Innovations in Snake Venom-Derived Therapeutics: A Systematic Review of Global Patents and Their Pharmacological Applications
Abstract
1. Introduction
2. Results
3. Discussion
3.1. Geographic Distribution of Snake Venom-Related Patents
3.2. Ten-Year Range Patent Distribution
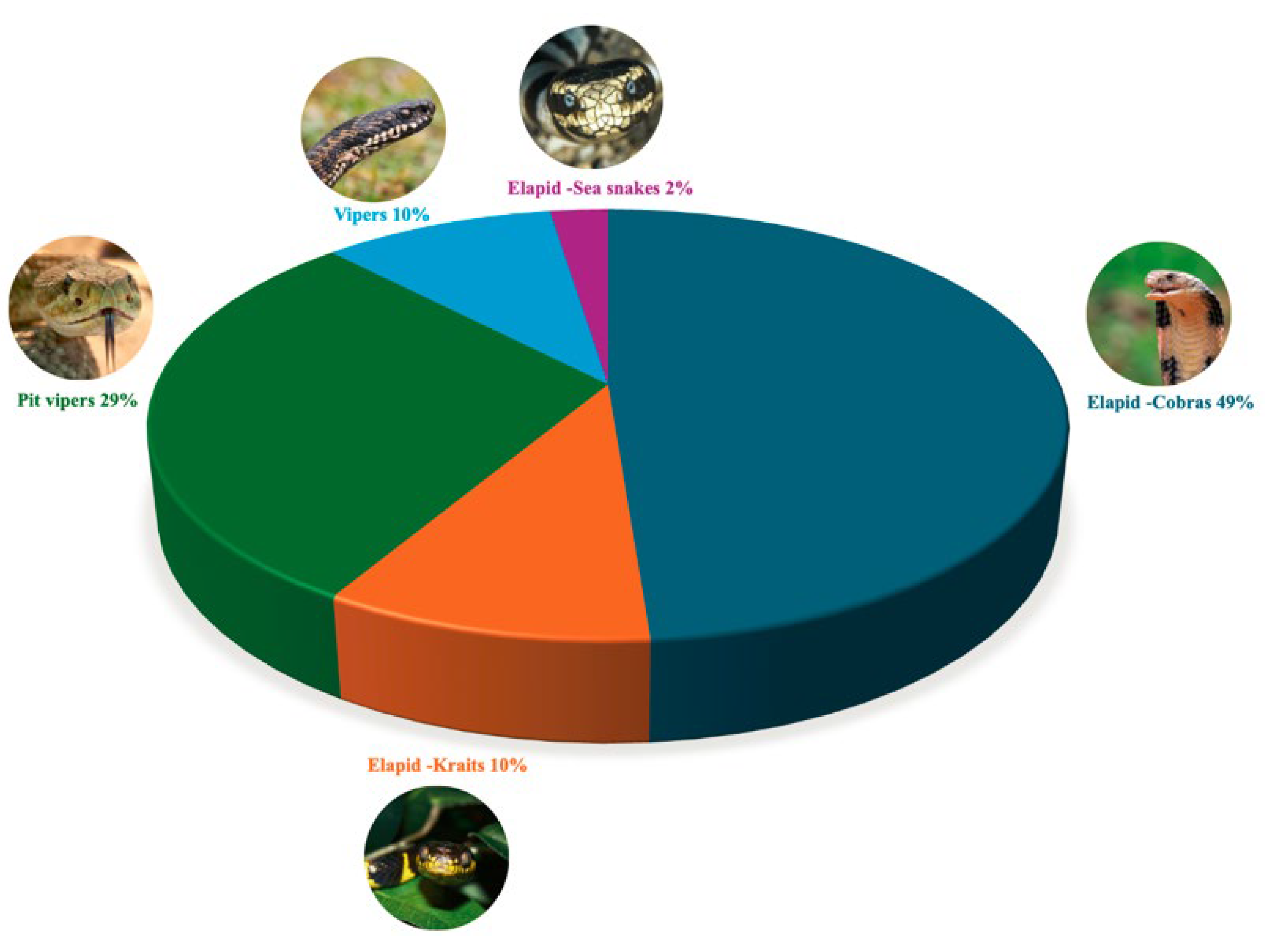
3.3. Most Used Snake Species in Pharmacological Innovations
| Family | Scientific Name | English Common Name | Pharmacological Activity Reported in Literature | Reference |
|---|---|---|---|---|
| Pit Viper | Agkistrodon piscivorus piscivorus | Northern Cottonmouth | Antithrombotic | [24] |
| Bothrops atrox | Fer-de-Lance, common lancehead | Anticoagulant | [25] | |
| Crotalus adamanteus | Eastern Diamondback Rattlesnake | Anticancer | [26] | |
| Crotalus durissus terrificus | South American rattlesnake | Anticancer, antimicrobial | [27] | |
| Gloydius intermedius | Central Asian pitviper | Anticoagulant and antiplatelet | [28] | |
| Protobothrops mucrosquamatus | Brown spotted pitviper | Anticoagulant and antiplatelet | [29] | |
| Trimeresurus fasciatus | Banded Pit Viper | Anticoagulant | [30] | |
| Trimeresurus stejnegeri | Chinese Green Tree Viper, Stejneger’s Bamboo pitviper | Virucidal activity | [31] | |
| Viper | Cerastes spp. | – | Antiplatelet activity | [32] |
| Daboia russelii | Russel’s Viper | Anticoagulant and antiplatelet | [33] | |
| Deinagkistrodon acutus | Chinese Moccasin, Hundred-pace viper | Antithrombotic, Antiplatelet | [34] | |
| Gloydius brevicaudus | Short-tailed Mamushi | Anticoagulant and antiplatelet | [28] | |
| Gloydius ussuriensis | Ussuri Mamushi | Anticoagulant and antiplatelet | [28] | |
| Elapid—Krait | Bungarus fasciatus | Banded Krait | Virucidal activity and Antibacterial | [25,31] |
| Bungarus multicinctus | Many-banded Krait | Immunogenic and anti-inflammatory | [35] | |
| Elapid—Cobra | Naja atra | Chinese Cobra | Anticoagulant | [36] |
| Naja kaouthia | Monocled Cobra, Monocellate Cobra | Antiviral, neuromodulatory and analgesic activities | [25] | |
| Naja melanoleuca | Central African Forest Cobra, Black and White Cobra | Antimicrobial and Antiviral | [37] | |
| Naja naja | Common cobra, Spectacled cobra | Virucidal activity | [31] | |
| Ophiophagus hannah | King Cobra | Analgesic | [25] | |
| Elapid—Sea snake | Hydrophis cyanocinctus | Annulated Sea Snake, Dusky-chinned giant sea snake | Antimicrobial and Anti-Inflammatory Activity | [38] |
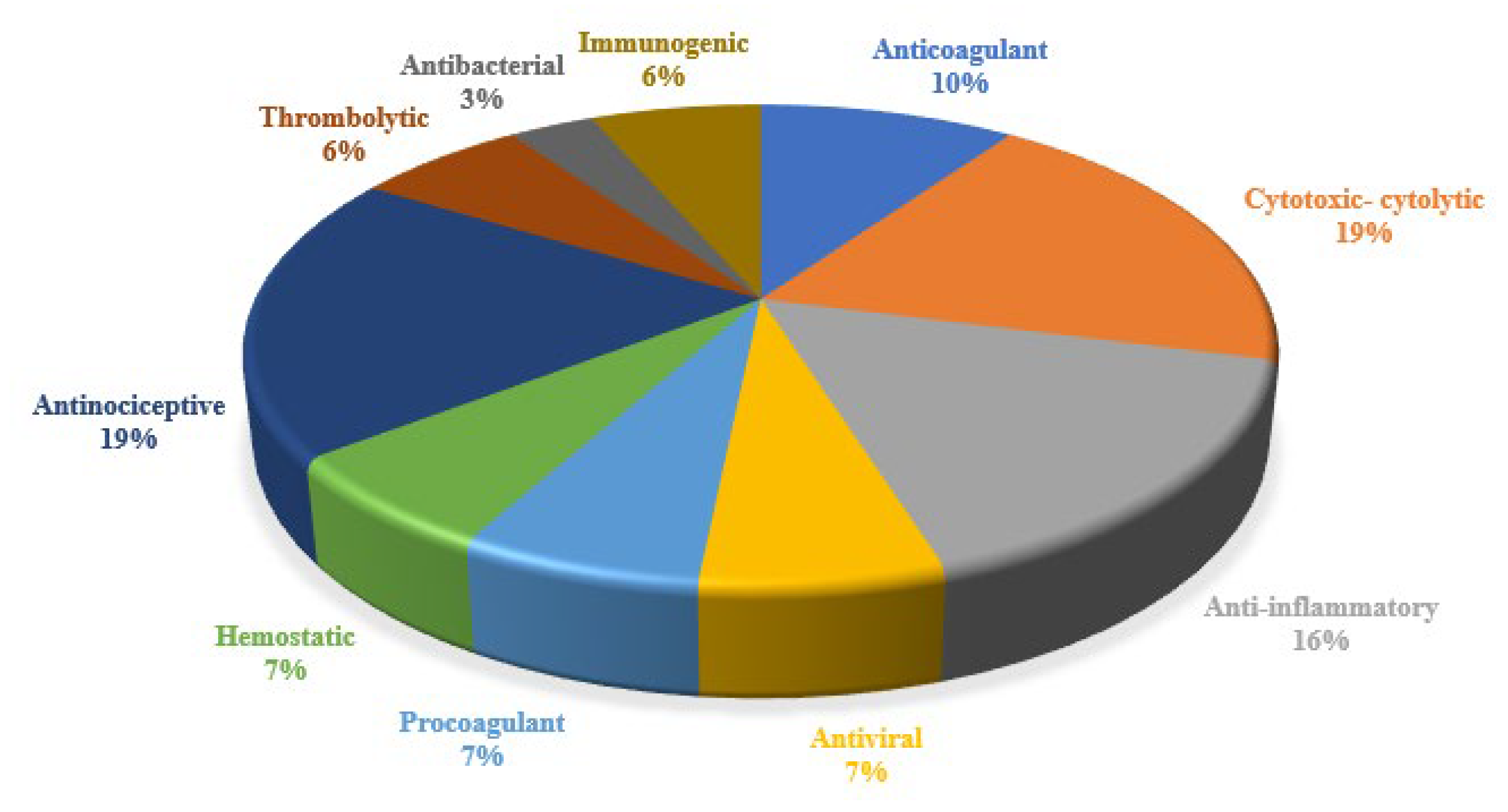
3.4. Therapeutic Indications, Applications, and Compounds Reported in Snake Venom Innovations
3.4.1. Anticoagulants and Hemostatic Agents
3.4.2. Metalloproteinases
3.4.3. Fibrinolytic Enzymes
3.4.4. Coagulation Factor Activators
3.5. Anti-Inflammatory Agents
3.6. Cancer Therapies
3.7. Cytotoxins and Polypeptides
3.8. Fusion Proteins
3.9. Pain Management
3.10. Antinociceptive Agents
3.11. Neurotoxins
3.12. Antimicrobial Agents
3.12.1. Antibacterial Agents
- Snake Venom Polypeptides
3.12.2. Anti-Viral Agents
- Broad-Spectrum Antivirals
3.13. Anti-Venom Innovations
- Neutralizing Anti-Venoms
3.14. Extraction and Stabilization Methods
4. Conclusions
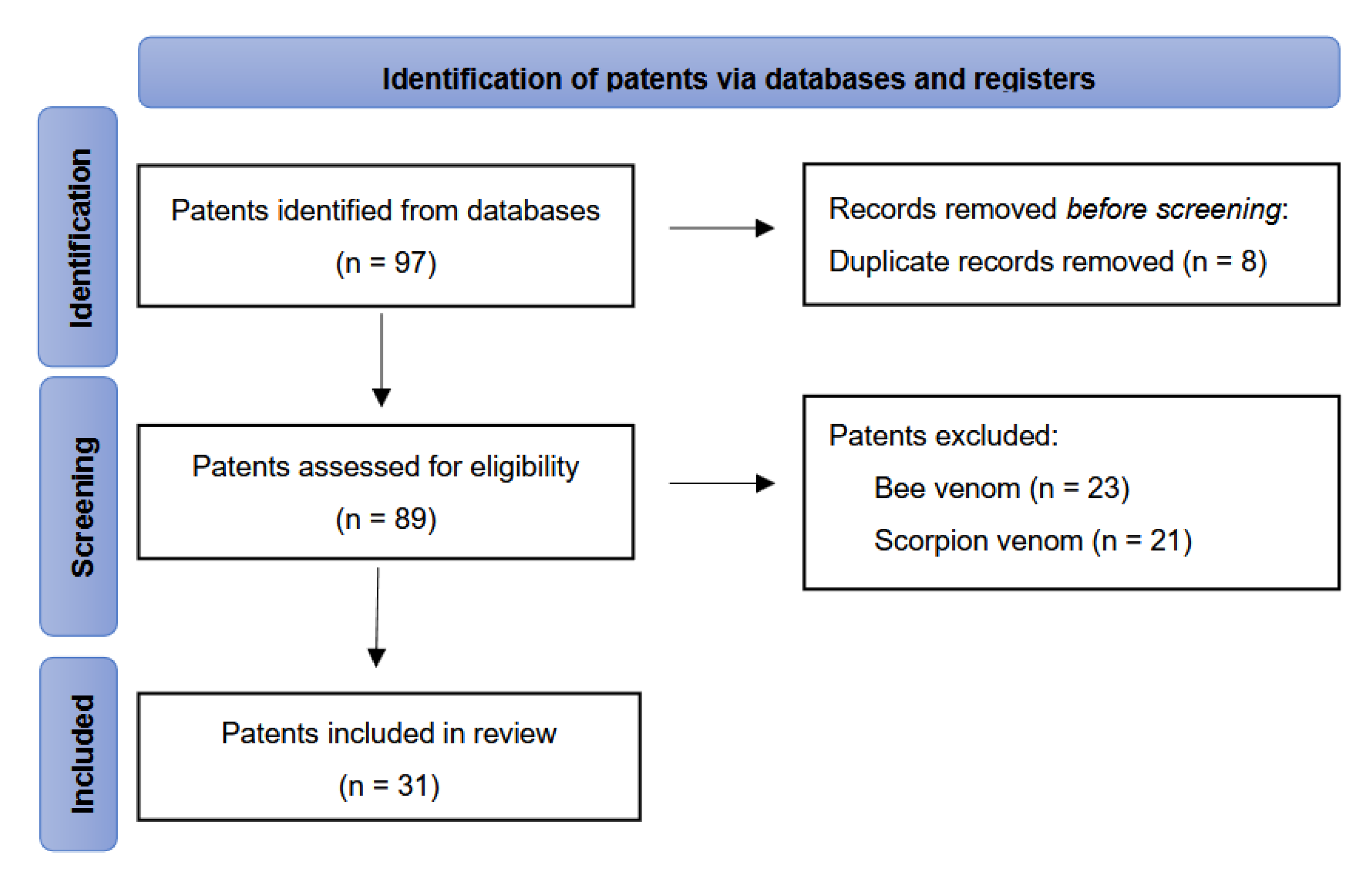
5. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Systematic Review Registration Statement
References
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Casewell, N.R.; Wüster, W.; Vonk, F.J.; Harrison, R.A.; Fry, B.G. Complex Cocktails: The Evolutionary Novelty of Venoms. Trends Ecol. Evol. 2013, 28, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y. Why Do We Study Animal Toxins? Zool. Res. 2015, 36, 183–222. [Google Scholar] [CrossRef]
- Vyas, V.K.; Brahmbhatt, K.; Bhatt, H.; Parmar, U. Therapeutic Potential of Snake Venom in Cancer Therapy: Current Perspectives. Asian Pac. J. Trop. Biomed. 2013, 3, 156–162. [Google Scholar] [CrossRef]
- Brahma, R.K.; Modahl, C.M.; Kini, R.M. Three-Finger Toxins. In Handbook of Venoms and Toxins of Reptiles; CRC Press: Boca Raton, FL, USA, 2021; pp. 177–194. [Google Scholar]
- Chen, P.-C.; Huang, M.-N.; Chang, J.-F.; Liu, C.-C.; Chen, C.-K.; Hsieh, C.-H. Snake Venom Proteome and Immuno-Profiling of the Hundred-Pace Viper, Deinagkistrodon acutus, in Taiwan. Acta Trop. 2019, 189, 137–144. [Google Scholar] [CrossRef]
- Bordon, K.d.C.F.; Cologna, C.T.; Fornari-Baldo, E.C.; Pinheiro-Júnior, E.L.; Cerni, F.A.; Amorim, F.G.; Anjolette, F.A.P.; Cordeiro, F.A.; Wiezel, G.A.; Cardoso, I.A.; et al. From Animal Poisons and Venoms to Medicines: Achievements, Challenges and Perspectives in Drug Discovery. Front. Pharmacol. 2020, 11, 1132. [Google Scholar] [CrossRef]
- Frangieh, J.; Rima, M.; Fajloun, Z.; Henrion, D.; Sabatier, J.-M.; Legros, C.; Mattei, C. Snake Venom Components: Tools and Cures to Target Cardiovascular Diseases. Molecules 2021, 26, 2223. [Google Scholar] [CrossRef]
- Lomonte, B.; Díaz, C.; Chaves, F.; Fernández, J.; Ruiz, M.; Salas, M.; Zavaleta, A.; Calvete, J.J.; Sasa, M. Comparative Characterization of Viperidae Snake Venoms from Perú Reveals Two Compositional Patterns of Phospholipase A2 Expression. Toxicon X 2020, 7, 100044. [Google Scholar] [CrossRef]
- Ojeda, P.G.; Ramírez, D.; Alzate-Morales, J.; Caballero, J.; Kaas, Q.; González, W. Computational Studies of Snake Venom Toxins. Toxins 2017, 10, 8. [Google Scholar] [CrossRef]
- Avella, I.; Wüster, W.; Luiselli, L.; Martínez-Freiría, F. Toxic Habits: An Analysis of General Trends and Biases in Snake Venom Research. Toxins 2022, 14, 884. [Google Scholar] [CrossRef]
- Estevão-Costa, M.-I.; Sanz-Soler, R.; Johanningmeier, B.; Eble, J.A. Snake Venom Components in Medicine: From the Symbolic Rod of Asclepius to Tangible Medical Research and Application. Int. J. Biochem. Cell Biol. 2018, 104, 94–113. [Google Scholar] [CrossRef] [PubMed]
- Espacenet-Bibliographic Data. Available online: https://www.epo.org/en (accessed on 22 July 2024).
- Ren, J.; Zhang, A.-H.; Wang, X.-J. Traditional Chinese Medicine for COVID-19 Treatment. Pharmacol. Res. 2020, 155, 104743. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Lanvin, B.; León, L.R.; Wunsch-Vincent, S. Global Innovation Index 2023: Innovation in the Face of Uncertainty; WIPO: Geneva, Switzerland, 2023; ISBN 9280533215. [Google Scholar]
- Bontekoe, F.E.; Wallot, M. World Intellectual Property Organization (WIPO). In Research Handbook on the European Union and International Organizations; Edward Elgar Publishing: Cheltenham, UK, 2019; pp. 275–292. ISBN 1786438933. [Google Scholar]
- Sofyantoro, F.; Yudha, D.S.; Lischer, K.; Nuringtyas, T.R.; Putri, W.A.; Kusuma, W.A.; Purwestri, Y.A.; Swasono, R.T. Bibliometric Analysis of Literature in Snake Venom-Related Research Worldwide (1933–2022). Animals 2022, 12, 2058. [Google Scholar] [CrossRef]
- OECD/FAO. OECD-FAO Agricultural Outlook 2022–2031; OECD Publishing: Paris, France, 2022. [Google Scholar]
- Utkin, Y.N. Modern Trends in Animal Venom Research-Omics and Nanomaterials. World J. Biol. Chem. 2017, 8, 4. [Google Scholar] [CrossRef]
- Markland, F.S.; Swenson, S. Snake Venom Metalloproteinases. Toxicon 2013, 62, 3–18. [Google Scholar] [CrossRef]
- Lomonte, B.; Calvete, J.J. Strategies in ‘Snake Venomics’ Aiming at an Integrative View of Compositional, Functional, and Immunological Characteristics of Venoms. J. Venom. Anim. Toxins Incl. Trop. Dis. 2017, 23, 26. [Google Scholar] [CrossRef]
- Casewell, N.R.; Jackson, T.N.W.; Laustsen, A.H.; Sunagar, K. Causes and Consequences of Snake Venom Variation. Trends Pharmacol. Sci. 2020, 41, 570–581. [Google Scholar] [CrossRef]
- Rao, W.-Q.; Kalogeropoulos, K.; E Allentoft, M.; Gopalakrishnan, S.; Zhao, W.-N.; Workman, C.T.; Knudsen, C.; Jiménez-Mena, B.; Seneci, L.; Mousavi-Derazmahalleh, M.; et al. The Rise of Genomics in Snake Venom Research: Recent Advances and Future Perspectives. GigaScience 2022, 11, giac024. [Google Scholar] [CrossRef]
- Dong, W.; Xiang, Y.; Kong, T.; Wang, H.; Peng, X.; Wang, S. Pharmaceutical Composition of 4-Aminoquinoline Derivative and Snake Venom Cytotoxin-CTX1. Espacenet-Bibliographic Data. Patent CN107737333A, 12 January 2017. [Google Scholar]
- Zhejing, S. Application of Postsynaptic Neurotoxin, Cardiotoxin, Cytotoxin, Phospholipase A2 and Crude Venom of Cobra in Resisting Virus Infection. Espacenet-Bibliographic Data. Patent CN111617108A, 9 April 2020. [Google Scholar]
- Vogel, C.; Willhem, F.P.; Rayes, J.; Lacroix-Desmazes, S. Uses of Humanized Cobra Venom Factor for Reducing or Preventing Immunogenicity. Espacenet-Bibliographic Data. Patent WO2015192101A1, 17 December 2015. [Google Scholar]
- Guo, Y.; Wu, J.; Jia, H.; Chen, W.; Shao, C.; Zhao, L.; Ma, J.; Li, R.; Zhong, Y.; Fang, F.; et al. Balancing the Expression and Production of a Heterodimeric Protein: Recombinant Agkisacutacin as a Novel Antithrombotic Drug Candidate. Sci. Rep. 2015, 5, 11730. [Google Scholar] [CrossRef]
- Waheed, H.; Moin, S.F.; Choudhary, M.I. Snake Venom: From Deadly Toxins to Life-Saving Therapeutics. Curr. Med. Chem. 2017, 24, 1874–1891. [Google Scholar] [CrossRef]
- Xiong, Y.; He, Q.; Yu, X.; Li, B.; Song, Z. The Anti-Ovarian Carcinoma Activity of L-Amino Acid Oxidase from Crotalus Adamanteus Venom in Vivo and in Vitro. Med. Oncol. 2022, 39, 112. [Google Scholar] [CrossRef] [PubMed]
- Falcao, C.B.; Radis-Baptista, G. Crotamine and Crotalicidin, Membrane Active Peptides from Crotalus durissus terrificus Rattlesnake Venom, and Their Structurally-Minimized Fragments for Applications in Medicine and Biotechnology. Peptides 2020, 126, 170234. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.-M.; Yang, Y.-E.; Chen, Y.; Cao, J.; Zhang, C.; Liu, L.-L.; Wang, Z.-Z.; Wang, X.-M.; Wang, Y.-M.; Tsai, I.-H. Transcriptome and Proteome of the Highly Neurotoxic Venom of Gloydius intermedius. Toxicon 2015, 107, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.-Y.; Ji, R.-S.; Yu, X.-Q.; Li, Y.-N.; Zhang, Q.-Y.; Sun, Q.-Y. A Novel Snake Venom C-Type Lectin-like Protein Modulates Blood Coagulation by Targeting von Willebrand Factor and Coagulation Factor IX. Sci. Rep. 2024, 14, 22962. [Google Scholar] [CrossRef]
- Liew, J.L.; Tan, N.H.; Tan, C.H. Proteomics and Preclinical Antivenom Neutralization of the Mangrove Pit Viper (Trimeresurus purpureomaculatus, Malaysia) and White-Lipped Pit Viper (Trimeresurus albolabris, Thailand) Venoms. Acta Trop. 2020, 209, 105528. [Google Scholar] [CrossRef]
- Hboub, H.; Mrid, R.B.; Bouchmaa, N.; Oukkache, N.; Fatimy, R. El An In-Depth Exploration of Snake Venom-Derived Molecules for Drug Discovery in Advancing Antiviral Therapeutics. Heliyon 2024, 10, e37321. [Google Scholar] [CrossRef]
- Chérifi, F.; Laraba-Djebari, F. Isolated Biomolecules of Pharmacological Interest in Hemostasis from Cerastes cerastes Venom. J. Venom. Anim. Toxins Incl. Trop. Dis. 2013, 19, 1–6. [Google Scholar] [CrossRef]
- Yasmin, R.; Chanchal, S.; Ashraf, M.Z.; Doley, R. Daboxin P, a Phospholipase A2 of Indian Daboia russelii Venom, Modulates Thrombin-Mediated Platelet Aggregation. J. Biochem. Mol. Toxicol. 2023, 37, e23476. [Google Scholar] [CrossRef]
- Li, B.X.; Dai, X.; Xu, X.R.; Adili, R.; Neves, M.A.D.; Lei, X.; Shen, C.; Zhu, G.; Wang, Y.; Zhou, H.; et al. In Vitro Assessment and Phase I Randomized Clinical Trial of Anfibatide a Snake Venom Derived Anti-Thrombotic Agent Targeting Human Platelet GPIbα. Sci. Rep. 2021, 11, 11663. [Google Scholar] [CrossRef]
- Wu, Y.; Liao, G.-Y.; Ke, H.-J.; Liu, P. Effects of Snake-Derived Phospholipase A2 Inhibitors on Acute Pancreatitis: In Vitro and in Vivo Characterization. Drug Des. Devel. Ther. 2020, 14, 4765–4774. [Google Scholar] [CrossRef]
- Zhong, X.-J.; Wang, C.-E.; Li, Y.-N.; Zhang, Q.-Y.; Sun, Q.-Y. Atrase A, a P-III Class Metalloproteinase Purified from Cobra Venom, Exhibits Potent Anticoagulant Activity by Inhibiting Coagulation Pathway and Activating the Fibrinolytic System. Heliyon 2024, 10, e30969. [Google Scholar] [CrossRef] [PubMed]
- Kalayci, S.; Iyigundogdu, Z.U.; Yazici, M.M.; Asutay, B.A.; Demir, O.; Sahin, F. Evaluation of Antimicrobial and Antiviral Activities of Different Venoms. Infect. Disord. Drug Targets 2016, 16, 44–53. [Google Scholar] [CrossRef]
- Wang, S.; Fan, L.; Pan, H.; Li, Y.; Zhao, X.; Qiu, Y.; Lu, Y. Identification and Characterization of a Novel Cathelicidin from Hydrophis cyanocinctus with Antimicrobial and Anti-Inflammatory Activity. Molecules 2023, 28, 2082. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.L.; Viegas, M.F.; da Silva, S.L.; Soares, A.M.; Ramos, M.J.; Fernandes, P.A. The Chemistry of Snake Venom and Its Medicinal Potential. Nat. Rev. Chem. 2022, 6, 451–469. [Google Scholar] [CrossRef] [PubMed]
- Koh, D.C.I.; Armugam, A.; Jeyaseelan, K. Snake Venom Components and Their Applications in Biomedicine. Cell. Mol. Life Sci. 2006, 63, 3030–3041. [Google Scholar] [CrossRef] [PubMed]
- Green, D. Coagulation Cascade. Hemodial. Int. 2006, 10 (Suppl. 2), S2–S4. [Google Scholar] [CrossRef]
- Reininger, A.J. Coagulation Activity of Platelets. Hamostaseologie 2007, 27, 247–250. [Google Scholar]
- Takeya, H.; Nishida, S.; Miyata, T.; Kawada, S.; Saisaka, Y.; Morita, T.; Iwanaga, S. Coagulation Factor X Activating Enzyme from Russell’s Viper Venom (RVV-X). A Novel Metalloproteinase with Disintegrin (Platelet Aggregation Inhibitor)-like and C-Type Lectin-like Domains. J. Biol. Chem. 1992, 267, 14109–14117. [Google Scholar] [CrossRef]
- Olaoba, O.T.; dos Santos, P.K.; Selistre-de-Araujo, H.S.; de Souza, D.H.F. Snake Venom Metalloproteinases (SVMPs): A Structure-Function Update. Toxicon X 2020, 7, 100052. [Google Scholar] [CrossRef]
- Sanchez, E.F.; Richardson, M.; Gremski, L.H.; Veiga, S.S.; Yarleque, A.; Niland, S.; Lima, A.M.; Estevao-Costa, M.I.; Eble, J.A. A Novel Fibrinolytic Metalloproteinase, Barnettlysin-I from Bothrops barnetti (Barnett’s Pitviper) Snake Venom with Anti-Platelet Properties. Biochim. Biophys. Acta-Gen. Subj. 2016, 1860, 542–556. [Google Scholar] [CrossRef]
- Girón, M.E.; Guerrero, B.; Salazar, A.M.; Sánchez, E.E.; Alvarez, M.; Rodríguez-Acosta, A. Functional Characterization of Fibrinolytic Metalloproteinases (Colombienases) Isolated from Bothrops colombiensis Venom. Toxicon 2013, 74, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Duarte, R.C.F.; Rios, D.R.A.; Leite, P.M.; Alves, L.C.; Magalhães, H.P.B.; das Graças Carvalho, M. Thrombin Generation Test for Evaluating Hemostatic Effects of Brazilian Snake Venoms. Toxicon 2019, 163, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Clare, R.H.; Dawson, C.A.; Westhorpe, A.; Albulescu, L.-O.; Woodley, C.M.; Mosallam, N.; Chong, D.J.W.; Kool, J.; Berry, N.G.; O’Neill, P.M. Snakebite Drug Discovery: High-Throughput Screening to Identify Novel Snake Venom Metalloproteinase Toxin Inhibitors. Front. Pharmacol. 2024, 14, 1328950. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Baby, D.; Rajguru, J.P.; Patil, P.B.; Thakkannavar, S.S.; Pujari, V.B. Inflammation and Cancer. Ann. Afr. Med. 2019, 18, 121–126. [Google Scholar] [CrossRef]
- Mancek-Keber, M. Inflammation-Mediating Proteases: Structure, Function in (Patho) Physiology and Inhibition. Protein Pept. Lett. 2014, 21, 1209–1229. [Google Scholar] [CrossRef]
- Park, J.Y.; Do, B.H.; Lee, J.S.; Yang, H.C.; Nguyen, A.N.; Krupa, M.; Kim, C.J.; Jang, Y.J.; Choe, H. Antinociceptive and Anti-Inflammatory Effects of Recombinant Crotamine in Mouse Models of Pain. Toxins 2021, 13, 707. [Google Scholar] [CrossRef]
- Vidya, V.; Achar, R.R.; Himathi, M.U.; Akshita, N.; Kameshwar, V.H.; Byrappa, K.; Ramadas, D. Venom Peptides-A Comprehensive Translational Perspective in Pain Management. Curr. Res. Toxicol. 2021, 2, 329–340. [Google Scholar] [CrossRef]
- Silvestrini, A.V.P.; de Macedo, L.H.; de Andrade, T.A.M.; Mendes, M.F.; Pigoso, A.A.; Mazzi, M.V. Intradermal Application of Crotamine Induces Inflammatory and Immunological Changes in Vivo. Toxins 2019, 11, 39. [Google Scholar] [CrossRef]
- Pal, S.K.; Gomes, A.; Dasgupta, S.C.; Gomes, A. Snake Venom as Therapeutic Agents: From Toxin to Drug Development. Indian J. Exp. Biol. 2002, 40, 1353–1358. [Google Scholar]
- Wild, C.; Weiderpass, E.; Stewart, B.W. World Cancer Report: Cancer Research for Cancer Prevention; IARC Press: Lyon, France, 2020; ISBN 9283204476. [Google Scholar]
- Li, L.; Huang, J.; Lin, Y. Snake Venoms in Cancer Therapy: Past, Present and Future. Toxins 2018, 10, 346. [Google Scholar] [CrossRef]
- Offor, B.C.; Piater, L.A. Snake Venom Toxins: Potential Anticancer Therapeutics. J. Appl. Toxicol. 2024, 44, 666–685. [Google Scholar] [CrossRef]
- Guo, X.; Fu, Y.; Peng, J.; Fu, Y.; Dong, S.; Ding, R.-B.; Qi, X.; Bao, J. Emerging Anticancer Potential and Mechanisms of Snake Venom Toxins: A Review. Int. J. Biol. Macromol. 2024, 269, 131990. [Google Scholar] [CrossRef]
- Pérez-Peinado, C.; Defaus, S.; Andreu, D. Hitchhiking with Nature: Snake Venom Peptides to Fight Cancer and Superbugs. Toxins 2020, 12, 255. [Google Scholar] [CrossRef]
- Lucena, S.; Castro, R.; Lundin, C.; Hofstetter, A.; Alaniz, A.; Suntravat, M.; Sánchez, E.E. Inhibition of Pancreatic Tumoral Cells by Snake Venom Disintegrins. Toxicon 2015, 93, 136–143. [Google Scholar] [CrossRef]
- Rodrigues, R.; Izidoro, L.F.; de Oliveira, R., Jr.; Soares, A.; Rodrigues, V.; Sampaio, S. Snake Venom Phospholipases A2: A New Class of Antitumor Agents. Protein Pept. Lett. 2009, 16, 894–898. [Google Scholar] [CrossRef]
- Jain, D.; Kumar, S. Snake Venom: A Potent Anticancer Agent. Asian Pacific J. Cancer Prev. 2012, 13, 4855–4860. [Google Scholar] [CrossRef]
- Montoya-Gómez, A.; Montealegre-Sánchez, L.; García-Perdomo, H.A.; Jiménez-Charris, E. Cervical Cancer and Potential Pharmacological Treatment with Snake Venoms. Mol. Biol. Rep. 2020, 47, 4709–4721. [Google Scholar] [CrossRef]
- McGeary, D.D.; McGeary, C.A.; Lippe, B. Interdisciplinary Chronic Pain Management. Am. Psychol. 2014, 69, 119–130. [Google Scholar]
- Hylands-White, N.; Duarte, R.V.; Raphael, J.H. An Overview of Treatment Approaches for Chronic Pain Management. Rheumatol. Int. 2017, 37, 29–42. [Google Scholar] [CrossRef]
- Aguggia, M. Neurophysiology of Pain. Neurol. Sci. 2003, 24 (Suppl. 2), S57–S60. [Google Scholar] [CrossRef]
- Messlinger, K.; Handwerker, H.O. Physiology of Pain. Schwerpunkt 2015, 29, 522–530. [Google Scholar] [CrossRef]
- Cañas, C.A.; Castaño-Valencia, S.; Castro-Herrera, F.; Cañas, F.; Tobón, G.J. Biomedical Applications of Snake Venom: From Basic Science to Autoimmunity and Rheumatology. J. Transl. Autoimmun. 2021, 4, 100076. [Google Scholar] [CrossRef]
- Chan, Y.S.; Cheung, R.C.F.; Xia, L.; Wong, J.H.; Ng, T.B.; Chan, W.Y. Snake Venom Toxins: Toxicity and Medicinal Applications. Appl. Microbiol. Biotechnol. 2016, 100, 6165–6181. [Google Scholar] [CrossRef]
- Leite dos Santos, G.G.; Casais e Silva, L.L.; Pereira Soares, M.B.; Villarreal, C.F. Antinociceptive Properties of Micrurus Lemniscatus Venom. Toxicon 2012, 60, 1005–1012. [Google Scholar] [CrossRef]
- Moreira, L.A.; Oliveira, L.P.; Magalhães, M.R.; Oliveira, S.A.M.; Oliveira-Neto, J.R.; Carvalho, P.M.G.; Carvalho, A.A.V.; Fajemiroye, J.O.; Cruz, A.C.; Cunha, L.C. Acute Toxicity, Antinociceptive, and Anti-Inflammatory Activities of the Orally Administered Crotamine in Mice. Naunyn. Schmiedebergs. Arch. Pharmacol. 2021, 394, 1703–1711. [Google Scholar] [CrossRef]
- Yap, M.K.K.; Tan, N.H.; Sim, S.M.; Fung, S.Y.; Tan, C.H. Pharmacokinetics of Naja sumatrana (Equatorial Spitting Cobra) Venom and Its Major Toxins in Experimentally Envenomed Rabbits. PLoS Negl. Trop. Dis. 2014, 8, e2890. [Google Scholar] [CrossRef]
- Morrison, L.; Zembower, T.R. Antimicrobial Resistance. Gastrointest. Endosc. Clin. NA 2020, 30, 619–635. [Google Scholar] [CrossRef]
- Breijyeh, Z.; Jubeh, B.; Karaman, R. Resistance of Gram-Negative Bacteria to Current Antibacterial Agents and Approaches to Resolve It. Molecules 2020, 25, 1340. [Google Scholar] [CrossRef]
- Parai, D.; Dey, P.; Mukherjee, S. Antimicrobial Peptides: An Approach to Combat Resilient Infections. Curr. Drug Discov. Technol. 2019, 17, 541–551. [Google Scholar] [CrossRef]
- Zambrano, P.; Xavier, L.C.; Santos, A.M.; Rossato, L.; da Costa, J.C.; Serafini, M.R.; Aragón, M.; Souto, R.B.; Alves, I.A. What Do We Have That Is New in Antifungal Peptides? A Patent Review. Future Microbiol. 2022, 17, 1421–1432. [Google Scholar] [CrossRef]
- Yacoub, T.; Rima, M.; Karam, M.; Sabatier, J.M.; Fajloun, Z. Antimicrobials from Venomous Animals: An Overview. Molecules 2020, 25, 2402. [Google Scholar] [CrossRef] [PubMed]
- Vargas, L.J.; Londoño, M.; Quintana, J.C.; Rua, C.; Segura, C.; Lomonte, B.; Núñez, V. An Acidic Phospholipase A2 with Antibacterial Activity from Porthidium nasutum Snake Venom. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2012, 161, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Oguiura, N.; Boni-Mitake, M.; Affonso, R.; Zhang, G. In Vitro Antibacterial and Hemolytic Activities of Crotamine, a Small Basic Myotoxin from Rattlesnake Crotalus Durissus. J. Antibiot. 2011, 64, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Macedo, J.M.; de Lima, A.M.; Kayano, A.M.; Souza, M.F.; da Silva Oliveira, I.; Garay, A.F.G.; Rocha, A.M.; Zuliani, J.P.; Soares, A.M. Literature Review on Crotalus Durissus Terrificus Toxins: From a Perspective of Structural Biology and Therapeutic Applications. Curr. Protein Pept. Sci. 2023, 24, 536–550. [Google Scholar] [CrossRef]
- Teixeira, S.C.; Borges, B.C.; Oliveira, V.Q.; Carregosa, L.S.; Bastos, L.A.; Santos, I.A.; Jardim, A.C.G.; Freire, F.M.; Freitas, L.M.; Rodrigues, V.M.; et al. Insights into the Antiviral Activity of Phospholipases A2 (PLA2s) from Snake Venoms. Int. J. Biol. Macromol. 2020, 164, 616–625. [Google Scholar] [CrossRef]
- Sanhajariya, S.; Duffull, S.B.; Isbister, G.K. Pharmacokinetics of Snake Venom. Toxins 2018, 10, 73. [Google Scholar] [CrossRef]
- León, G.; Vargas, M.; Segura, Á.; Herrera, M.; Villalta, M.; Sánchez, A.; Solano, G.; Gómez, A.; Sánchez, M.; Estrada, R. Current Technology for the Industrial Manufacture of Snake Antivenoms. Toxicon 2018, 151, 63–73. [Google Scholar] [CrossRef]
- Laustsen, A.H. Antivenom in the Age of Recombinant DNA Technology. In Handbook of Venoms and Toxins of Reptiles; CRC Press: Boca Raton, FL, USA, 2021; pp. 499–510. [Google Scholar]
- Pucca, M.B.; Cerni, F.A.; Janke, R.; Bermúdez-Méndez, E.; Ledsgaard, L.; Barbosa, J.E.; Laustsen, A.H. History of Envenoming Therapy and Current Perspectives. Front. Immunol. 2019, 10, 1598. [Google Scholar] [CrossRef]
- Pereañez, J.A.; Preciado, L.M.; Fernández, J.; Camacho, E.; Lomonte, B.; Castro, F.; Cañas, C.A.; Galvis, C.; Castaño, S. Snake Venomics, Experimental Toxic Activities and Clinical Characteristics of Human Envenomation by Bothrocophias myersi (Serpentes: Viperidae) from Colombia. J. Proteom. 2020, 220, 103758. [Google Scholar] [CrossRef]
- Tasima, L.J.; Hatakeyama, D.M.; Serino-Silva, C.; Rodrigues, C.F.B.; de Lima, E.O.V.; Sant’Anna, S.S.; Grego, K.F.; de Morais-Zani, K.; Sanz, L.; Calvete, J.J.; et al. Comparative Proteomic Profiling and Functional Characterization of Venom Pooled from Captive Crotalus durissus terrificus Specimens and the Brazilian Crotalic Reference Venom. Toxicon 2020, 185, 26–35. [Google Scholar] [CrossRef]
- Santos, A.P.D.; de Araújo, T.G.; Rádis-Baptista, G. Nanoparticles Functionalized with Venom-Derived Peptides and Toxins for Pharmaceutical Applications. Curr. Pharm. Biotechnol. 2020, 21, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Bello, C.A.; Hermogenes, A.L.N.; Magalhaes, A.; Veiga, S.S.; Gremski, L.H.; Richardson, M.; Sanchez, E.F. Isolation and Biochemical Characterization of a Fibrinolytic Proteinase from Bothrops leucurus (White-Tailed Jararaca) Snake Venom. Biochimie 2006, 88, 189–200. [Google Scholar] [CrossRef] [PubMed]



| No | Cod. | Institution | Year/Country | Species | Active Compound | Activity and Mechanism | Administration Route | Dose | Assay |
|---|---|---|---|---|---|---|---|---|---|
| 1 | CN105567666 (A) | Pharmaceutical industry | 2016/China | Naja atra | PIII type metalloproteinase | Anticoagulant: The target protein has the activity of hydrolyzing fibrinogen α chain, and this activity can be completely inhibited by metal chelators EDTA, EGTA, 1,10-phenanthroline and reducing agent DTT. | I.V | 0.3 and 3.0 mg/kg | Activity: In vitro anticoagulation platelet aggregation rate on rabbits Anticoagulant effect in rats |
| 2 | CN106632686 (A) | University | 2017/China | Trimeresurus fasciatus | Anti-IL-4R single-chain antibody and a snake venom L-amino acid oxidase fusion protein. | Citotoxic: Direct killing and apoptosis-inducing effects on a variety of cancer cells, selectivity lung cancer | I.P. | 100, 50, 20 mg/kg | Activity: MTT assay, effects of fusion protein on human lung adenocarcinoma cell line, effects of anti-IL-4R single-chain antibody and LAAO fusion protein on tumor inhibition rate and life extension rate of mice bearing human lung adenocarcinoma Safety: Cell line H460 |
| 3 | CN113388020 (A) | Research institute | 2021/China | Deinagkistrodon acutus | Peptide DAvp-1 | Anti-inflammatory: Antagonize TNF-α | I.P | 500 μg/kg | Activity: Ulcerative colitis mouse model. |
| 4 | CN104434981 (A) | Pharmaceutical industry | 2015/China | Naja atra | Physically treated cobra venom | Anti-inflammatory: Inhibit acute lung inflammation induced by lipopolysaccharide | Orally | 10–3000 μg/kg animal/1 to 1000 μg/kg per person. | Activity: Acute lung injury induced by LPS model |
| 5 | CN111617108 (A) | Pharmaceutical industry | 2020/China | Naja naja, King cobra, Bengal cobra, Bungarus, Coral snake, Black mamba | Elapheidae postsynaptic neurotoxin, cardiotoxin, cytotoxin, phospholipase A2 | Anti-viral: Membrane toxins can easily pass through the Membrane structure and then destroy internal structures such as mitochondria and lysosomes, reversibly bind to nicotinic acetylcholine receptors | NA | 1.03, 0.39, 0.23, 0.32 and 0.89 (unit: μg/mL) | Activity: Virus plaque |
| 6 | CN115957303 (A) | University | 2023/China | Hydrophis cyanocinctus | Polypeptide Hc-CATH | Anti-viral: Destroy the viral envelope, induce the leakage of the viral genome, and thus inactivate viral particles | I.V | 5 mg/kg | Activity: Zika in vitro/in vivo infection, Safety: Cytotoxicity assay in Vero cells |
| 7 | WO2023168743 (A1) | Pharmaceutical industry | 2023/WIPO | NA | Viper venom hemocoagulase (Slounase) | Procoagulant and hemostatic: Reverse the anticoagulant effect of FXa inhibitors and completely restore thrombosis in human blood. | NA | 0.014–0.077 U/mL | Activity: In vitro thromboelastography |
| 8 | CN108079285 (A) | Pharmaceutical industry | 2018/China | Bothrops moojeni | L-aminobutanedioic acid stabilizer for snake venom enzyme: Defibrase | Stabilizer: for making snake venom enzyme preparation safer, more effective, more stable. | NA | NA | Stability Test |
| 9 | US2019336572 (A1) | Research institute | 2019/United states | Naja tripudians, N. siamensis, N. naja, N. atra, N. kaouthia, and O. hannah | Cobra venom | Antinociceptive: Target the cholinergic system by blocking the activity of acetylcholine | Orally | 0.1 to about 0.5 mg/mL | Stability Test, Activity: Formalin, hot-plate and acetic acid writhing tests |
| 10 | KR20190102909 (A) | Pharmaceutical industry | 2019/Republic of Korea | Naja atra | Low molecular peptide isolated from the heat-treated snake venom | Anti-inflammatory: NA | NA | 50 μg/mL | Safety: Cytotoxicity |
| 11 | US2022362356 (A1) | Pharmaceutical industry | 2022/United states | Agkistrodon piscivorus piscivorus or Naja melanoleuca | Extract of snake venom | Anti-inflammatory: Increase the level of regulatory T cells, increased all of the level of each combination of CD4 positive, CD25 positive, can improve the inflammatory symptoms of rheumatoid arthritis. | Orally/skin application | 0.1 to 50 mg/kg | Activity: Arthritis model mice |
| 12 | KR20220170290 (A) | University | 2022/Republic of Korea | Republic of Korea pit viper (Gloydius brevicaudus, Gloydius intermedius, Gloydius ussuriensis) | Anti-viper serum/anti-viper viper serum | Anti-venom: venom neutralizing effect | I.M | 50 μL/100 μL) | Safety: Lethality test |
| 13 | US2015110770 (A1) | Research institute | 2015/United states | Crotalus durissus terrificus | Crotoxin compositions | Cytolytic: NA | I.V | 0.0012–0.01 mg/kg | Activity: Anti-tumor activity both in vitro and in vivo, Safety: LD50 |
| 14 | CN110724678 (A) | University | 2020/China | Agkistrodon acutus | Agkistrodon halys venom fibrinolysin- plasmin | Thrombolytic: Remove the fibrin gel blocks deposited in the thrombus on the blood vessel wall, dissolve the thrombus | Intravenous drip | 25 μg/kg, 50 μg/kg and 100 μg/kg | Activity: Determination of fibrinolytic enzyme activity, thrombolytic assay, Anticoagulant effect in mice |
| 15 | CN108273067 (A) | Pharmaceutical industry | 2018/China | Bothrops atrox | Glutamic acid stabilizer for snake venom enzyme: Defibrase | NA | NA | NA | Accelerated stability test |
| 16 | CN108743924 (A) | Pharmaceutical industry | 2018/China | NA | Snake venom coagulation factor activator | Procoagulant: clotting factor, blood coagulation X factor activator. | NA | NA | NA |
| 17 | CN105497873 (A) | University | 2016/China | Cobra or Agkistrodon | Light-controlled targeted snake venom polypeptide zinc nanoformulation | Antinociceptive: Central effect. | I.P | 2 mg/kg | Activity: Hot plate |
| 18 | CN109943554 (A) | Pharmaceutical industry | 2019/China | Vipera ruselli, Bothrops, Deinagkistrodon, Bungarus, Cerastes, Calloselasma, Ophiophagus, Crotalus adamanteus and/or Naja | Coagulation factor X activator from snake venom. | Hemostatic: Factor X activators activate factor X at the site of blood vessel damage, promoting the generation of thrombin. | NA | NA | NA |
| 19 | NZ753297 (A) | Pharmaceutical industry | 2021/New Zealand | Agkistrodon acutus | Recombinant Agkisacutacin | Antiplatelet: Inhibiting platelet aggregation | NA | 2 μg | Activity: GPIb binding activity and antiplatelet agglutination activity |
| 20 | CN109929020 (A) | Pharmaceutical industry | 2019/China | Naja, Naja atra | Neurotoxin | Antinociceptive: NA | NA | NA | NA |
| 21 | KR20190007161 (A) | Pharmaceutical industry | 2019/Republic of Korea | Naja atra, Naja kaouthia | Modified cobra venom | Anti-inflammatory and antinociceptive: NA | Orally, external and injectable | 300 μg/kg | Activity: Mouse Ear Swelling Assay Safety: LD50 |
| 22 | CN107737333 (A) | Pharmaceutical industry | 2018/China | Naja atra | 4-aminoquinoline derivative and cytotoxin-CTX1 | Anti-tumor: CTXs-mediated cancer cell damage is achieved by destroying lysosomes, induce apoptosis and necrosis of a variety of tumor cells. | NA | NA | Activity: Citotoxicity breast cancer cell line MCF7, human acute myeloid leukemia cell line KG1a |
| 23 | CN107929717 (A) | Pharmaceutical industry | 2018/China | Naja atra | Siramesin and snake venom cytotoxin-CTX1 | Anti-tumor: Significant synergistic effect on the growth inhibition of the MCF7 tumor line and could effectively induce late apoptosis and necrosis of MCF7 cells. | NA | NA | Activity: Citotoxicity cell line MCF7, Changes in reactive oxygen species during cell death |
| 24 | CN115594746 (A) | University | 2023/China | Agkistrodon acutus | C-type lectin-like protein | Anti-platelet and anti-coagulation: Effect by prolonging TT, APTT and PT pathways. | I.V | 0.5 μg/g and 1.5 μg/g | Activity: Anticoagulant effect on mice, Platelet count, Coagulation function assay, Tail bleeding time determination |
| 25 | CN117756907 (A) | Pharmaceutical industry | 2024/China | Naja atra | Cobra-peptide and Substance A | Antitumor: inhibits the proliferation of various cells, induce early apoptosis. | NA | NA | Activity: Human cervical cancer cell line Hela cells, Flow cytometry assay for cell apoptosis Stability test |
| 26 | CN107098956 (A) | University | 2017/China | Naja atra | Cytotoxin-4N | Cytotoxic: Activation or cause apoptosis | NA | NA | Activity: Effect on the inhibition of HSC-T6 cell proliferation, Effects on apoptosis of HSC-T6 cells |
| 27 | WO2017190263 (A1) | Pharmaceutical industry | 2017/WIPO | Naja atra | C fragment polypeptide | Antinociceptive: NA | I.M | 0.5 mg/mL | Activity: Postoperative Pain in Rats, Von Frey test |
| 28 | CN114409757 (A) | Pharmaceutical industry | 2022/China | Naja | Cobra venom neurotoxin | Antinociceptive: High affinity for N-type acetylcholine receptors and can block the transmission of nerve impulse signals at the neuromuscular junction | I.M | 23.3 μg/kg | Activity: Hot plate test |
| 29 | CN117343131 (A) | University | 2024/China | Naja atra, Gloydius brevicaudus, Deinagkistrodon, Trimeresurus stejnegeri, Bungarus multicinctus, Bungarus fasciatus, Protobothrops mucrosquamatus. | Snake venom polypeptide | Antibacterial: NA | Orally/injected | 100 mg/kg | Activity: MIC E.coli, Acute toxicity test in mice, Subacute toxicity test in mice, Hemolytic test. Safety: LD50 Stability test |
| 30 | CN105861476 (A) | University | 2016/China | Naja atra | PIII type metalloproteinase- Atrase A/Atrase B | Thrombolytic: Hydrolyze the α chain of fibrinogen and show anti-platelet aggregation activity | I.V | 3.0 mg/kg–0.3 mg/kg | Activity: Determination of fibrinolytic function |
| 31 | US2017128544 (A1) | University | 2017/United states | Naja naja, Naja kaouthia | Humanized cobra venom factor | Immunogenic: Induce a humoral or a cell mediated response of the immune system | I.P | 250 μg/kg–500 μg/kg | Activity: Murine model of age-related macular degeneration, murine model of gastrointestinal ischemia reperfusion injury. |
| Application Number [Reference] | Amino Acids Sequence | Activity |
|---|---|---|
| CN107737333 (A) [13] | LKCNKLIPIA SKTCPAGKNL CYKMFMMSDLTIPVKRGCID VCPKNSLLVK YVCCNTDRCN | Anti-tumor |
| CN115957303 (A) [13] | KFFKRLLKSVRRAVKKFRKKPRLIGLSTLL | Anti-viral |
| CN109929020 (A) [13] | LECHNQQSSQTPTTTGCSGGETNCYKKRWRDHRGYRTERGCGCPSVKNGIEINCCTTDRCNN | Analgesic |
| CN117756907 (A) [13] | LECHNQQSSQ TPTTTGCSGG ETNCYKKRWRDHRGYRTERG CGCPSVKNGI EINCCTTDRC NN | Antitumor |
| WO2017190263 (A1) [13] | KDHRGTRIER | Analgesic |
| CN114409757 (A) [13] | LECHNQQSSQTPTTTGCSGGETNCYKKRWRDHRGYRTERGCGCPSVKDGIEINCCTTDRCNN | Analgesic |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zona Rubio, D.C.; Aragón, D.M.; Almeida Alves, I. Innovations in Snake Venom-Derived Therapeutics: A Systematic Review of Global Patents and Their Pharmacological Applications. Toxins 2025, 17, 136. https://doi.org/10.3390/toxins17030136
Zona Rubio DC, Aragón DM, Almeida Alves I. Innovations in Snake Venom-Derived Therapeutics: A Systematic Review of Global Patents and Their Pharmacological Applications. Toxins. 2025; 17(3):136. https://doi.org/10.3390/toxins17030136
Chicago/Turabian StyleZona Rubio, Diana Carolina, Diana Marcela Aragón, and Izabel Almeida Alves. 2025. "Innovations in Snake Venom-Derived Therapeutics: A Systematic Review of Global Patents and Their Pharmacological Applications" Toxins 17, no. 3: 136. https://doi.org/10.3390/toxins17030136
APA StyleZona Rubio, D. C., Aragón, D. M., & Almeida Alves, I. (2025). Innovations in Snake Venom-Derived Therapeutics: A Systematic Review of Global Patents and Their Pharmacological Applications. Toxins, 17(3), 136. https://doi.org/10.3390/toxins17030136






