1. Introduction
Botulinum toxin (BoNT) is a neurotoxin whose primary site of action is nerve terminals and cranial nuclei. It reduces muscle hyperactivity by inhibiting the release of acetylcholine at the neuromuscular junction (it cleaves the 25 kDa protein associated with the synaptosome, which is necessary for vesicle docking and neurotransmitter release) [
1]. As a result, BoNT injections can reduce spasticity by temporarily paralyzing muscle activity and decreasing muscle hypertonia [
2]. Currently, BoNT is a first-line agent for treating focal and multifocal spasticity [
3]. A large amount of research has shown that BoNT injections are effective in correcting muscle imbalances, decreasing muscle tone, and enhancing muscle function, especially in cases of hereditary spastic paraplegias (HSPs) [
4], stroke [
5], dystonia [
6], spinal cord injury [
7], cerebral palsy [
8], and multiple sclerosis [
9].
There are significant pharmacological differences between botulinum toxin (BoNT) preparations, particularly in terms of potency and the duration of action [
10] BoNT is commercially available in France under two serotypes, A and B. Type A botulinum toxin is effective in very small amounts, with a long-lasting effect that can last for several months. These properties explain why it has been used to block neuromuscular junctions in a targeted manner through local injections [
11]. It is manufactured and distributed by different laboratories (
Table 1).
The units used to quantify the activity of each toxin are specific to the laboratory preparing the formulation in question. In the OnaA and AboA formulations, the neurotoxin is associated with a larger protein complex containing accessory proteins, whereas the IncoA formulation contains a purified neurotoxin, free from complexing proteins, and thus exhibits a higher specific biological activity [
12]. Since each dosage is specific to the laboratory and there are no clear differences in terms of efficacy between the different preparations, their comparability remains the subject of ongoing debate [
13].
IncoA was proven to be as effective as OnaA, with a comparable adverse event profile when a clinical conversion ratio of 1:1 or 1:1.2 was applied [
14,
15,
16]. Therefore, clinical analyses demonstrate that the clinical conversion rate between OnaA and IncoA is very close to 1:1. For the conversion from OnaA to AboA, the commonly used conversion factor is ≤1:3. Studies using this conversion report clinical equivalence [
17,
18,
19]. When the conversion factor is close to 1:3, AboA shows superior efficacy [
20,
21,
22]. When the conversion ratio exceeds 1:3, AboA demonstrates both superior efficacy and a longer duration of action compared to OnaA, though with more adverse events [
23,
24,
25]. While the most frequent conversion ratios are 1:3 or 1:4 [
26], in clinical practice, they can vary from 1:1 [
27] to 1:11 [
28]. This broad spectrum of conversion ratios reflects actual clinical practice, where the treating physician decides the number of muscles to be treated and the dosage, based on the patient’s medical condition, impairment profile, and treatment objectives [
13]. There is no uniform treatment strategy, and the doses used have varied significantly over the years. These total doses are not evidence-based but rather based on “expert opinion” and primarily on small clinical trials conducted by researchers [
29]. Thus, there is considerable variation in clinical practices across injection sites, botulinum toxin-A brand selection, and dosing [
30].
Despite the existence of dosage conversion guidelines, it is commonly accepted that these conversions should not be interchanged with other botulinum toxin preparations [
31]. However, an observational study following a switch from AboA to OnaA in 48 patients demonstrated a perceived better efficacy with OnaA, with 19 adverse events reported during the AboA phase and none during the OnaA phase [
32]. In another retrospective study, 87 patients who switched from AboA to ObnaA or vice versa were analyzed. No statistically significant differences in outcome measures were observed [
25].
Injections are sometimes accompanied by adverse effects, with reported rates varying across studies. For children with cerebral palsy, the incidence of adverse effects ranges from 8.7% of subjects, without distinction between toxin brands [
33], to as high as 23.2% [
34]. However, the study by Wabbels et al., 2011 [
35] found no differences in the frequency of adverse events between OnaA and IncoA, a finding supported by Saad et al., 2014 [
36] or Dressler et al., 2014 [
37].
Based on the available data, it cannot be conclusively stated that switching from one toxin brand to another should be avoided entirely due to loss of effectiveness or an increase in adverse effects. However, the findings suggest that such a switch can be feasible if it is carefully prepared, particularly in terms of dosage adjustments and patient-specific considerations. Proper monitoring and individualized treatment planning appear to be key factors in ensuring the switch is both effective and well-tolerated.
To address this question, an opportunity arose in France. On 1 July 2023, a publication in the Official Journal announced that only one toxin (AboA, Dysport) would continue to be reimbursed, compelling physicians to modify the toxin brand and injection patterns for their patients. Subsequently, on 25 July, a second brand (IncoA, Xeomin) was also approved for reimbursement. On 28 September 2023, the Official Journal reintroduced reimbursement for Botox toxin. Injection follow-up data are traditionally collected by the teams at the Saint-Hélier Rehabilitation department (54 rue St Helier, Rennes, France); it was determined that these data would serve as the foundation for a retrospective study.
This article adheres to the STROBE guidelines for the reporting of observational studies.
4. Discussion
The primary objective of this study was to enhance knowledge about toxin changes and injection patterns. The results show that even when subjected to unprepared changes, the transition is possible without worsening adverse events. However, individual clinical dimensions remain a central focus of toxin injection interventions. It is difficult to generalize these results.
4.1. Study Limitations and Biases
As a retrospective study, this analysis is subject to several inherent biases and limitations that must be acknowledged. The retrospective nature restricted the ability to control for confounding variables, as data collection depended on previously recorded clinical information, which may have lacked uniformity or completeness. Additionally, the absence of randomization and the reliance on real-world data made it challenging to establish causal relationships between the toxin switch and clinical outcomes. Recall bias and potential inconsistencies in follow-up duration across patients further limit the generalizability of the findings. Despite these limitations, this study provides valuable insights into the clinical practice of botulinum toxin switching, emphasizing the need for prospective, controlled studies to validate these observations.
4.2. Weighting of Adverse Events and Negative Perceptions
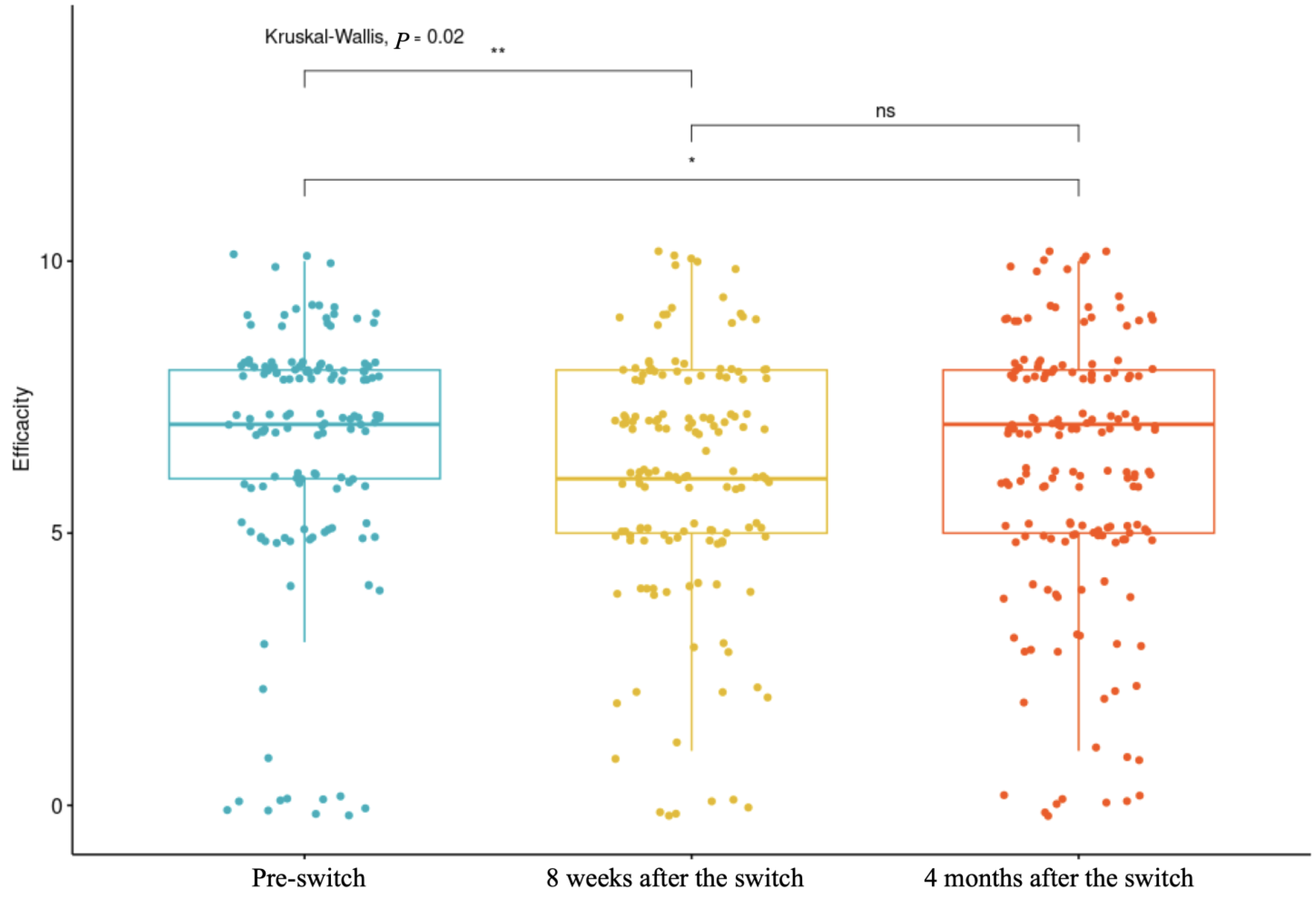
The number of individuals reporting that the new toxin was less effective than the previous one was higher than those reporting improved efficacy (32.75% vs. 20.47%). These findings must be interpreted with caution, as placebo and nocebo effects have been shown to influence outcomes when switching molecules during treatment. Studies have suggested that there is a strong placebo effect in the reduction in spasticity [
5]. Similarly to placebo effects, the nocebo effect is a cognitive and idiosyncratic phenomenon with specific biological underpinnings. It is mediated by distinct neurotransmitters within well-mapped brain regions, likely located in the limbic system network. Zis et al. (2018) [
39] demonstrated that adverse events related to drug treatments are widespread across various neurological disorders, particularly in conditions such as headaches, Parkinson’s disease, Alzheimer’s disease, depression, epilepsy, multiple sclerosis, and motor neuron disease. In Parkinson’s disease, it has also been shown that motor performance can be modulated in two opposing directions by placebos and nocebos, depending on positive or negative expectations regarding motor function [
40]. A strong nocebo effect could negatively impact treatment adherence and overall efficacy [
41].
The rates of adverse events (AEs) in placebo groups (nocebo AE rates) range from 25% in randomized controlled trials (RCTs) investigating symptomatic treatments for multiple sclerosis to nearly 80% in RCTs on motor neuron disease [
42]. The grouped discontinuation rates due to AEs in placebo groups (nocebo discontinuation rates) vary from 2% in RCTs on multiple sclerosis to almost 10% in RCTs on Parkinson’s disease. In the observational study by Wetwittayakhlang et al. (2024) [
43], involving patients who underwent a biosimilar switch, a nocebo effect was observed in 13.3% of cases, with a treatment discontinuation rate of 4.8%. Similarly, switching from branded medications to generics has been associated with an increase in side effects attributable to the nocebo effect [
44].
During a switch, it is also possible that physicians administering the injections may have inadvertently heightened patients’ awareness of potential side effects as part of switch monitoring. However, learning about anticipated symptoms may lead to heightened self-focused attention, potentially resulting in the perception or reporting of a greater number of such symptoms [
45,
46]. In the context of a biosimilar switch for anti-inflammatory treatment, Tweehuysen et al. (2017) [
47] reported that 24% of patients discontinued the new treatment due to an increase in subjective complaints, including a higher number of perceived painful joints and/or subjective adverse events. These outcomes are likely partially explained by nocebo effects and/or incorrect causal attributions.
However, adopting an effective communication strategy can significantly reduce the nocebo effect [
42]. In an experimental setting, Varelmann et al. (2010) [
48] demonstrated this during childbirth. Before administering an epidural injection, women were told one of two statements: “We are going to administer a local anesthetic that will numb the area, and you will feel comfortable during the procedure”, or “You are going to feel a big bee sting; this is the most painful part of the procedure”. Women reported significantly higher pain levels associated with the second statement.
A structured communication program during a biosimilar switch can mitigate potential nocebo effects. For instance, in the Netherlands, a “One Voice” package [
49] provides health institutions with guidance on language and terminology, ensuring a standardized and unified approach to communication about biosimilar medications from the receptionist to the physician.
4.3. Clinical Impact of Botulinum Toxin Switching in Spasticity Management
Switching botulinum toxin formulations in the treatment of spasticity can have significant clinical implications, particularly in patients who exhibit suboptimal responses, develop tolerance, or experience adverse effects with their initial toxin. The ability to transition between different botulinum toxin products allows for personalized treatment strategies, potentially improving efficacy and patient satisfaction. Clinical observations suggest that switching may enhance therapeutic outcomes by leveraging differences in pharmacological properties, such as potency, diffusion characteristics, and immunogenicity profiles. Additionally, it provides an opportunity to optimize the treatment regimen for patients who may benefit from alternate dosing schedules or the improved targeting of specific muscle groups. However, the switch requires careful assessment of prior treatment history, dosage equivalence, and patient-specific factors to minimize risks and ensure therapeutic continuity. This retrospective analysis sheds light on the practical considerations and benefits associated with toxin switching, ultimately supporting more flexible and effective management of spasticity in diverse clinical contexts.
4.4. Limtation
This study has certain limitations. Firstly, the data come from only one center, so it is possible that the procedure associated with the care pathway developed by the center and used by the injecting physicians is related to the low number of adverse events. The second limitation is that the diversity of injection sites and injection guidance techniques does not allow for the identification of sites or techniques associated with lack of efficacy and satisfaction. The third limitation is that we do not have complete data on previous treatment courses and injection frequency. However, recent animal studies have shown that repeated injections lead to persistent atrophy in muscle fibers with fibrosis [
50,
51]. Some of the failures could be due not to the switch but to the natural progression associated with repeated injections.
5. Conclusions
In conclusion, this study found that transitioning between different toxin brands is feasible without increasing adverse effects, provided there is appropriate monitoring and an individualized approach. Dose adjustments varied, highlighting the importance of personalizing treatment. While the majority of patients (57.06%) chose to continue with their new treatment, a significant number (42.93%) preferred to return to their initial treatment. This suggests that factors such as habit, perceived efficacy, and individual responses to toxins play a crucial role in patient decisions. The perceived efficacy varied, with some patients experiencing improvement and others deterioration. However, the median perceived efficacy remained stable, which could be attributed to placebo and nocebo effects. Nocebo effects possibly influence the perception of efficacy and side effects during treatment changes. Despite some differences observed between the groups, these were not statistically significant. These results underline the importance of an individualized approach and careful monitoring during botulinum toxin changes. Although this study has limitations due to its retrospective nature, it provides valuable insights into real-world clinical practice and emphasizes the need for controlled prospective studies to validate these findings. In practice, physicians should consider patient preferences, adjust dosages precisely, and closely monitor potential adverse effects. Effective communication strategies should also be implemented to minimize nocebo effects and improve tolerance to treatment changes. Ultimately, this study suggests that toxin changes are possible, but their success depends on several patient-specific factors and rigorous clinical follow-up.
6. Materials and Methods
6.1. Context
The Saint-Hélier Foundation (54 rue St Helier, Rennes, France) includes, among other services, a functional rehabilitation department that treats approximately 5000 patients per year. It is also a specialized consultation center in physical medicine that routinely administers botulinum toxin injections. The switch applied to all injections administered after 1 July 2023. Reimbursement for Botox toxin was reintroduced on 28 September 2023. The protocol was subsequently written and submitted for approval. This study received a favorable opinion from the ethics committee of Rennes University Hospital on 15 November 2023. Following the approval of the ethics committee, patients who had undergone the switch and were still being followed at the Saint-Hélier rehabilitation department were contacted to obtain their consent for the collection of their data starting from the time of the switch.
6.2. Population
The inclusion criteria for this study required participants to be capable of providing their free and informed non-opposition to the use of their data within the framework of the study. Eligible participants were adult men or women over the age of 18 who presented with spasticity necessitating botulinum toxin injections in the upper and/or lower limbs. Exclusion criteria included individuals under legal protection measures such as guardianship, curatorship, or judicial safeguard, as well as pregnant or breastfeeding women. Additionally, patients who explicitly opposed the use of their data were not included in the study.
6.3. Variables and Measurements
Several criteria were analyzed using the patients’ medical records. The data collected included the patient’s pathology and information regarding the change in botulinum toxin, both before and after the switch. The doses injected prior to and following the change were also documented. Subjective evaluations by patients were assessed using a numerical scale (NS) to measure the perceived effectiveness of the previous injection on the day of reinjection, as well as their tolerance to the toxin at its peak efficacy (5 to 8 weeks post-injection) and at the time of reinjection (3 to 6 months post-injection).
Adverse effects were recorded, including their type, if present. Patients also provided subjective evaluations of the toxin’s effectiveness at peak efficacy and at the time of reinjection using the NS. Comparisons were made to assess any difference in effectiveness before and after the toxin change. Additionally, evaluations were performed using a 3-point Likert scale (worse/same/better) for the perceived effectiveness of the toxin at its peak and at the time of reinjection.
Finally, during the injection following the toxin change, the type of toxin reinjected and its total dose were documented.
6.4. Bias
Eight physicians specializing in physical medicine and rehabilitation (MPR) at the Pôle Saint Helier routinely administer botulinum toxin injections and thus participated in this study as injectors. The results were evaluated considering this diversity in order to eliminate any operator bias.
6.5. Sample
No sample size calculation was performed. The goal was to collect as many data as possible during the six-month period between the suspension and reintroduction of reimbursement. However, based on the number of patients receiving botulinum toxin injections at the Pôle Saint Helier center, it was expected that approximately 200 to 250 patient records would be analyzed over this six-month period.
6.6. Statistical Consideration
Statistical analyses included a description of the population based on their pathologies and a description of the type of switch with the brands and doses used. The normality of variables was assessed to determine the use of parametric or non-parametric tests. A comparison of satisfaction, tolerance, and adverse effects was conducted across the three evaluation time points: the first evaluated injection efficacy before the switch, the second evaluated efficacy approximately 8 weeks after the switch, and the third evaluated efficacy approximately 4 months after the switch. Predictive factors of satisfaction were analyzed using multivariate analyses. No data imputation was performed for missing data. The statistics were conducted using RStudio v. 2024.12.1-563.