Occurrence and Levels of Emerging Alternaria Mycotoxins Detected in Spices and Herbs Marketed in Italy
Abstract
1. Introduction
2. Results
2.1. Method Performance
2.2. Occurrence of Alternaria Mycotoxins in Different Types of Spices and Herbs
3. Discussion
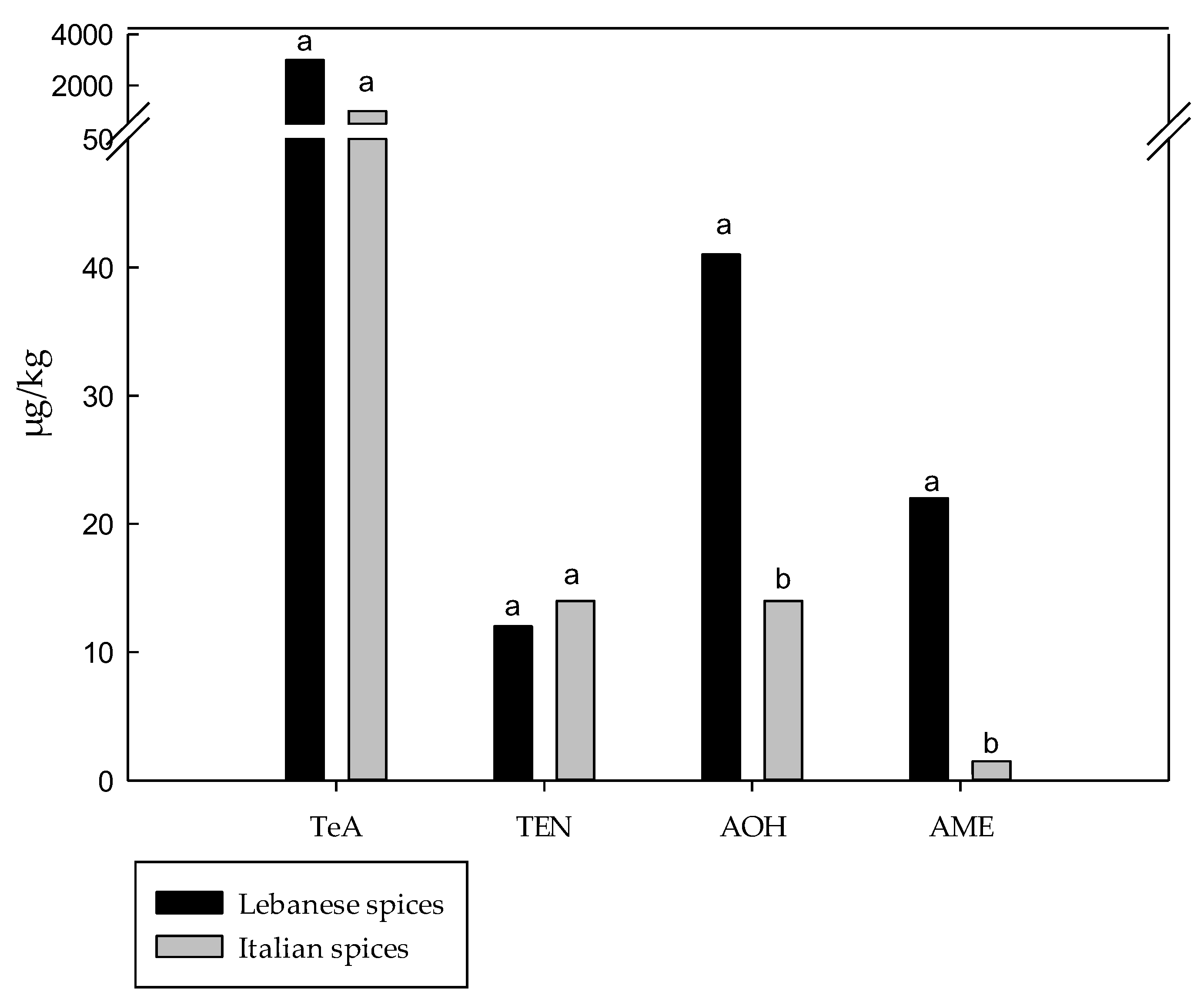
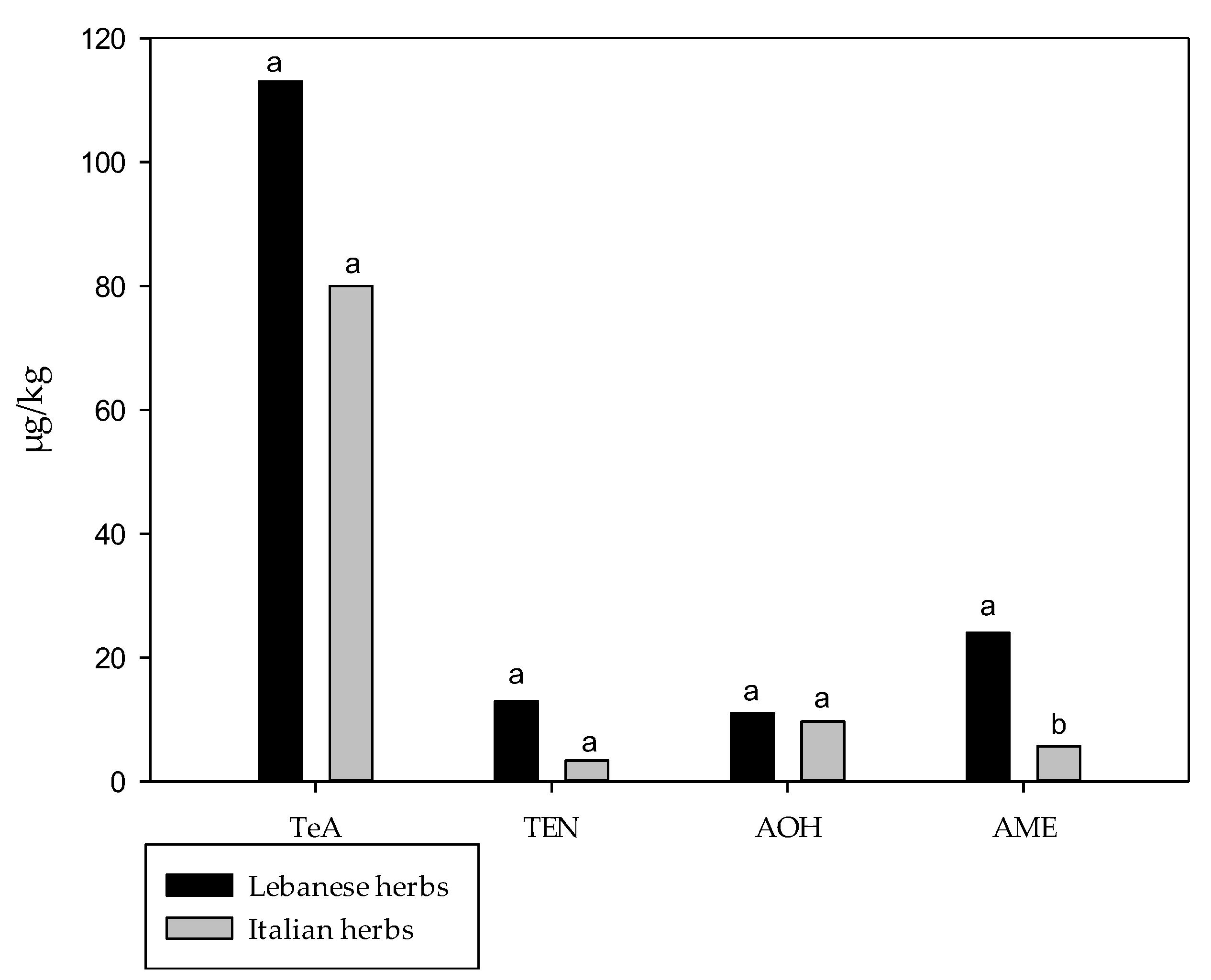
3.1. Comparison of Spices and Herbs Marketed in Italy and Lebanon
3.2. Comparison with the Scientific Literature Data
4. Conclusions
5. Materials and Methods
5.1. Sampling
5.2. Chemicals and Reagents
5.3. Determination of Alternaria Mycotoxins
5.3.1. Mycotoxins Extraction
5.3.2. LC-MS/MS Equipment and Parameters
5.3.3. Evaluation of Results
5.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hamad, G.M.; Mehany, T.; Simal-Gandara, J.; Abou-Alella, S.; Esua, O.J.; Abdel-Wahhab, M.A.; Hafez, E.E. A Review of Recent Innovative Strategies for Controlling Mycotoxins in Foods. Food Control 2023, 144, 109350. [Google Scholar] [CrossRef]
- Fernandes, C.; Casadevall, A.; Gonçalves, T. Mechanisms of Alternaria Pathogenesis in Animals and Plants. FEMS Microbiol. Rev. 2023, 47, fuad061. [Google Scholar] [CrossRef]
- Arcella, D.; Eskola, M.; Gómez Ruiz, J.A. Dietary Exposure Assessment to Alternaria Toxins in the European Population. EFSA J. 2016, 14, e04654. [Google Scholar] [CrossRef]
- Somma, S.; Amatulli, M.T.; Masiello, M.; Moretti, A.; Logrieco, A.F. Alternaria Species Associated to Wheat Black Point Identified through a Multilocus Sequence Approach. Int. J. Food Microbiol. 2019, 293, 34–43. [Google Scholar] [CrossRef]
- Patriarca, A. Alternaria in Food Products. Curr. Opin. Food Sci. 2016, 11, 1–9. [Google Scholar] [CrossRef]
- Crudo, F.; Varga, E.; Aichinger, G.; Galaverna, G.; Marko, D.; Dall’Asta, C.; Dellafiora, L. Co-Occurrence and Combinatory Effects of Alternaria Mycotoxins and Other Xenobiotics of Food Origin: Current Scenario and Future Perspectives. Toxins 2019, 11, 640. [Google Scholar] [CrossRef]
- Scientific Opinion on the Risks for Animal and Public Health Related to the Presence of Alternaria Toxins in Feed and Food. EFSA J. 2011, 9, 2407. [CrossRef]
- den Hollander, D.; Holvoet, C.; Demeyere, K.; De Zutter, N.; Audenaert, K.; Meyer, E.; Croubels, S. Cytotoxic Effects of Alternariol, Alternariol Monomethyl-Ether, and Tenuazonic Acid and Their Relevant Combined Mixtures on Human Enterocytes and Hepatocytes. Front. Microbiol. 2022, 13, 849243. [Google Scholar] [CrossRef]
- Solfrizzo, M. Recent Advances on Alternaria Mycotoxins. Curr. Opin. Food Sci. 2017, 17, 57–61. [Google Scholar] [CrossRef]
- Fehr, M.; Pahlke, G.; Fritz, J.; Christensen, M.O.; Boege, F.; Altemöller, M.; Podlech, J.; Marko, D. Alternariol Acts as a Topoisomerase Poison, Preferentially Affecting the IIalpha Isoform. Mol. Nutr. Food Res. 2009, 53, 441–451. [Google Scholar] [CrossRef]
- Ostry, V. Alternaria Mycotoxins: An Overview of Chemical Characterization, Producers, Toxicity, Analysis and Occurrence in Foodstuffs. World Mycotoxin J. 2008, 1, 175–188. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA) Management of Left-Censored Data in Dietary Exposure Assessment of Chemical Substances. EFSA J. 2010, 8, 1557. [CrossRef]
- Commission Recommendation (EU) 2022/553 of 5 April 2022 on Monitoring the Presence of Alternaria Toxins in Food. Off. J. Eur. Union 2022, 9, 90–92.
- Nguegwouo, E.; Sone, L.E.; Tchuenchieu, A.; Tene, H.M.; Mounchigam, E.; Njayou, N.F.; Nama, G.M. Ochratoxin A in Black Pepper, White Pepper and Clove Sold in Yaoundé (Cameroon) Markets: Contamination Levels and Consumers’ Practices Increasing Health Risk. Int. J. Food Contam. 2018, 5, 1. [Google Scholar] [CrossRef]
- Vyhnánek, T.; Hanáček, P.; Šafránková, I.; Dordević, B.; Beranová, H.; Trojan, V.; Havel, L. Molecular Detection of Fungi in Paprika, Chili Powder and Black Pepper. Acta Univ. Agric. Silvic. Mendel. Brun. 2018, 66, 927–937. [Google Scholar] [CrossRef]
- Iha, M.H.; Trucksess, M.W. Management of Mycotoxins in Spices. J. AOAC Int. 2019, 102, 1732–1739. [Google Scholar] [CrossRef]
- Kabak, B.; Dobson, A.D.W. Mycotoxins in Spices and Herbs-An Update. Crit. Rev. Food Sci. Nutr. 2017, 57, 18–34. [Google Scholar] [CrossRef]
- Gialluisi, K.; El Darra, N.; Nicoletti, M.G.; Solfrizzo, M.; Gambacorta, L. Natural Occurrence of Main Mycotoxins in Herbs and Spices Commercialized in Italy. Foods 2025, 14, 1889. [Google Scholar] [CrossRef]
- Gambacorta, L.; El Darra, N.; Fakhoury, R.; Logrieco, A.F.; Solfrizzo, M. Incidence and Levels of Alternaria Mycotoxins in Spices and Herbs Produced Worldwide and Commercialized in Lebanon. Food Control 2019, 106, 106724. [Google Scholar] [CrossRef]
- Potortì, A.G.; Tropea, A.; Lo Turco, V.; Pellizzeri, V.; Belfita, A.; Dugo, G.; Di Bella, G. Mycotoxins in Spices and Culinary Herbs from Italy and Tunisia. Nat. Prod. Res. 2020, 34, 167–171. [Google Scholar] [CrossRef]
- Lattanzio, V.M.T.; Verdini, E.; Sdogati, S.; Bibi, R.; Ciasca, B.; Pecorelli, I. Monitoring Alternaria Toxins in Italian Food to Support Upcoming Regulation. Food Addit. Contam. Part B Surveill. 2022, 15, 42–51. [Google Scholar] [CrossRef]
- Gambacorta, L.; Magistá, D.; Perrone, G.; Murgolo, S.; Logrieco, A.F.; Solfrizzo, M. Co-Occurrence of Toxigenic Moulds, Aflatoxins, Ochratoxin A, Fusarium and Alternaria Mycotoxins in Fresh Sweet Peppers (Capsicum annuum) and Their Processed Products. World Mycotoxin J. 2018, 11, 159–173. [Google Scholar] [CrossRef]
- Asam, S.; Lichtenegger, M.; Liu, Y.; Rychlik, M. Content of the Alternaria Mycotoxin Tenuazonic Acid in Food Commodities Determined by a Stable Isotope Dilution Assay. Mycotoxin Res. 2012, 28, 9–15. [Google Scholar] [CrossRef]
- Mujahid, C.; Savoy, M.C.; Baslé, Q.; Woo, P.M.; Ee, E.C.Y.; Mottier, P.; Bessaire, T. Levels of Alternaria Toxins in Selected Food Commodities Including Green Coffee. Toxins 2020, 12, 595. [Google Scholar] [CrossRef] [PubMed]
- da Cruz Cabral, L.; Terminiello, L.; Fernández Pinto, V.; Fog Nielsen, K.; Patriarca, A. Natural Occurrence of Mycotoxins and Toxigenic Capacity of Alternaria Strains from Mouldy Peppers. Int. J. Food Microbiol. 2016, 236, 155–160. [Google Scholar] [CrossRef]
- Hashem, M.; Alamri, S. Contamination of Common Spices in Saudi Arabia Markets with Potential Mycotoxin-Producing Fungi. Saudi J. Biol. Sci. 2010, 17, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Siruguri, V.; Bhat, R.V. Assessing Intake of Spices by Pattern of Spice Use, Frequency of Consumption and Portion Size of Spices Consumed from Routinely Prepared Dishes in Southern India. Nutr. J. 2015, 14, 7. [Google Scholar] [CrossRef] [PubMed]
- Commission Decision 2002/657/EC of 12 August 2002 Implementing Council Directive 96/23/EC Concerning the Performance of Analytical Methods and the Interpretation of Results. Available online: https://eur-lex.europa.eu/eli/dec/2002/657/oj/eng (accessed on 6 October 2025).

| Alternaria Mycotoxins | LOD (µg/kg) | LOQ (µg/kg) |
|---|---|---|
| TeA | 1.0 | 3.4 |
| AOH | 2.2 | 7.4 |
| AME | 0.8 | 2.7 |
| TEN | 3.2 | 11.2 |
| ALT | 5.4 | 17.9 |
| (a) | Spices (n = 72) | TeA | AME | AOH | TEN | ALT |
| Mean (µg/kg) | 1040.3 | 1.4 | 5.0 | 12.3 | nd | |
| Median (µg/kg) | 0.5 | 0.4 | 1.1 | 1.6 | nd | |
| Max (µg/kg) | 12,611.8 | 16.9 | 35.6 | 359.5 | nd | |
| n. positives | 33 | 20 | 18 | 14 | 0 | |
| % positives | 46 | 28 | 25 | 19 | 0 | |
| (b) | Herbs (n = 20) | TeA | AME | AOH | TEN | ALT |
| Mean (µg/kg) | 93.1 | 5.0 | 8.0 | 4.8 | nd | |
| Median (µg/kg) | 25.7 | 0.4 | 1.1 | 1.6 | nd | |
| Max (µg/kg) | 938.8 | 65.4 | 57.9 | 23.9 | nd | |
| n. positives | 12 | 8 | 7 | 4 | 0 | |
| % positives | 60 | 40 | 35 | 20 | 0 |
| (a) | Spices (72) | TeA μg/kg (n.pos) | TEN μg/kg (n.pos) | AOH μg/kg (n.pos) | AME μg/kg (n.pos) | ALT μg/kg (n.pos) | Sum of Means μg/kg |
| Flax seeds (2) | 10,261.9 (2) | 17.8 (1) | 1.1 (0) | 0.4 (0) | 2.7 (0) | 10,283.9 | |
| Paprika (3) | 9640.6 (3) | 36.6 (3) | 13.0 (2) | 5.1 (3) | 2.7 (0) | 9698.0 | |
| Red chili (4) | 4274.9 (4) | 14.0 (1) | 3.6 (1) | 2.4 (3) | 2.7 (0) | 4297.6 | |
| Licorice (1) | 1158.3 (1) | 53.6 (1) | 20.1 (1) | 3.8 (1) | 2.7 (0) | 1238.5 | |
| Hemp seeds (1) | 985.2 (1) | 105.6 (1) | 1.1 (0) | 0.4 (0) | 2.7 (0) | 1095.0 | |
| Mix spices (5) | 841.0 (2) | 7.2 (2) | 1.1 (0) | 0.4 (0) | 2.7 (0) | 852.4 | |
| Cumin (2) | 228.6 (2) | 180.5 (2) | 1.1 (0) | 0.4 (0) | 2.7 (0) | 413.3 | |
| Sichuan pepper (1) | 251.4 (1) | 1.6 (0) | 18.5 (1) | 3.4 (1) | 2.7 (0) | 277.6 | |
| Coriander seeds (1) | 227.4 (1) | 1.6 (0) | 15.3 (1) | 6.1 (1) | 2.7 (0) | 253.1 | |
| Mustard seeds (1) | 104.9 (1) | 28.7 (1) | 1.1 (0) | 0.4 (0) | 2.7 (0) | 137.8 | |
| Nutmeg (3) | 109.0 (2) | 1.6 (0) | 1.1 (0) | 0.4 (0) | 2.7 (0) | 114.8 | |
| Sunflower seeds (1) | 59.5 (1) | 1.6 (0) | 1.1 (0) | 0.4 (0) | 2.7 (0) | 65.3 | |
| Cinnamon (5) | 36.3 (2) | 1.6 (0) | 13.6 (4) | 1.6 (2) | 2.7 (0) | 55.8 | |
| Chia seeds (1) | 37.0 (1) | 1.6 (0) | 12.4 (1) | 0.4 (0) | 2.7 (0) | 54.1 | |
| Turmeric (3) | 39.1 (2) | 1.6 (0) | 1.1 (0) | 0.4 (0) | 2.7 (0) | 44.9 | |
| Garlic (7) | 16.7 (3) | 3.0 (1) | 10.0 (3) | 3.2 (4) | 2.7 (0) | 35.6 | |
| Sesame seeds (2) | 17.2 (2) | 1.6 (0) | 5.9 (1) | 3.8 (1) | 2.7 (0) | 31.2 | |
| Ginger (3) | 20.7 (1) | 1.6 (0) | 1.1 (0) | 0.4 (0) | 2.7 (0) | 26.5 | |
| Black pepper (5) | 4.4 (1) | 1.6 (0) | 6.1 (2) | 0.8 (2) | 2.7 (0) | 15.6 | |
| Onion (5) | 0.5 (0) | 2.4 (1) | 4.5 (1) | 1.3 (1) | 2.7 (0) | 11.4 | |
| Fennel seeds (3) | 0.5 (0) | 2.9 (1) | 1.1 (0) | 0.4 (0) | 2.7 (0) | 7.6 | |
| Cardamon seeds (1) | 0.5 (0) | 2.9 (1) | 1.1 (0) | 0.4 (0) | 2.7 (0) | 7.6 | |
| Green pepper (2) | 0.5 (0) | 1.6 (0) | 1.1 (0) | 0.8 (1) | 2.7 (0) | 6.7 | |
| White pepper (3) | 0.5 (0) | 1.6 (0) | 1.1 (0) | 0.4 (0) | 2.7 (0) | 6.3 | |
| Juniper berries (1) | 0.5 (0) | 1.6 (0) | 1.1 (0) | 0.4 (0) | 2.7 (0) | 6.3 | |
| Pumpkin seeds (1) | 0.5 (0) | 1.6 (0) | 1.1 (0) | 0.4 (0) | 2.7 (0) | 6.3 | |
| Poppy seeds (1) | 0.5 (0) | 1.6 (0) | 1.1 (0) | 0.4 (0) | 2.7 (0) | 6.3 | |
| Anice seeds (1) | 0.5 (0) | 1.6 (0) | 1.1 (0) | 0.4 (0) | 2.7 (0) | 6.3 | |
| Fenugreek (1) | 0.5 (0) | 1.6 (0) | 1.1 (0) | 0.4 (0) | 2.7 (0) | 6.3 | |
| Cloves (2) | 0.5 (0) | 1.6 (0) | 1.1 (0) | 0.4 (0) | 2.7 (0) | 6.3 | |
| (b) | Herbs (20) | TeA μg/kg (n.pos) | TEN μg/kg (n.pos) | AOH μg/kg (n.pos) | AME μg/kg (n.pos) | ALT μg/kg (n.pos) | Sum of Means μg/kg |
| Basil (3) | 398.5 (2) | 13.5 (2) | 1.1 (0) | 0.4 (0) | 2.7 (0) | 416.2 | |
| Sage (2) | 111.1 (2) | 1.6 (0) | 37.9 (2) | 39.1 (2) | 2.7 (0) | 192.4 | |
| Oregano (3) | 91.1 (3) | 5.5 (1) | 8.2 (1) | 2.7 (2) | 2.7 (0) | 110.2 | |
| Mix herbs (1) | 51.9 (1) | 1.6 (0) | 13.5 (1) | 5.0 (1) | 2.7 (0) | 74.7 | |
| Mint (1) | 19.7 (1) | 1.6 (0) | 10.9 (1) | 1.3 (1) | 2.7 (0) | 36.2 | |
| Chives (1) | 0.5 (0) | 17.5 (1) | 10.7 (1) | 0.4 (0) | 2.7 (0) | 31.8 | |
| Parsley (3) | 16.7 (1) | 1.6 (0) | 1.1 (0) | 0.4 (0) | 2.7 (0) | 22.5 | |
| Dill (1) | 14.9 (1) | 1.6 (0) | 1.1 (0) | 0.4 (0) | 2.7 (0) | 20.7 | |
| Thyme (1) | 0.5 (0) | 1.6 (0) | 12.4 (1) | 1.3 (1) | 2.7 (0) | 18.5 | |
| Rosemary (3) | 10.9 (1) | 1.6 (0) | 1.1 (0) | 0.7 (1) | 2.7 (0) | 17.0 | |
| Marjoram (1) | 0.5 (0) | 1.6 (0) | 1.1 (0) | 0.4 (0) | 2.7 (0) | 6.3 |
| SPICES | |||||||
|---|---|---|---|---|---|---|---|
| Fruits | Seeds | Roots | Bark | Berries | Bulbs | Buds | |
| red chili (3) | chia (1) | licorice (1) | cinnamon (5) | green pepper (2) | onion (5) | cloves (2) | |
| paprika (3) | sunflower (1) | ginger (3) | black pepper (5) | garlic (7) | |||
| pumpkin (1) | turmeric (3) | pink pepper (1) | |||||
| mustard (1) | white pepper (3) | ||||||
| sesame (2) | Sichuan pepper (1) | ||||||
| flax (2) | juniper (1) | ||||||
| anise (1) | |||||||
| fennel (3) | |||||||
| cardamon (1) | |||||||
| poppy (1) | |||||||
| hemp (1) | |||||||
| nutmeg (3) | |||||||
| cumin (2) | |||||||
| coriander (1) | |||||||
| fenugreek (1) | |||||||
| N. of samples | 6 | 22 | 7 | 5 | 13 | 12 | 2 |
| Mycotoxins | Weighted mean of means (μg/kg) | ||||||
| TeA | 7668.2 | 1034.6 | 191.1 | 36.3 | 22.2 | 9.9 | 0.5 |
| AOH | 8.7 | 2.7 | 3.9 | 13.6 | 4.4 | 7.5 | 1.1 |
| AME | 4.1 | 1.0 | 0.9 | 1.6 | 0.9 | 2.4 | 0.4 |
| TEN | 27.3 | 25.5 | 9.0 | 1.6 | 1.6 | 2.7 | 1.6 |
| ALT | 2.7 | 2.7 | 2.7 | 2.7 | 2.7 | 2.7 | 2.7 |
| Sum | 7711.0 | 1066.4 | 207.6 | 55.8 | 31.7 | 25.3 | 6.3 |
| Study | Matrix | Max TeA Level (µg/kg) |
|---|---|---|
| Present study | Paprika | 12,611.8 |
| Arcella et al., 2016 [3] | Paprika | 8800 |
| Asam et al., 2012 [23] | Paprika | 2900 |
| Mujahid et al., 2020 [24] | Paprika | 18,856 |
| da Cruz Cabral et al., 2016 [25] | Peppers | 11,422 |
| Present study | Herbs | 938.8 |
| Mujahid et al., 2020 [24] | Herbs | 748 |
| Spices (67) | Mix Spices (5) | Herbs (19) | Mix Herbs (1) |
|---|---|---|---|
| Pink pepper (1) | Mix pepper 1 (1) | Chive (1) | Mix herbs 5 (1) |
| Green pepper (2) | Mix Berberè 2 (1) | Dill (1) | |
| Black pepper (5) | Curry 3 (2) | Sage (2) | |
| White pepper (3) | Mix Creola 4 (1) | Basil (3) | |
| Sichuan pepper (1) | Parsley (3) | ||
| Juniper berries (1) | Marjoram (1) | ||
| Sunflower seeds (1) | Rosmary (3) | ||
| Pumpkin seeds (1) | Thyme (1) | ||
| Mustard seeds (1) | Oregano (3) | ||
| Sesame seeds (2) | Mint (1) | ||
| Flax seeds (2) | |||
| Anise seeds (1) | |||
| Fennel seeds (3) | |||
| Chia seeds (1) | |||
| Cardamom seeds (1) | |||
| Poppy seeds (1) | |||
| Hemp seeds (1) | |||
| Cumin (2) | |||
| Coriander (1) | |||
| Licorice (1) | |||
| Fenugreek (1) | |||
| Paprika (3) | |||
| Red chili (3) | |||
| Nutmegs (3) | |||
| Cinnamon (5) | |||
| Ginger (3) | |||
| Curcuma (3) | |||
| Cloves (2) | |||
| Onion (5) | |||
| Garlic (7) |
| Analyte | Precursor Ion | Q1 (m/z) | Q3 (m/z) | DP (V) | EP (V) | CE(V) | CXP (V) |
|---|---|---|---|---|---|---|---|
| TeA | [TeA-H]− | 196.2 | 111.7 | −100 | −4 | −34 | −10 |
| 139.1 | −28 | ||||||
| 69.0 * | −62 | ||||||
| 82.8 | −58 | ||||||
| AOH | [AOH-H]− | 257.2 | 215.0 | −150 | −3 | −35 | −15 |
| 147.1 | −44 | ||||||
| 185.2 * | −38 | ||||||
| 156.9 | −40 | ||||||
| AME | [AME-H]− | 271.4 | 256.0 | −120 | −10 | −30 | −18 |
| 227.9 | −40 | −16 | |||||
| 213.2 | −50 | −15 | |||||
| 183.0 * | −55 | −12 | |||||
| TEN | [TEN-H]− | 413.5 | 141.1 | −150 | −5 | −30 | −15 |
| 213.8 * | −150 | −35 | |||||
| 271.2 | −160 | −23 | |||||
| 339.2 | −160 | −39 | |||||
| ALT | [ALT-H]− | 291.4 | 202.9 | −160 | −5 | −45 | −15 |
| 248.0 | −35 | ||||||
| 160.9 * | −58 | ||||||
| 188.8 | −46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gialluisi, K.; Nicoletti, M.G.; El Darra, N.; Solfrizzo, M.; Gambacorta, L. Occurrence and Levels of Emerging Alternaria Mycotoxins Detected in Spices and Herbs Marketed in Italy. Toxins 2025, 17, 552. https://doi.org/10.3390/toxins17110552
Gialluisi K, Nicoletti MG, El Darra N, Solfrizzo M, Gambacorta L. Occurrence and Levels of Emerging Alternaria Mycotoxins Detected in Spices and Herbs Marketed in Italy. Toxins. 2025; 17(11):552. https://doi.org/10.3390/toxins17110552
Chicago/Turabian StyleGialluisi, Katia, Maria Giovanna Nicoletti, Nada El Darra, Michele Solfrizzo, and Lucia Gambacorta. 2025. "Occurrence and Levels of Emerging Alternaria Mycotoxins Detected in Spices and Herbs Marketed in Italy" Toxins 17, no. 11: 552. https://doi.org/10.3390/toxins17110552
APA StyleGialluisi, K., Nicoletti, M. G., El Darra, N., Solfrizzo, M., & Gambacorta, L. (2025). Occurrence and Levels of Emerging Alternaria Mycotoxins Detected in Spices and Herbs Marketed in Italy. Toxins, 17(11), 552. https://doi.org/10.3390/toxins17110552





