Botulinum Toxin Combined with Robot-Assisted Therapy for Post-Stroke Spasticity: A Systematic Review
Abstract
1. Introduction
2. Results
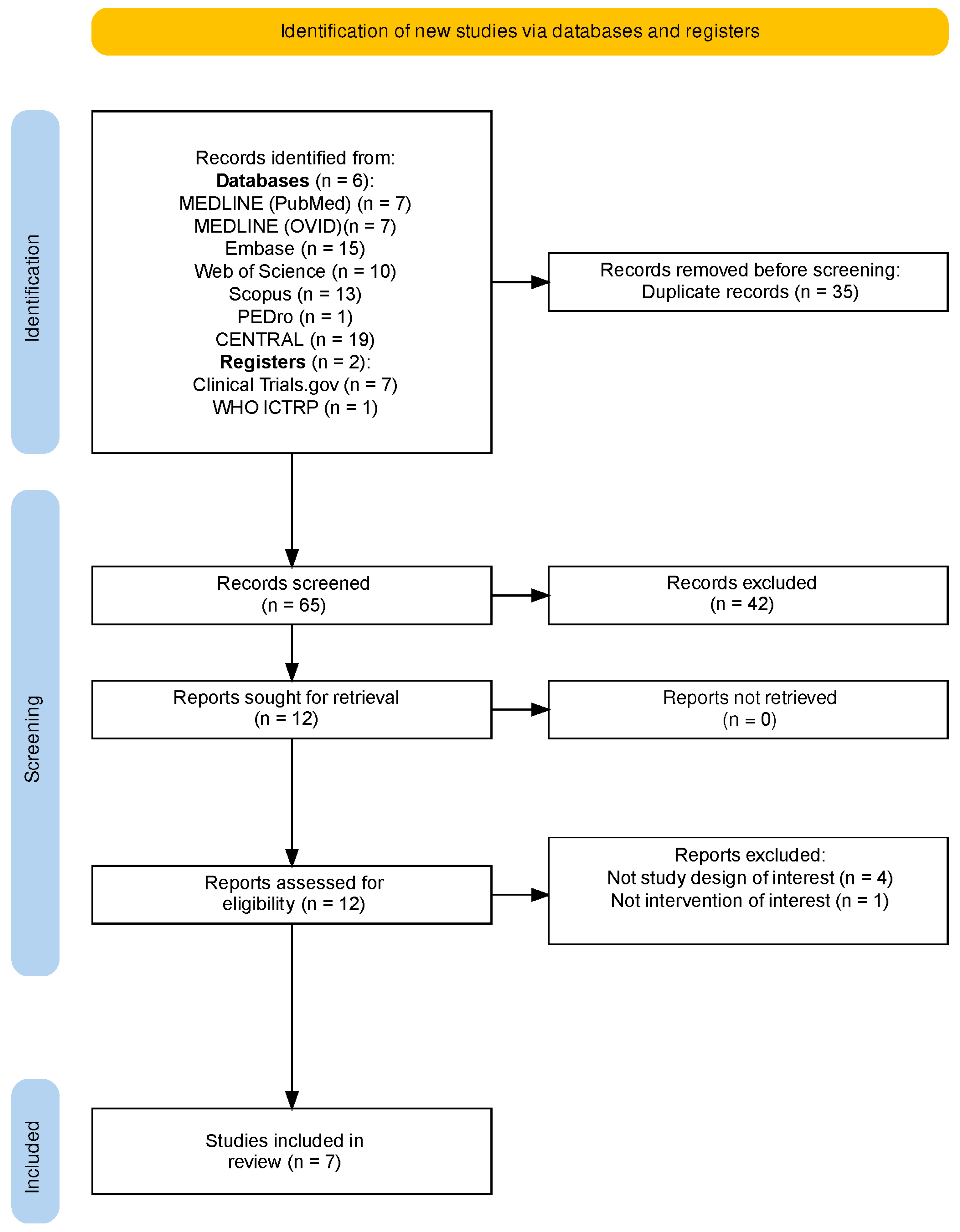
2.1. Study Selection
2.2. Study Characteristics
2.3. Participant Characteristics
2.4. Interventions Characteristics
2.4.1. BoNT-A Administration
2.4.2. Rehabilitation Protocols
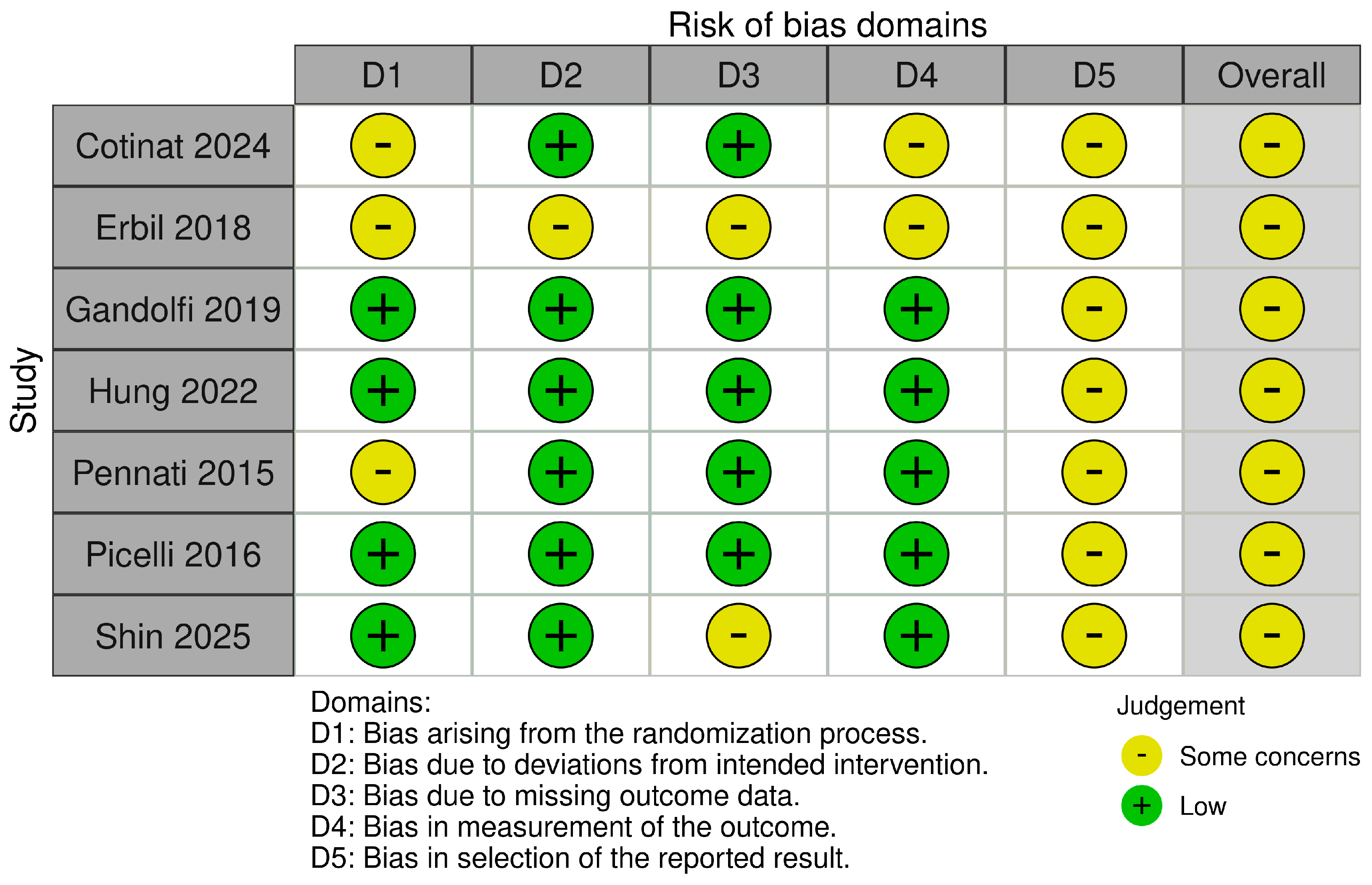
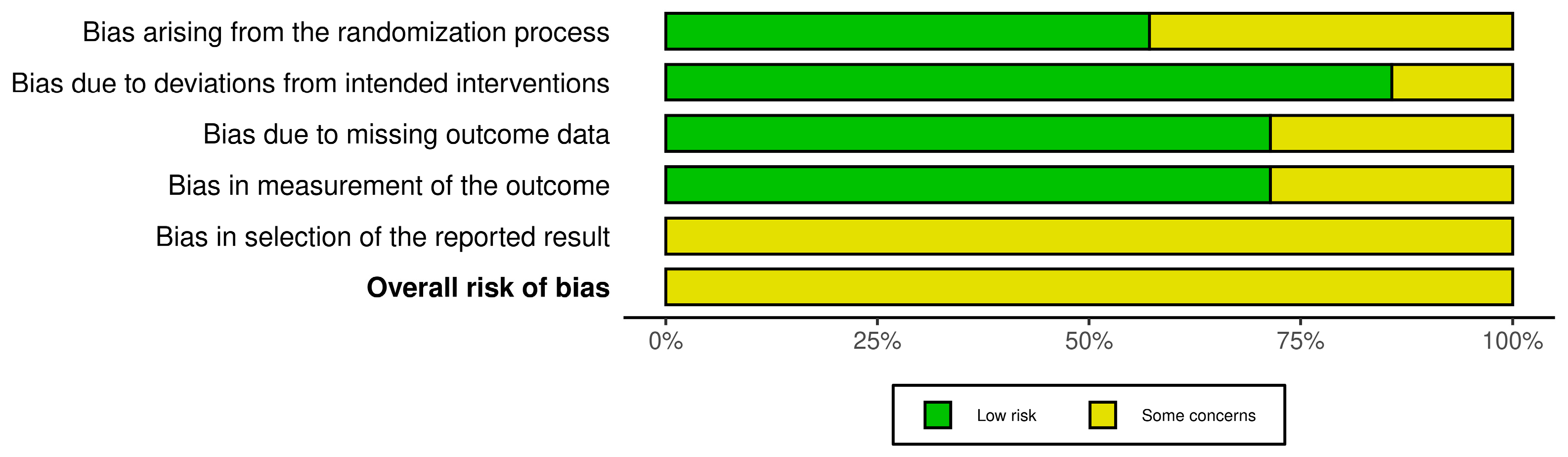
2.5. Risk of Bias Assessment
2.6. Effects of Interventions
2.6.1. Primary Outcomes: MAS
2.6.2. Motor Function
2.6.3. Functional Outcomes, Daily Activities, and Quality of Life
2.6.4. Adverse Events
2.7. Synthesis of Results
3. Discussion
Limitations
4. Conclusions
5. Materials and Methods
5.1. Search Strategy
5.2. Eligibility Criteria
5.3. Study Selection and Data Extraction
5.4. Risk of Bias
5.5. Data Synthesis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feigin, V.L.; Brainin, M.; Norrving, B.; Martins, S.O.; Pandian, J.; Lindsay, P.; Grupper, M.F.; Rautalin, I. World Stroke Organization: Global Stroke Fact Sheet 2025. Int. J. Stroke 2025, 20, 132. [Google Scholar] [CrossRef]
- Lance, J. Symposium Synopsis. In Spasticity: Disordered Motor Control; Felman, R.G., Young, R.R., Koella, W.P., Eds.; Book Medic; Chicago Year Book Medical Publications: Chicago, IL, USA, 1980; pp. 485–494. [Google Scholar]
- Wissel, J.; Manack, A.; Brainin, M. Toward an Epidemiology of Poststroke Spasticity. Neurology 2013, 80, S13–S19. [Google Scholar] [CrossRef] [PubMed]
- Facciorusso, S.; Spina, S.; Picelli, A.; Baricich, A.; Francisco, G.E.; Molteni, F.; Wissel, J.; Santamato, A. The Role of Botulinum Toxin Type-A in Spasticity: Research Trends from a Bibliometric Analysis. Toxins 2024, 16, 184. [Google Scholar] [CrossRef]
- Andringa, A.; van de Port, I.; van Wegen, E.; Ket, J.; Meskers, C.; Kwakkel, G. Effectiveness of Botulinum Toxin Treatment for Upper Limb Spasticity Poststroke Over Different ICF Domains: A Systematic Review and Meta-Analysis. Arch. Phys. Med. Rehabil. 2019, 100, 1703–1725. [Google Scholar] [CrossRef] [PubMed]
- Mehrholz, J.; Pohl, M.; Platz, T.; Kugler, J.; Elsner, B. Electromechanical and Robot-Assisted Arm Training for Improving Activities of Daily Living, Arm Function, and Arm Muscle Strength after Stroke. Cochrane Database Syst. Rev. 2018, 9, CD006876. [Google Scholar] [CrossRef]
- Veerbeek, J.M.; Langbroek-Amersfoort, A.C.; Van Wegen, E.E.H.; Meskers, C.G.M.; Kwakkel, G. Effects of Robot-Assisted Therapy for the Upper Limb after Stroke. Neurorehabil Neural Repair. 2017, 31, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Howard, I.M.; Patel, A.T. Spasticity Evaluation and Management Tools. Muscle Nerve 2023, 67, 272–283. [Google Scholar] [CrossRef]
- Paolucci, T.; Agostini, F.; Mangone, M.; Bernetti, A.; Pezzi, L.; Liotti, V.; Recubini, E.; Cantarella, C.; Bellomo, R.G.; D’Aurizio, C.; et al. Robotic Rehabilitation for End-Effector Device and Botulinum Toxin in Upper Limb Rehabilitation in Chronic Post-Stroke Patients: An Integrated Rehabilitative Approach. Neurol. Sci. 2021, 42, 5219–5229. [Google Scholar] [CrossRef]
- Kinnear, B.Z.; Lannin, N.A.; Cusick, A.; Harvey, L.A.; Rawicki, B. Rehabilitation Therapies After Botulinum Toxin-A Injection to Manage Limb Spasticity: A Systematic Review. Phys. Ther. 2014, 94, 1569–1581. [Google Scholar] [CrossRef]
- Hara, T.; Momosaki, R.; Niimi, M.; Yamada, N.; Hara, H.; Abo, M. Botulinum Toxin Therapy Combined with Rehabilitation for Stroke: A Systematic Review of Effect on Motor Function. Toxins 2019, 11, 707. [Google Scholar] [CrossRef]
- Elia, A.E.; Filippini, G.; Calandrella, D.; Albanese, A. Botulinum Neurotoxins for Post-Stroke Spasticity in Adults: A Systematic Review. Mov. Disord. 2009, 24, 801–812. [Google Scholar] [CrossRef]
- Allart, E.; Mazevet, D.; Idée, S.; Boyer, F.C.; Bonan, I. Adjunct Therapies after Botulinum Toxin Injections in Spastic Adults: Systematic Review and SOFMER Recommendations. Ann. Phys. Rehabil. Med. 2022, 65, 101544. [Google Scholar] [CrossRef]
- Mills, P.B.; Dossa, F. Transcutaneous Electrical Nerve Stimulation for Management of Limb Spasticity: A Systematic Review. Am. J. Phys. Med. Rehabil. 2016, 95, 309–318. [Google Scholar] [CrossRef]
- Shin, J.H.; Park, G.; Kim, H.; Cho, D.Y.; Kwon, S. Combined Effects and Timing of Robotic Training and Botulinum Toxin on Upper Limb Spasticity and Motor Function: A Single-blinded Randomized Controlled Pilot Study. J. Neuroeng. Rehabil. 2025, 22, 50. [Google Scholar] [CrossRef]
- Gandolfi, M.; Valè, N.; Dimitrova, E.K.; Mazzoleni, S.; Battini, E.; Filippetti, M.; Picelli, A.; Santamato, A.; Gravina, M.; Saltuari, L.; et al. Effectiveness of Robot-Assisted Upper Limb Training on Spasticity, Function and Muscle Activity in Chronic Stroke Patients Treated with Botulinum Toxin: A Randomized Single-Blinded Controlled Trial. Front. Neurol. 2019, 10, 41. [Google Scholar] [CrossRef]
- Hung, J.W.; Yen, C.L.; Chang, K.C.; Chiang, W.C.; Chuang, I.C.; Pong, Y.P.; Wu, W.C.; Wu, C.Y. A Pilot Randomized Controlled Trial of Botulinum Toxin Treatment Combined with Robot-Assisted Therapy, Mirror Therapy, or Active Control Treatment in Patients with Spasticity Following Stroke. Toxins 2022, 14, 415. [Google Scholar] [CrossRef] [PubMed]
- Pennati, G.V.; Da Re, C.; Messineo, I.; Bonaiuti, D. How Could Robotic Training and Botolinum Toxin Be Combined in Chronic Post Stroke Upper Limb Spasticity? A Pilot Study. Eur. J. Phys. Rehabil. Med. 2015, 51, 381–387. [Google Scholar]
- Erbil, D.; Tugba, G.; Murat, T.H.; Melike, A.; Merve, A.; Cagla, K.; Mehmetali, Ç.C.; Akay, Ö.; Nigar, D. Effects of Robot-Assisted Gait Training in Chronic Stroke Patients Treated by Botulinum Toxin-a: A Pivotal Study. Physiother. Res. Int. 2018, 23, e1718. [Google Scholar] [CrossRef] [PubMed]
- Picelli, A.; Bacciga, M.; Melotti, C.; La Marchina, E.; Ferrari, F.; Pontillo, A.; Corradi, J.; Tamburin, S.; Saltuari, L.; Corradini, C.; et al. Combined Effects of Robot-Assisted Gait Training and Botulinum Toxin Type A on Spastic Equinus Foot in Patients with Chronic Stroke: A Pilot, Single Blind, Randomized Controlled Trial. Eur. J. Phys. Rehabil. Med. 2016, 52, 759–766. [Google Scholar] [PubMed]
- Cotinat, M.; Celerier, M.; Arquillière, C.; Flipo, M.; Prieur-Blanc, N.; Viton, J.M.; Bensoussan, L. Robotic Gait Training and Botulinum Toxin Injection Improve Gait in the Chronic Post-Stroke Phase: A Randomized Controlled Trial. Ann. Phys. Rehabil. Med. 2024, 67, 101785. [Google Scholar] [CrossRef]
- Saini, M.; Singh, N.; Kumar, N.; Kumaran, S.; Mehndiratta, A.; Srivastava, M.P. Cortical Reorganization in Stroke Patients Using Upper-Limb Robotic Rehabilitation Therapy. J. Neurol. Sci. 2021, 429, 118758. [Google Scholar] [CrossRef]
- Veverka, T.; Hlustík, P.; Tomásová, Z.; Hok, P.; Otruba, P.; Král, M.; Tüdös, Z.; Zapletalová, J.; Herzig, R.; Krobot, A.; et al. BoNT-A Related Changes of Cortical Activity in Patients Suffering from Severe Hand Paralysis with Arm Spasticity Following Ischemic Stroke. J. Neurol. Sci. 2012, 319, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Xiao, H.; Zhu, Z.; Guan, Y.; Wang, Y. Research Progress in the Use of Botulinum Toxin Type a for Post-Stroke Spasticity Rehabilitation: A Narrative Review. Ann. Med. 2025, 57, 2521427. [Google Scholar] [CrossRef]
- Subramanian, S.K.; Feldman, A.G.; Levin, M.F. Spasticity May Obscure Motor Learning Ability after Stroke. J. Neurophysiol. 2018, 119, 5–20. [Google Scholar] [CrossRef]
- Facciorusso, S.; Guanziroli, E.; Brambilla, C.; Spina, S.; Giraud, M.; Molinari Tosatti, L.; Santamato, A.; Molteni, F.; Scano, A. Muscle Synergies in Upper Limb Stroke Rehabilitation: A Scoping Review. Eur. J. Phys. Rehabil. Med. 2024, 60, 767–792. [Google Scholar] [CrossRef]
- Brin, M.F.; James, C.; Maltman, J. Botulinum Toxin Type A Products Are Not Interchangeable: A Review of the Evidence. Biologics 2014, 8, 227–241. [Google Scholar] [CrossRef]
- Gassert, R.; Dietz, V. Rehabilitation Robots for the Treatment of Sensorimotor Deficits: A Neurophysiological Perspective. J. Neuroeng. Rehabil. 2018, 15, 46. [Google Scholar] [CrossRef] [PubMed]
- Shambushankar, A.K.; Jose, J.; Gnanasekaran, S.; Kaur, G. Cost-Effectiveness of Telerehabilitation Compared to Traditional In-Person Rehabilitation: A Systematic Review and Meta-Analysis. Cureus 2025, 17, e79028. [Google Scholar] [CrossRef]
- Spina, S.; Facciorusso, S.; Cinone, N.; Santoro, L.; Castagna, A.; Ramella, M.; Molteni, F.; Santamato, A. Integrating Telemedicine in Botulinum Toxin Type-A Treatment for Spasticity Management: Perspectives and Challenges from Italian Healthcare Professionals. Toxins 2024, 16, 529. [Google Scholar] [CrossRef]
- Ryu, J.S.; Lee, J.W.; Lee, S., II; Chun, M.H. Factors Predictive of Spasticity and Their Effects on Motor Recovery and Functional Outcomes in Stroke Patients. Top. Stroke Rehabil. 2010, 17, 380–388. [Google Scholar] [CrossRef]
- Wissel, J.; Verrier, M.; Simpson, D.M.; Charles, D.; Guinto, P.; Papapetropoulos, S.; Sunnerhagen, K.S. Post-Stroke Spasticity: Predictors of Early Development and Considerations for Therapeutic Intervention. PMR 2015, 7, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Gingrich, N.; Bosancich, J.; Schmidt, J.; Sakakibara, B.M. Capability, Opportunity, Motivation, and Social Participation after Stroke. Top. Stroke Rehabil. 2023, 30, 423–435. [Google Scholar] [CrossRef]
- Facciorusso, S.; Spina, S.; Picelli, A.; Baricich, A.; Molteni, F.; Santamato, A. May Spasticity-Related Unpleasant Sensations Interfere with Daily Activities in People with Stroke and Traumatic Brain Injury? Secondary Analysis from the CORTOX Study. J. Clin. Med. 2024, 13, 1720. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed]
| Author, Year | Study Aim | Study Design | Participants (N, Characteristics) |
|---|---|---|---|
| Pennati et al. [18] (2015) | Verify how combined short robotic training and chemical neurolysis reduces spasticity and improves function in chronic UL. | Prospective, single-blind RCT, pilot. | N = 15 (Robot only = 8, BoNT-A + Robot = 7). Chronic post-stroke (≥6 mo), severe UL spastic paresis. |
| Picelli et al. [20] (2016) | Evaluate combined effects of RAT and BoNT-A on spastic equinus foot. | Pilot, single-blind RCT. | N = 22 (Group 1 = 11, Group 2 = 11). Adult outpatients, spastic equinus due to chronic stroke (≥6 mo), FAC ≥ 4. |
| Erbil et al. [19] (2018) | Investigate combined effects of RAT + PT vs. PT only on balance and gait after BoNT-A. | Prospective RCT. | N = 48 (final N = 29 RAT, N = 14 Control). Chronic stroke (≥6 mo), received BoNT-A for LE spasticity, ambulatory, BBS ≥ 20. |
| Gandolfi et al. [16] (2019) | Evaluate effects of Robot-assisted UL training on UL spasticity, function, muscle strength, sEMG after BoNT. | Single-blind RCT. | N = 32 (EG = 16, CG = 16). Chronic post-stroke (≥6 mo), UL spastic hemiparesis, MAS (shoulder and elbow) ≤ 3 and ≥1+. |
| Hung et al. [17] (2022) | Investigate effects of RAT, MT, or AC combined with BoNT-A on motor recovery, spasticity, daily function. | Pilot RCT. | N = 37 (RT = 13, MT = 12, AC = 12). Chronic (≥6 mo) spastic hemiplegic stroke, MAS > 1 (UE), FMA 17–56. |
| Cotinat et al. [21] (2024) | Compare efficacy of RAT vs. PT on gait after BoNT-A in triceps surae; assess timing. | RCT, cross-over. | N = 33 (15A, 18B). Chronic stroke (≥6 mo), triceps surae spasticity inducing gait impairment. |
| Shin et al. [15] (2025) | Evaluate combined effects of RAT& BONT-A on UL motor function/spasticity and investigate optimal timing of administration. | Single-blinded, 4-arm RCT, pilot study | N = 42 enrolled, 40 completed. Chronic stroke (≥6 mo), ULS, FMA ≤ 45, MAS elbow flexor ≥ 1+. |
| First Author, Year | Target Limb BoNT-A Type and Dosage | Robotic Device | Experimental Group | Control Group | Outcome Measures | Key Results on Spasticity | Key Results— Secondary Outcomes |
|---|---|---|---|---|---|---|---|
| Pennati et al. [18], 2015 | Upper Limb Abo Individual dose titration. | ReoGo | BoNT-A + RAT: 2 days/wk, 60 min/sessions– 10 sessions | RAT only: 10 sessions (60 min each, 2–3×/wk) ReoGo UL training. | FMA, B&B Test, MAS, FIM, Euro-Qol, sEMG. | Greater mean decrease in MAS for the BoNT-A + RAT group (change in −0.86) compared to the RAT only group (change of −0.14). | Both groups improved in FMA (RAT only: 8.25; BoNT-A + RAT: 5.29). B&B: RAT only > BoNT-A + RAT. sEMG: Both groups showed improved muscle activation, reduced co-contraction. |
| Picelli et al. [20], 2016 | Lower Limb Abo 250 U per muscle part (Total 750 U). | G-EO System | BoNT-A + ES + RAT: 5 consecutive days, 30 min/day | BoNT-A + Elec. Stim only: No RAT. | MAS, Tardieu scale, 6MWT. | Both groups significantly reduced their MAS scores (p < 0.5). No significant between-group difference was found (p = 0.852). | 6MWT: Group 1 (RAT) > Group 2 (BoNT-A only) for T1-T0 change (diff ~26 m, p = 0.045). |
| Erbil et al. [19], 2018 | Lower Limb Abo At least 300 U to plantar flexor group. | RoboGait | BoNT-A + RAT + PT: 3 wks, weekdays. 30 min RAT + 60 min PT per session. | BoNT-A + PT only: 3 wks, weekdays. 90 min PT per session. | MAS, Tardieu Scale, BBS, TUG, RVGA. | Both groups showed significant reductions in MAS. There was no significant between-group difference for MAS (p = 0.288 at week 12). | RAT group > Control for TUG, BBS, RVGA change from baseline at Wk6 and Wk12 (p < 0.01 for all). |
| Gandolfi et al. [16], 2019 | Upper Limb Ona/Abo/Inco Dosage based on spasticity severity. | Armotion | BoNT-A + RAT: 2 days/wk, 45 min/session (10 min passive mob/stretch + 35 min RAT)–10 sessions | BoNT-A + PT-: 5 wks, 2×/wk, 45 min/session. 10 min passive mob/stretch + 35 min conventional UL exercises. | MAS, FMA, MRC, sEMG (EG only). | Both groups significantly reduced their MAS scores (p = 0.008). No significant between-group difference was found (p > 0.05). | EG > CG for MRC total and specific movements (p < 0.05). sEMG (EG): Biceps activation changes. |
| Hung et al. [17], 2022 | Upper Limb Ona ~306–330 IU total | Manu-Track | BoNT-A + RAT: 3 days/wk, 75 min/session (45 min RAT + 30 min functional practice)- 24 sessions | BoNT-A + Mirror therapy: 8 wks, 3×/wk, 75 min/session. 45 min Mirror Therapy + 30 min functional practice. BoNT-A + PT: 8 wks, 3×/wk, 75 min/session. 45 min conventional task-oriented training + 30 min functional practice. | FMA, MAS, MAL-AOU/QOM, Arm activity level. | All three groups significantly reduced their MAS scores. No significant between-group difference was found (p = 0.841). | All 3 groups improved FMA, MAL post-treatment. No between-group difference post-treatment. At 3-mo FU, PT > RT/MT for MAL-QOM (p = 0.033). No between-group difference for arm activity level. |
| Cotinat et al. [21], 2024 | Lower Limb Ona 200–204 UI, Inco 200 UI | Lokomat | BoNT-A + RAT then PT: 5 days/wk, 45 min (15 min stretch, 30 min RAT)–10 sessions then 2 wks PT | BoNT-A + PT then RAT: 2 wks PT (5×/wk, 45 min: 15 min stretch, 30 min PT), then 2 wks RAT | 6MWT, 10mWT, TUG, BBS, MAS. | Both groups showed significant reductions in MAS. No significant between-group difference in MAS. | RAT > PT for 10 mWT. PT -then- RAT group had better BBS by W8 (p = 0.003). W0-W4: RAT > for 6MWT (33 m, p = 0.007). W0-W8: RAT -first group maintained 30 m 6MWT advantage (p = 0.019). |
| Shin et al. [15], 2025 | Upper Limb Ona Total 300 U | InMotion ARM | BoNT-A + RAT at W0: 5 days/wk, 30 min/sessions from W0–W4–20 sessions | BoNT-A at W0, RAT at W4: BONT-A at W0, then Robot -UL (5 days/wk, 30 min/session–20 sessions) from W4-W8. RAT at W0, BONT-A at W4: RAT (5 days/wk, 30 min/session from W0–W4–20 sessions), then BoNT-A at W4 BoNT-A + RAT at W4: 5 days/wk, 30 min/session from W4–W8–20 sessions | FMA, MAS, Robotic Kinematic Parameters, SIS | W0–W4: groups receiving BoNT-A (either alone or with robot) had a significantly greater spasticity reduction than the groups that did not (p < 0.01). W0–W8: no significant difference between the different timing schedules. | W0–W4: No significant Time x Group interactions for FMA. W0-W8: Group BONT-A at W0, RAT at W4 showed the most substantial and significant improvement in FMA and kinematic parameters compared to the reference group (p = 0.042). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Facciorusso, S.; Spina, S.; Filippetti, M.; Reebye, R.; Francisco, G.E.; Santamato, A. Botulinum Toxin Combined with Robot-Assisted Therapy for Post-Stroke Spasticity: A Systematic Review. Toxins 2025, 17, 569. https://doi.org/10.3390/toxins17120569
Facciorusso S, Spina S, Filippetti M, Reebye R, Francisco GE, Santamato A. Botulinum Toxin Combined with Robot-Assisted Therapy for Post-Stroke Spasticity: A Systematic Review. Toxins. 2025; 17(12):569. https://doi.org/10.3390/toxins17120569
Chicago/Turabian StyleFacciorusso, Salvatore, Stefania Spina, Mirko Filippetti, Rajiv Reebye, Gerard E. Francisco, and Andrea Santamato. 2025. "Botulinum Toxin Combined with Robot-Assisted Therapy for Post-Stroke Spasticity: A Systematic Review" Toxins 17, no. 12: 569. https://doi.org/10.3390/toxins17120569
APA StyleFacciorusso, S., Spina, S., Filippetti, M., Reebye, R., Francisco, G. E., & Santamato, A. (2025). Botulinum Toxin Combined with Robot-Assisted Therapy for Post-Stroke Spasticity: A Systematic Review. Toxins, 17(12), 569. https://doi.org/10.3390/toxins17120569