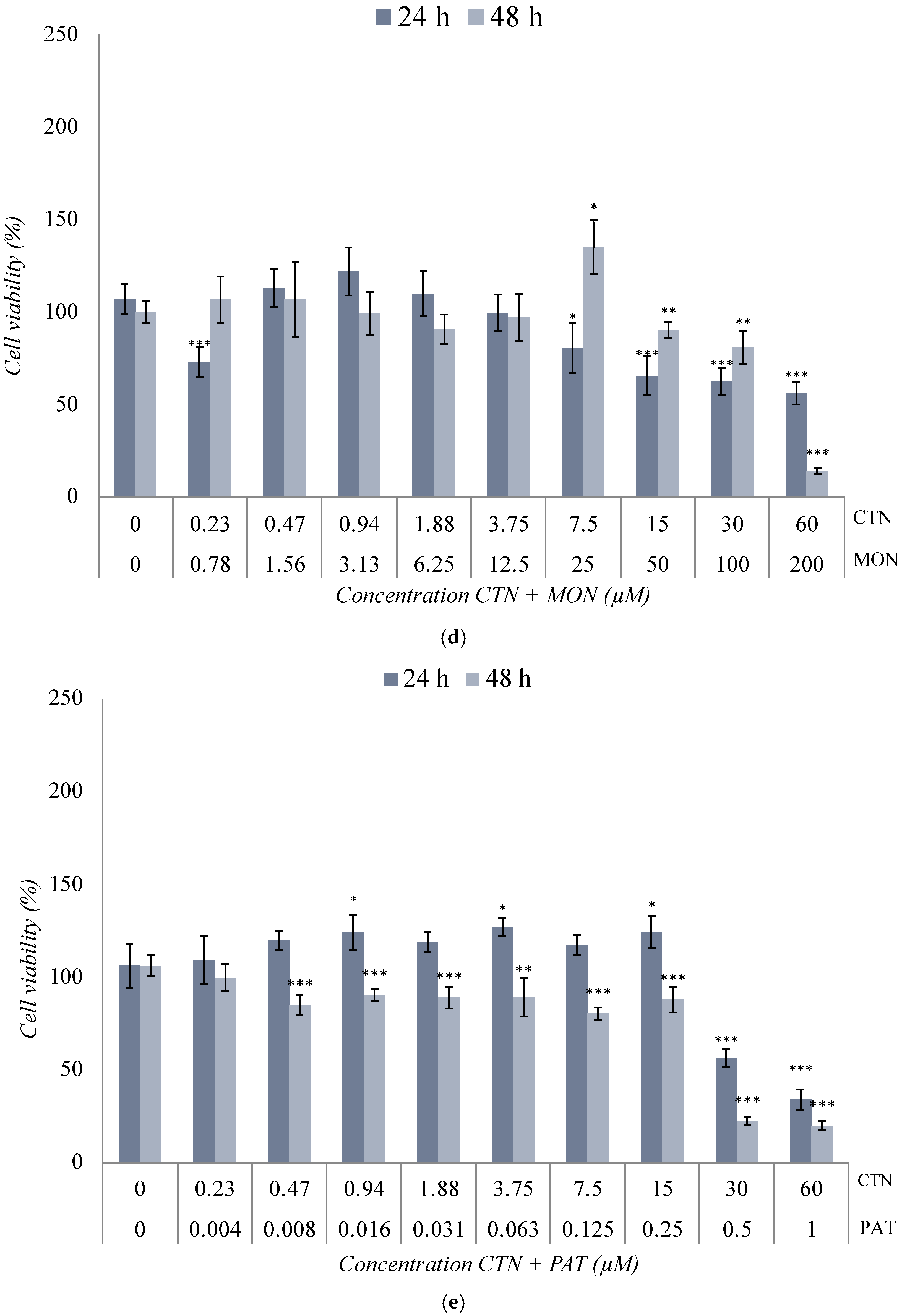
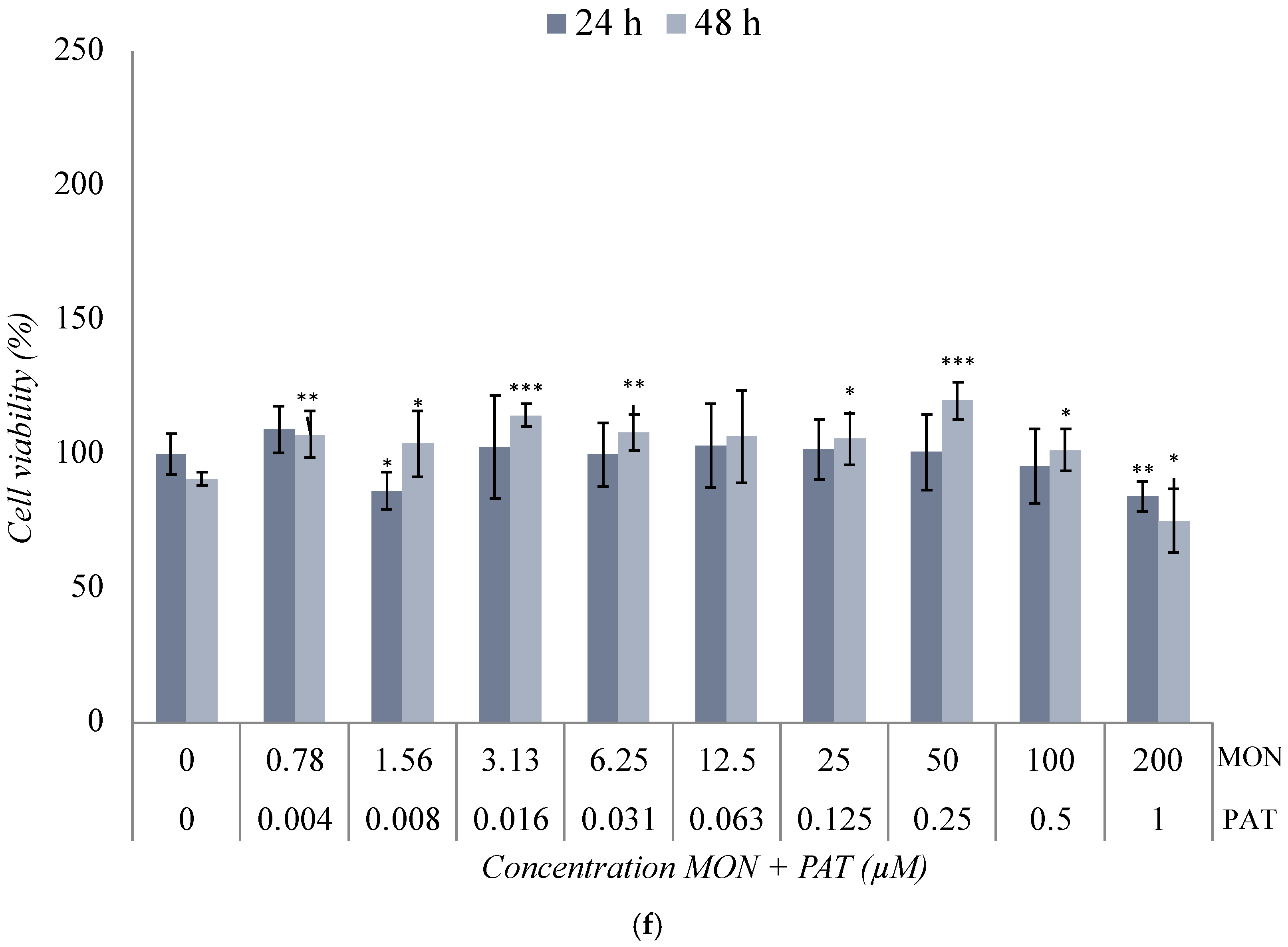
2.3. Cytotoxicity Assay of the Binary Co-Exposure Treatment of BEA, CTN, MON, and PAT in the SH-SY5Y Cell Line
3. Discussion
3.1. Cytotoxicity Assays of Individual Treatment with BEA, CTN, MON, and PAT
3.2. Cytotoxicity Assays of the Binary Co-Exposure Treatment of BEA, CTN, MON, and PAT
4. Conclusions
5. Materials and Methods
5.1. Chemical Reagents
5.2. Cell Culture
5.3. Mycotoxin Exposure
5.4. MTT Assay
5.5. Statical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Malir, F.; Pickova, D.; Toman, J.; Grosse, Y.; Ostry, V. Hazard Characterisation for Significant Mycotoxins in Food. Mycotoxin Res. 2023, 39, 81–93. [Google Scholar] [CrossRef]
- Ali, S.; Freire, L.G.D.; Rezende, V.T.; Noman, M.; Ullah, S.; Abdullah; Badshah, G.; Afridi, M.S.; Tonin, F.G.; de Oliveira, C.A.F. Occurrence of Mycotoxins in Foods: Unraveling the Knowledge Gaps on Their Persistence in Food Production Systems. Foods 2023, 12, 4314. [Google Scholar] [CrossRef]
- Gruber-Dorninger, C.; Novak, B.; Nagl, V.; Berthiller, F. Emerging Mycotoxins: Beyond Traditionally Determined Food Contaminants. J. Agric. Food Chem. 2017, 65, 7052–7070. [Google Scholar] [CrossRef] [PubMed]
- Prosperini, A.; Juan-García, A.; Font, G.; Ruiz, M.J. Beauvericin-Induced Cytotoxicity via ROS Production and Mitochondrial Damage in Caco-2 Cells. Toxicol. Lett. 2013, 222, 204–211. [Google Scholar] [CrossRef]
- Abudayyak, M.; Karaman, E.F.; Ozden, S. Mechanisms Underlying Citrinin-Induced Toxicity via Oxidative Stress and Apoptosis-Mediated by Mitochondrial-Dependent Pathway in SH-SY5Y Cells. Drug Chem. Toxicol. 2023, 46, 944–954. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.W.; Klich, M. Mycotoxins. Clin. Microbiol. Rev. 2003, 16, 497–516. [Google Scholar] [CrossRef] [PubMed]
- Zhong, L.; Carere, J.; Lu, Z.; Lu, F.; Zhou, T. Toxins Patulin in Apples and Apple-Based Food Products: The Burdens and the Mitigation Strategies. Toxins 2018, 10, 475. [Google Scholar] [CrossRef]
- Karsauliya, K.; Yahavi, C.; Pandey, A.; Bhateria, M.; Sonker, A.K.; Pandey, H.; Sharma, M.; Singh, S.P. Co-Occurrence of Mycotoxins: A Review on Bioanalytical Methods for Simultaneous Analysis in Human Biological Samples, Mixture Toxicity and Risk Assessment Strategies. Toxicon 2022, 218, 25–39. [Google Scholar] [CrossRef]
- Smith, M.C.; Madec, S.; Coton, E.; Hymery, N. Natural Co-Occurrence of Mycotoxins in Foods and Feeds and Their in Vitro Combined Toxicological Effects. Toxins 2016, 8, 94. [Google Scholar] [CrossRef]
- Stanciu, O.; Juan, C.; Miere, D.; Loghin, F.; Mañes, J. Occurrence and Co-Occurrence of Fusarium Mycotoxins in Wheat Grains and Wheat Flour from Romania. Food Control 2017, 73, 147–155. [Google Scholar] [CrossRef]
- Zouaoui, N.; Mallebrera, B.; Berrada, H.; Abid-Essefi, S.; Bacha, H.; Ruiz, M.J. Cytotoxic Effects Induced by Patulin, Sterigmatocystin and Beauvericin on CHO–K1 Cells. Food Chem. Toxicol. 2016, 89, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Jestoi, M.; Rokka, M.; Yli-Mattila, T.; Parikka, P.; Rizzo, A.; Peltonen, K.; Jestoi, M.; Rokka, M.; Yli-Mattila, T.; Parikka, P.; et al. Presence and Concentrations of the Fusarium-Related Mycotoxins Beauvericin, Enniatins and Moniliformin in Finnish Grain Samples. Food Addit. Contam. 2004, 21, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Juan, C.; Mañes, J.; Juan-García, A.; Moltó, J.C.; Juan, C.; Mañes, J.; Juan-García, A.; Moltó, J.C. Multimycotoxin Analysis in Oat, Rice, Almond and Soy Beverages by Liquid Chromatography-Tandem Mass Spectrometry. Appl. Sci. 2022, 12, 3942. [Google Scholar] [CrossRef]
- Juan, C.; Ritieni, A.; Mañes, J. Occurrence of Fusarium Mycotoxins in Italian Cereal and Cereal Products from Organic Farming. Food Chem. 2013, 141, 1747–1755. [Google Scholar] [CrossRef]
- Juan, C.; Covarelli, L.; Beccari, G.; Colasante, V.; Mañes, J. Simultaneous Analysis of Twenty-Six Mycotoxins in Durum Wheat Grain from Italy. Food Control 2016, 62, 322–329. [Google Scholar] [CrossRef]
- Kostecki, M.; Szczęsna, J.; Chełkowski, J.; Wiśniewska, H. Beauvericin and Moniliformin Production by Polish Isolates of Fusarium Subglutinans and Natural Coocurrence of Both Mycotoxins in Maize Samples. Environ. Sci. Agric. Food Sci. 1995, 13, 1. [Google Scholar]
- Stanciu, O.; Juan, C.; Miere, D.; Loghin, F.; Mañes, J. Presence of Enniatins and Beauvericin in Romanian Wheat Samples: From Raw Material to Products for Direct Human Consumption. Toxins 2017, 9, 189. [Google Scholar] [CrossRef]
- Ostry, V.; Malir, F.; Cumova, M.; Kyrova, V.; Toman, J.; Grosse, Y.; Pospichalova, M.; Ruprich, J. Investigation of Patulin and Citrinin in Grape Must and Wine from Grapes Naturally Contaminated by Strains of Penicillium Expansum. Food Chem. Toxicol. 2018, 118, 805–811. [Google Scholar] [CrossRef]
- Agahi, F.; Juan, C.; Font, G.; Juan-García, A. In Silico Methods for Metabolomic and Toxicity Prediction of Zearalenone, α-Zearalenone and β-Zearalenone. Food Chem. Toxicol. 2020, 146, 111818. [Google Scholar] [CrossRef]
- Agahi, F.; Juan-García, A.; Font, G.; Juan, C. Study of Enzymatic Activity in Human Neuroblastoma Cells SH-SY5Y Exposed to Zearalenone’s Derivates and Beauvericin. Food Chem. Toxicol. 2021, 152, 112227. [Google Scholar] [CrossRef]
- Agahi, F.; Font, G.; Juan, C.; Juan-García, A. Individual and Combined Effect of Zearalenone Derivates and Beauvericin Mycotoxins on SH-SY5Y Cells. Toxins 2020, 12, 212. [Google Scholar] [CrossRef]
- Agahi, F.; Juan, C.; Font, G.; Juan-García, A. Neurotoxicity of Zearalenone’s Metabolites and Beauvericin Mycotoxins via Apoptosis and Cell Cycle Disruption. Toxicology 2021, 456, 152784. [Google Scholar] [CrossRef] [PubMed]
- Agahi, F.; Álvarez-Ortega, N.; Font, G.; Juan-García, A.; Juan, C. Oxidative Stress, Glutathione, and Gene Expression as Key Indicators in SH-SY5Y Cells Exposed to Zearalenone Metabolites and Beauvericin. Toxicol. Lett. 2020, 334, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Martí-Quijal, F.J.; Castagnini, J.M.; Barba, F.J.; Ruiz, M.J. Effect of Spirulina and Fish Processing By-Products Extracts on Citrinin-Induced Cytotoxicity in SH-SY5Y Cells. Foods 2024, 13, 1932. [Google Scholar] [CrossRef]
- Martí-Quijal, F.J.; Franco-Campos, F.; Barba, F.J.; Ruiz, M.J. Citrinin-Induced Cellular Damage: Insights from SH-SY5Y Cell Line Studies. Foods 2025, 14, 356. [Google Scholar] [CrossRef]
- Tsai, J.F.; Wu, T.S.; Yu, F.Y.; Liu, B.H. Neurotoxicity of Mycotoxin Citrinin: Novel Evidence in Developing Zebrafish and Underlying Mechanisms in Human Neuron Cells. Food Chem. Toxicol. 2023, 171, 113543. [Google Scholar] [CrossRef]
- Ficheux, A.S.; Sibiril, Y.; Parent-Massin, D. Effects of Beauvericin, Enniatin b and Moniliformin on Human Dendritic Cells and Macrophages: An in Vitro Study. Toxicon 2013, 71, 1–10. [Google Scholar] [CrossRef]
- Han, J.; Jin, C.; Zhong, Y.; Zhu, J.; Liu, Q.; Sun, D.; Feng, J.; Xia, X.; Peng, X. Involvement of NADPH Oxidase in Patulin-Induced Oxidative Damage and Cytotoxicity in HEK293 Cells. Food Chem. Toxicol. 2021, 150, 112055. [Google Scholar] [CrossRef] [PubMed]
- Jayashree, G.V.; Rachitha, P.; Raghavendra, V.B.; Kandikattu, H.K. Patulin Induced Neuronal Cell Damage in Human Neuroblastoma SH-SY5Y Cells. Toxicol. Rep. 2025, 14, 101886. [Google Scholar] [CrossRef]
- Zhang, B.; Liang, H.; Huang, K.; Li, J.; Xu, D.; Huang, C.; Li, Y. Cardiotoxicity of Patulin Was Found in H9c2 Cells. Toxicon 2022, 207, 21–30. [Google Scholar] [CrossRef]
- Flajs, D.; Peraica, M. Toxicological Properties of Citrinin. Arch. Ind. Hyg. Toxicol. 2009, 60, 457–464. [Google Scholar] [CrossRef] [PubMed]
- de Sá, S.V.M.; Faria, M.A.; Fernandes, J.O.; Cunha, S.C. Investigating the Individual and Mixture Cytotoxicity of Co-Occurring Aflatoxin B1, Enniatin B, and Sterigmatocystin on Gastric, Intestinal, Hepatic, and Renal Cellular Models. Food Chem. Toxicol. 2024, 188, 114640. [Google Scholar] [CrossRef]
- Huang, Y.L.; Pan, W.L.; Cai, W.W.; Ju, J.Q.; Sun, S.C. Exposure to Citrinin Induces DNA Damage, Autophagy, and Mitochondria Dysfunction during First Cleavage of Mouse Embryos. Environ. Toxicol. 2021, 36, 2217–2224. [Google Scholar] [CrossRef]
- Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Grasl-Kraupp, B.; Hogstrand, C.; et al. Risks to Human and Animal Health Related to the Presence of Moniliformin in Food and Feed. EFSA J. 2018, 16, e05082. [Google Scholar] [CrossRef]
- Sanhueza, C.E.P.; Degrossi, M.C. Moniliformina, Una Micotoxina de Fusarium. Sci. Fungorum. 2004, 3, 103–112. [Google Scholar] [CrossRef]
- IARC Agents Classifed by the IARC Monographs, Volumes 1–135. Available online: https://monographs.iarc.who.int/list-of-classifications/ (accessed on 24 January 2025).
- Kim, H.M.; Choi, H.Y.; Cho, G.H.; Im, J.H.; Hong, E.Y.; Chun, H.S. Natural Thiols, but Not Thioethers, Attenuate Patulin-Induced Endoplasmic Reticulum Stress in HepG2 Cells. Toxins 2021, 13, 727. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.H.; Yu, F.Y.; Wu, T.S.; Li, S.Y.; Su, M.C.; Wang, M.C.; Shih, S.M. Evaluation of Genotoxic Risk and Oxidative DNA Damage in Mammalian Cells Exposed to Mycotoxins, Patulin and Citrinin. Toxicol. Appl. Pharmacol. 2003, 191, 255–263. [Google Scholar] [CrossRef]
- Bevardi, M.; Petrović, M.; Markov, K.; Bošnir, J. How Sulphur Dioxide and Storage Temperature Contribute to Patulin Degradation in Homemade Apple Juice. Arch. Ind. Hyg. Toxicol. 2018, 69, 258–263. [Google Scholar] [CrossRef]
- Ramalingam, S.; Bahuguna, A.; Kim, M. The Effects of Mycotoxin Patulin on Cells and Cellular Components. Trends Food Sci. Technol. 2019, 83, 99–113. [Google Scholar] [CrossRef]
- Hsu, S.S.; Lin, Y.S.; Chio, L.M.; Liang, W.Z. Evaluation of the Mycotoxin Patulin on Cytotoxicity and Oxidative Stress in Human Glioblastoma Cells and Investigation of Protective Effect of the Antioxidant N-Acetylcysteine (NAC). Toxicon 2023, 221, 106957. [Google Scholar] [CrossRef]
- de Sá, S.V.M.; Sousa Monteiro, C.; Fernandes, J.O.; Pinto, E.; Faria, M.A.; Cunha, S.C. Evaluating the Human Neurotoxicity and Toxicological Interactions Impact of Co-Occurring Regulated and Emerging Mycotoxins. Food Res. Int. 2024, 184, 114239. [Google Scholar] [CrossRef] [PubMed]
- Sobral, M.M.C.; Faria, M.A.; Cunha, S.C.; Ferreira, I.M.P.L.V.O. Toxicological Interactions between Mycotoxins from Ubiquitous Fungi: Impact on Hepatic and Intestinal Human Epithelial Cells. Chemosphere 2018, 202, 538–548. [Google Scholar] [CrossRef] [PubMed]
- Marin, M.L.; Murtha, J.; Dong, W.; Pestka, J.J. Effects of Mycotoxins on Cytokine Production and Proliferation in EL-4 Thymoma Cells. J. Toxicol. Environ. Health 1996, 48, 379–396. [Google Scholar] [CrossRef]
- Wild, C.P.; Gong, Y.Y. Mycotoxins and Human Disease: A Largely Ignored Global Health Issue. Carcinogenesis 2010, 31, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Zain, M.E. Impact of Mycotoxins on Humans and Animals. J. Saudi Chem. Soc. 2011, 15, 129–144. [Google Scholar] [CrossRef]
- Doi, K.; Uetsuka, K. Mechanisms of Mycotoxin-Induced Neurotoxicity through Oxidative Stress-Associated Pathways. Int. J. Mol. Sci. 2011, 12, 5213. [Google Scholar] [CrossRef]
- Gayathri, L.; Dhivya, R.; Dhanasekaran, D.; Periasamy, V.S.; Alshatwi, A.A.; Akbarsha, M.A. Hepatotoxic Effect of Ochratoxin A and Citrinin, Alone and in Combination, and Protective Effect of Vitamin E: Invitro Study in HepG2 Cell. Food Chem. Toxicol. 2015, 83, 151–163. [Google Scholar] [CrossRef]
- Zhang, B.; Peng, X.; Li, G.; Xu, Y.; Xia, X.; Wang, Q. Oxidative Stress Is Involved in Patulin Induced Apoptosis in HEK293 Cells. Toxicon 2015, 94, 1–7. [Google Scholar] [CrossRef]
- Ferrer, E.; Juan-García, A.; Font, G.; Ruiz, M.J. Reactive Oxygen Species Induced by Beauvericin, Patulin and Zearalenone in CHO-K1 Cells. Toxicol. Vitr. 2009, 23, 1504–1509. [Google Scholar] [CrossRef]
- Manyes, L.; Escrivá, L.; Ruiz, M.J.; Juan-García, A. Beauvericin and Enniatin B Effects on a Human Lymphoblastoid Jurkat T-Cell Model. Food Chem. Toxicol. 2018, 115, 127–135. [Google Scholar] [CrossRef]
- Nielsen, T.S.; Sørensen, I.F.; Sørensen, J.L.; Søndergaard, T.E.; Purup, S. Cytotoxic and Apoptotic Effect of Mycotoxins in Human Small Intestinal Cells. J. Anim. Sci. 2016, 94, 234–237. [Google Scholar] [CrossRef]
- Lopez-Suarez, L.; Al Awabdh, S.; Coumoul, X.; Chauvet, C. The SH-SY5Y Human Neuroblastoma Cell Line, a Relevant in Vitro Cell Model for Investigating Neurotoxicology in Human: Focus on Organic Pollutants. Neurotoxicology 2022, 92, 131–155. [Google Scholar] [CrossRef] [PubMed]
- Penalva-Olcina, R.; Juan, C.; Fernández-Franzón, M.; Juan-García, A. Effectiveness of Beetroot Extract in SH-SY5Y Neuronal Cell Protection against Fumonisin B1, Ochratoxin A and Its Combination. Food Chem. Toxicol. 2022, 165, 113164. [Google Scholar] [CrossRef] [PubMed]
- Xicoy, H.; Wieringa, B.; Martens, G.J.M. The SH-SY5Y Cell Line in Parkinson’s Disease Research: A Systematic Review. Mol. Neurodegener. 2017, 12, 10. [Google Scholar] [CrossRef]
- Mallebrera, B.; Juan-Garcia, A.; Font, G.; Ruiz, M.J. Mechanisms of Beauvericin Toxicity and Antioxidant Cellular Defense. Toxicol. Lett. 2016, 246, 28–34. [Google Scholar] [CrossRef]
- Fernández-Blanco, C.; Frizzell, C.; Shannon, M.; Ruiz, M.J.; Connolly, L. An in Vitro Investigation on the Cytotoxic and Nuclear Receptor Transcriptional Activity of the Mycotoxins Fumonisin B1 and Beauvericin. Toxicol. Lett. 2016, 257, 1–10. [Google Scholar] [CrossRef]
- Juan-García, A.; Carbone, S.; Ben-Mahmoud, M.; Sagratini, G.; Mañes, J. Beauvericin and Ochratoxin A Mycotoxins Individually and Combined in HepG2 Cells Alter Lipid Peroxidation, Levels of Reactive Oxygen Species and Glutathione. Food Chem. Toxicol. 2020, 139, 111247. [Google Scholar] [CrossRef]
- Springler, A.; Vrubel, G.J.; Mayer, E.; Schatzmayr, G.; Novak, B. Effect of Fusarium-Derived Metabolites on the Barrier Integrity of Differentiated Intestinal Porcine Epithelial Cells (IPEC-J2). Toxins 2016, 8, 345. [Google Scholar] [CrossRef]
- Oh, S.-Y.; Boermans, H.J.; Swamy, H.V.L.N.; Sharma, B.S.; Karrow, N.A. Immunotoxicity of Penicillium Mycotoxins on Viability and Proliferation of Bovine Macrophage Cell Line (BOMACs). Open Mycol. J. 2012, 6, 11–16. [Google Scholar] [CrossRef]
- Salah, A.; Bouaziz, C.; Amara, I.; Abid-Essefi, S.; Bacha, H. Eugenol Protects against Citrinin-Induced Cytotoxicity and Oxidative Damages in Cultured Human Colorectal HCT116 Cells. Environ. Sci. Pollut. Res. Int. 2019, 26, 31374–31383. [Google Scholar] [CrossRef]
- Aydin, Y.; Orta Yilmaz, B.; Yildizbayrak, N.; Korkut, A.; Arabul Kursun, M.; Irez, T.; Erkan, M. Evaluation of Citrinin-Induced Toxic Effects on Mouse Sertoli Cells. Drug Chem. Toxicol. 2021, 44, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Cetin, Y.; Bullerman, L.B. Cytotoxicity of Fusarium Mycotoxins to Mammalian Cell Cultures as Determined by the MTT Bioassay. Food Chem. Toxicol. 2005, 43, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.C.; Madec, S.; Troadec, S.; Coton, E.; Hymery, N. Effects of Fusariotoxin Co-Exposure on THP-1 Human Immune Cells. Cell Biol. Toxicol. 2018, 34, 191–205. [Google Scholar] [CrossRef]
- Smith, M.C.; Hymery, N.; Troadec, S.; Pawtowski, A.; Coton, E.; Madec, S. Hepatotoxicity of Fusariotoxins, Alone and in Combination, towards the HepaRG Human Hepatocyte Cell Line. Food Chem. Toxicol. 2017, 109, 439–451. [Google Scholar] [CrossRef]
- Lu, S.; Liu, S.; Cui, J.; Liu, X.; Zhao, C.; Fan, L.; Yin, S.; Hu, H. Combination of Patulin and Chlorpyrifos Synergistically Induces Hepatotoxicity via Inhibition of Catalase Activity and Generation of Reactive Oxygen Species. J. Agric. Food Chem. 2019, 67, 11474–11480. [Google Scholar] [CrossRef]
- Alves, I.; Oliveira, N.G.; Laires, A.; Rodrigues, A.S.; Rueff, J. Induction of Micronuclei and Chromosomal Aberrations by the Mycotoxin Patulin in Mammalian Cells: Role of Ascorbic Acid as a Modulator of Patulin Clastogenicity. Mutagenesis 2000, 15, 229–234. [Google Scholar] [CrossRef]
- Diao, E.; Hou, H.; Hu, W.; Dong, H.; Li, X. Removing and Detoxifying Methods of Patulin: A Review. Trends Food Sci. Technol. 2018, 81, 139–145. [Google Scholar] [CrossRef]
- Pal, S.; Singh, N.; Dev, I.; Sharma, V.; Jagdale, P.R.; Ayanur, A.; Ansari, K.M. TGF-β/Smad Signaling Pathway Plays a Crucial Role in Patulin-Induced pro-Fibrotic Changes in Rat Kidney via Modulation of Slug and Snail Expression. Toxicol. Appl. Pharmacol. 2022, 434, 115819. [Google Scholar] [CrossRef]
- Battilani, P.; Palumbo, R.; Giorni, P.; Dall’Asta, C.; Dellafiora, L.; Gkrillas, A.; Toscano, P.; Crisci, A.; Brera, C.; De Santis, B.; et al. Mycotoxin Mixtures in Food and Feed: Holistic, Innovative, Flexible Risk Assessment Modelling Approach: MYCHIF. EFSA Support. Publ. 2020, 17, 1757E. [Google Scholar] [CrossRef]
- Grenier, B.; Oswald, I.P. Mycotoxin Co-Contamination of Food and Feed: Meta-Analysis of Publications Describing Toxicological Interactions. World Mycotoxin J. 2011, 4, 285–313. [Google Scholar] [CrossRef]
- Skrzydlewski, P.; Twarużek, M.; Grajewski, J. Cytotoxicity of Mycotoxins and Their Combinations on Different Cell Lines: A Review. Toxins 2022, 14, 244. [Google Scholar] [CrossRef]
- Stoev, S.D. Food Security and Foodborne Mycotoxicoses—What Should Be the Adequate Risk Assessment and Regulation? Microorganisms 2024, 12, 580. [Google Scholar] [CrossRef]
- Timm, M.; Saaby, L.; Moesby, L.; Hansen, E.W. Considerations Regarding Use of Solvents in in Vitro Cell Based Assays. Cytotechnology 2013, 65, 887–894. [Google Scholar] [CrossRef]
- Penalva-Olcina, R.; Juan, C.; Fernández-Franzón, M.; Juan-García, A. Involvement of Pro-Inflammatory Mediators and Cell Cycle Disruption in Neuronal Cells Induced by Gliotoxin and Ochratoxin A after Individual and Combined Exposure. Toxicol. Lett. 2024, 393, 24–32. [Google Scholar] [CrossRef]
- Juan-García, A.; Juan, C.; König, S.; Ruiz, M.J. Cytotoxic Effects and Degradation Products of Three Mycotoxins: Alternariol, 3-Acetyl-Deoxynivalenol and 15-Acetyl-Deoxynivalenol in Liver Hepatocellular Carcinoma Cells. Toxicol. Lett. 2015, 235, 8–16. [Google Scholar] [CrossRef]
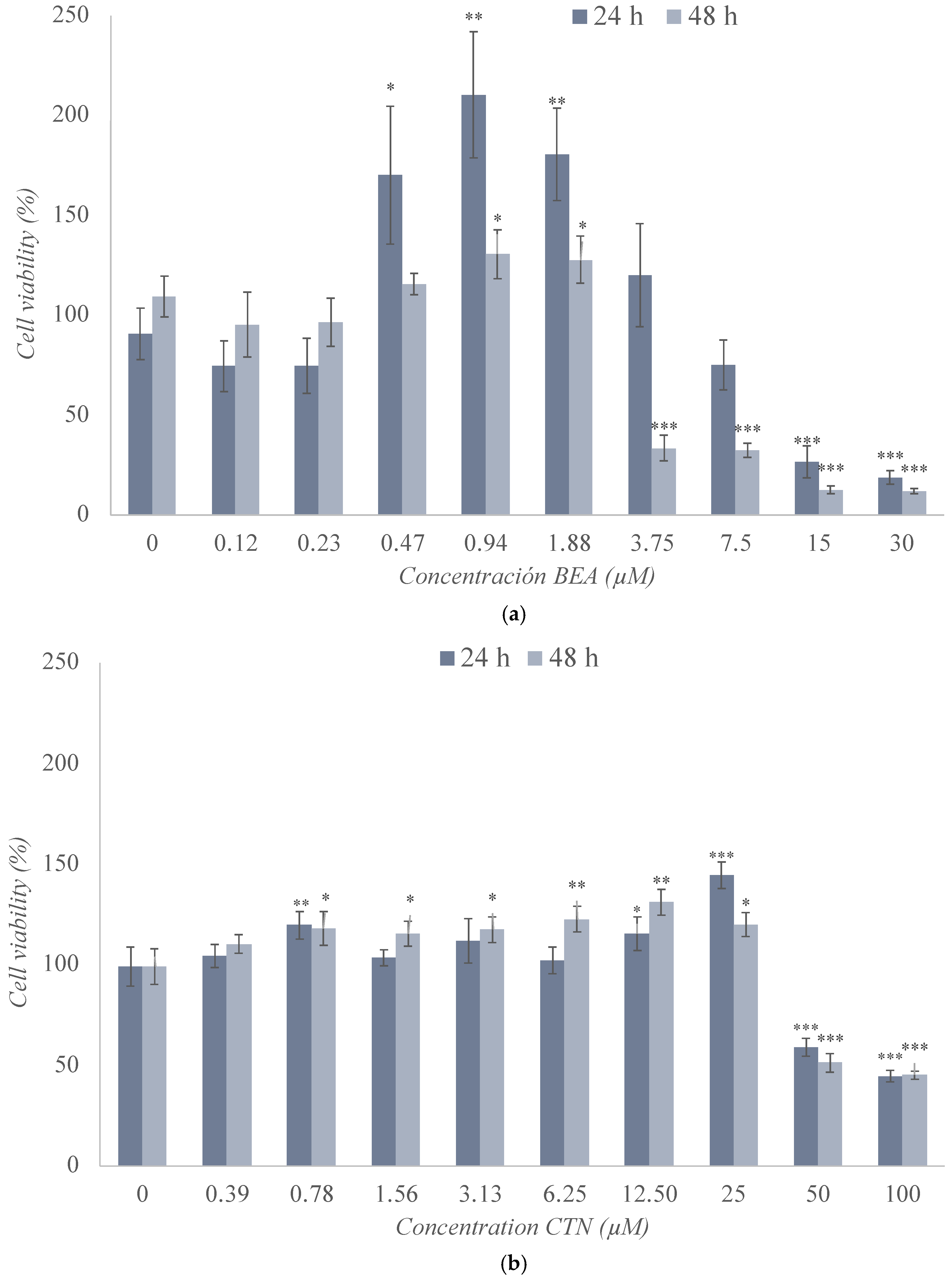
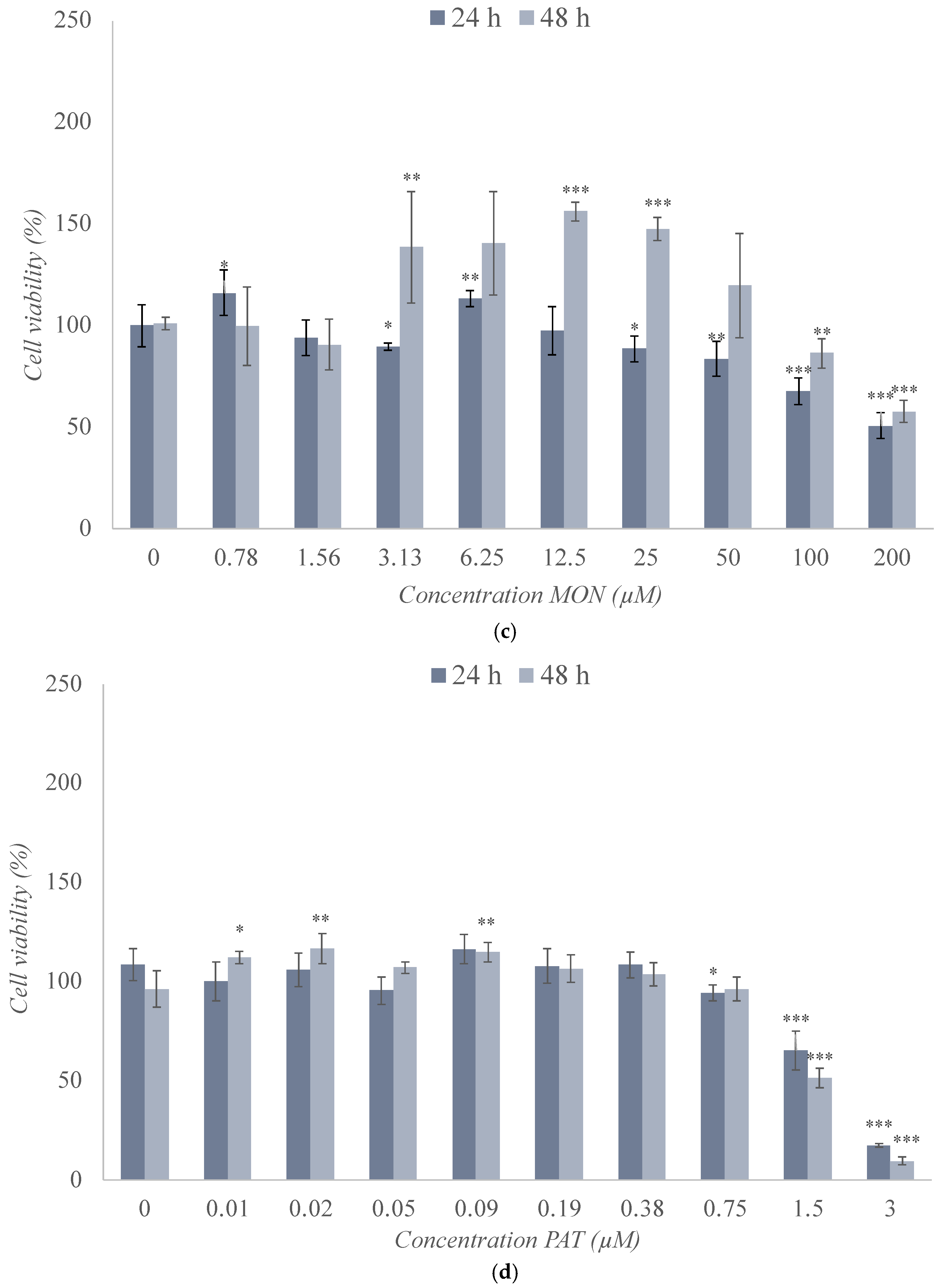
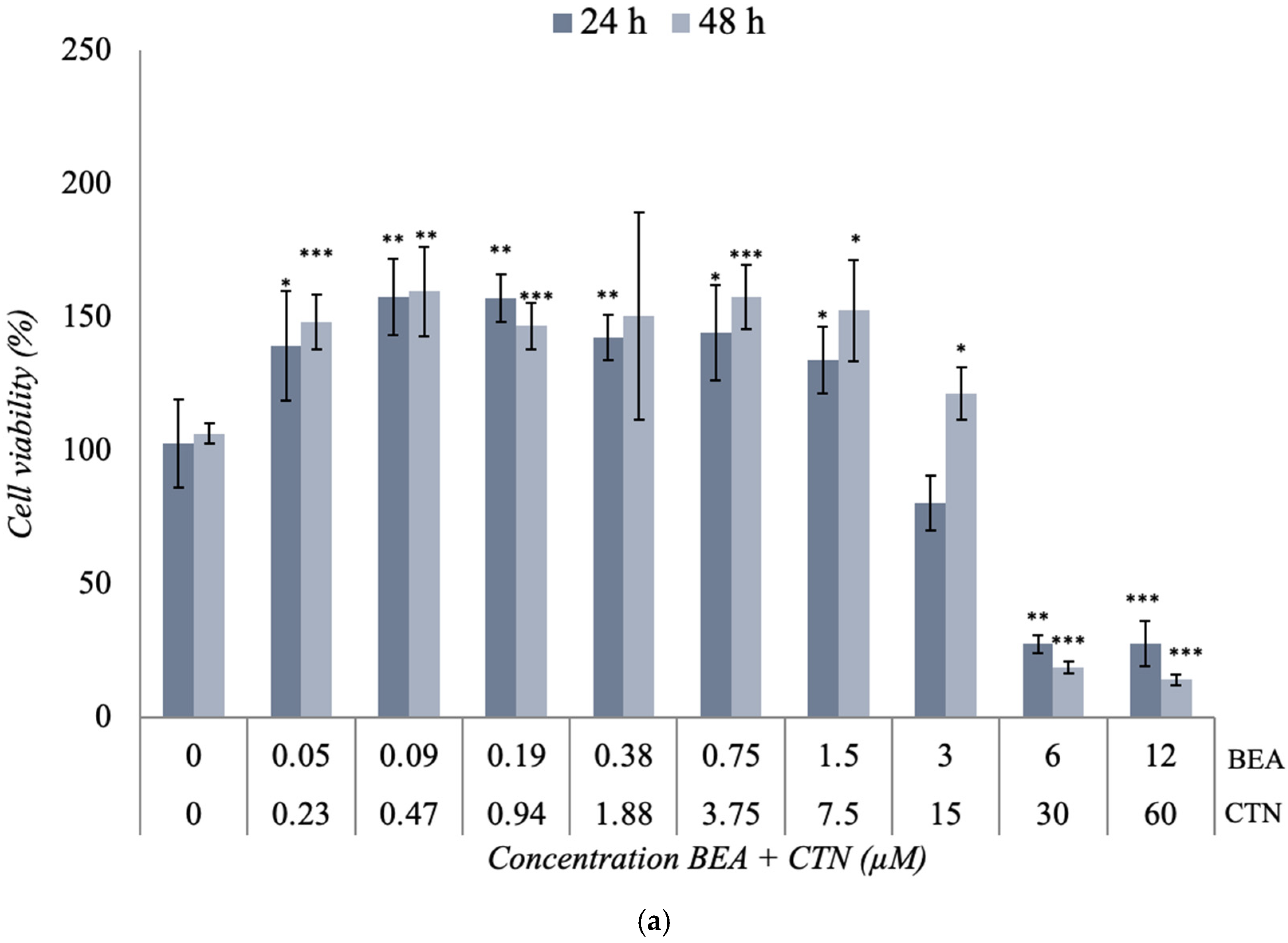

| Mycotoxin | Exposure Time 24 h IC50 (Mean ± SD) (μM) | Exposure Time 48 h IC50 (Mean ± SD) (μM) |
|---|---|---|
| BEA | (12 ± 8) | (3.25 ± 6.36) |
| CTN | (80 ± 2) | (50 ± 4) |
| MON | (200 ± 6) | N/A |
| PAT | (2.5 ± 1) | (1.5 ± 4.5) |
| BEA + CTN | [4.5 + 22.5] ± 3.4 | [4.8 + 24] ± 2.2 |
| BEA + MON | [4.5 + 75] ± 8.5 | [3 + 50] ± 3 |
| BEA + PAT | [5.5 + 1.15] ± 4.02 | [4.5 + 0.94] ± 3.27 |
| CTN + MON | N/A | [45 + 15] ± 2 |
| CTN + PAT | [45 + 0.75] ± 4.80 | [22.5 + 0.38] ± 1.98 |
| MON + PAT | N/A | N/A |
| Mycotoxin (Dilution Ratios) | Concentration Range (μM) |
|---|---|
| BEA | 0.12–30 |
| CTN | 0.39–100 |
| MON | 0.78–200 |
| PAT | 0.01–3 |
| BEA + CTN (1:5) | 0.28–72 |
| BEA + MON (3:50) | 0.83–212 |
| BEA + PAT (6:1.25) | 0.06–14.5 |
| CTN + MON (3:10) | 1.01–260 |
| CTN + PAT (60:1) | 0.234–61 |
| MON + PAT (200:1) | 0.784–201 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moyano-López, C.; Bridgeman, L.; Juan, C.; Juan-García, A. Cytotoxic Profiles of Beauvericin, Citrinin, Moniliformin, and Patulin and Their Binary Combinations: A Literature-Based Comparison and Experimental Validation in SH-SY5Y Cells. Toxins 2025, 17, 143. https://doi.org/10.3390/toxins17030143
Moyano-López C, Bridgeman L, Juan C, Juan-García A. Cytotoxic Profiles of Beauvericin, Citrinin, Moniliformin, and Patulin and Their Binary Combinations: A Literature-Based Comparison and Experimental Validation in SH-SY5Y Cells. Toxins. 2025; 17(3):143. https://doi.org/10.3390/toxins17030143
Chicago/Turabian StyleMoyano-López, Claudia, Luna Bridgeman, Cristina Juan, and Ana Juan-García. 2025. "Cytotoxic Profiles of Beauvericin, Citrinin, Moniliformin, and Patulin and Their Binary Combinations: A Literature-Based Comparison and Experimental Validation in SH-SY5Y Cells" Toxins 17, no. 3: 143. https://doi.org/10.3390/toxins17030143
APA StyleMoyano-López, C., Bridgeman, L., Juan, C., & Juan-García, A. (2025). Cytotoxic Profiles of Beauvericin, Citrinin, Moniliformin, and Patulin and Their Binary Combinations: A Literature-Based Comparison and Experimental Validation in SH-SY5Y Cells. Toxins, 17(3), 143. https://doi.org/10.3390/toxins17030143







